Abstract
Rationale
Previously, we reported that the GABAA receptor containing α5 subunit played a significant role in the reinforcing actions of EtOH in rats selectively bred to consume alcohol. However, the role of the α5 receptor in regulating the neurobehavioral effects of EtOH in outbred rats is not known.
Objective
In the present study, RY024, a novel benzodiazepine (BDZ) inverse agonist with high affinity (Kd~ 0.40 nM) and selectivity (~67.3-fold) for the α5 receptor, was investigated for its capacity to antagonize EtOH’s reinforcing, motor impairing, and sedative effects in Long-Evans rats.
Methods
Rats were trained to lever press for EtOH under a fixed-ratio 1 schedule of reinforcement. Subsequent studies evaluated EtOH’s motor-impairing effects in an oscillating bar task, while EtOH’s sedative effects were measured in the open field.
Results
RY024 (0.125−3.5 mg/kg; IP) markedly reduced EtOH-maintained responding with no effects on water responding, except for the highest dose. RY024 (3.0−15 mg/kg; IP) also reversed the motor impairing effects of a moderate (0.75 g/kg), and intoxicating EtOH dose (1.25 g/kg) in the absence of intrinsic effects. Finally, RY024 (7.5 mg/kg) attenuated the sedation produced by the 1.25 g/kg EtOH dose; however, it failed to attenuate the sedation induced by the 0.75 g/kg EtOH dose. Given alone, RY024 exhibited intrinsic effects in the open field.
Conclusion
The results suggest the GABAA receptor containing α5 subtype plays an important role in regulating the reinforcing, motor-impairing, and sedative effects of alcohol in outbred rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The γ-aminobutyric acid (GABA) system has been suggested to be the best evidence for a “single” neurotransmitter in regulating the neurobehavioral effects of ethanol (EtOH) (Draski and Deitrich 1996; June et al. 1998e). These effects include EtOH’s euphoric (McBride and Li 1998; June et al. 2001), motor impairing, sedative (Draski and Deitrich 1996; June et al. 1998d, 1998e), and anxiolytic (Liljequist and Engel 1984; Koob et al. 1986; June et al. 1998b) actions. Most studies have employed negative GABAergic modulators such as benzodiazepine (BDZ) inverse agonists and competitive GABA antagonists to implicate GABA in EtOH’s actions (for review, see Jackson and Nutt 1995; also Draski and Deitrich 1996). While these compounds have proven to be useful pharmacological probes to delineate the GABA systems, these agents are classified as “non-selective” GABA antagonists (Barnard et al. 1998), and are not capable of delineating the specific roles of the various GABAA receptor subtypes in alcohol’s neurobehavioral effects.
Over the past 5 years, we have synthesized a series of 8-substituted imidazobenzodiazepines (Lui et al. 1995, 1996; Skolnick et al. 1997) developed from the partial inverse agonist Ro15-4513 (Suzdak et al. 1986) [see Table 1]. These agents exhibit both high affinity (Ki ~0.4–2.7 nM), and selectivity (~58- to 75-fold) for the α5 receptor. Previously, we reported that direct hippocampal infusions of one of the ligands (i.e. RY023) was highly effective in antagonizing the reinforcing actions of EtOH in alcohol-preferring rats (June et al. 2001). However, the role of the α5 receptor in regulating other behavioral effects of EtOH in outbred rats is not known.
While a variety of behavioral tests exist to examine the intoxicating effects of EtOH in rodents, several previous studies (Lê and Israel 1994; Bell et al. 2001) suggest that the oscillating bar represents a sensitive measure to evaluate EtOH’s motor impairing effects (i.e. ataxia). In the oscillating bar task, the ability of the rat to maintain its balance is related to the frequency of oscillations. Unlike previously learned measures, which are time demanding and labor intensive, a measurable criterion on the oscillating bar tasks can be reached in 1–2 days rather than weeks. Furthermore, the oscillating bar task has been specifically designed to evaluate low to moderate doses of EtOH (0.25–0.75 g/kg), and has also been suggested to be a behavioral tool suitable for investigation of the neurochemical mechanisms of both low and high alcohol doses (Lê and Israel 1994).
Similar to the oscillating bar, the open field apparatus can also be used to make inferences about the neurochemical mechanisms of low and high intoxicating doses of alcohol. However, unlike the oscillating bar, which measures ataxia, the open field is typically used to evaluate locomotor sedation (for review, see Draski and Deitrich 1996). Previous research has suggested the motor impairing and the locomotor sedation produced by BDZs are regulated by different receptor isoforms (Rudolph et al. 1999). It is possible that employing multiple dependent measures and pharmacological probes which target selective receptor subtypes, such strategies could be useful in delineating potential neurochemical mechanisms of alcohol intoxication.
“Intrinsic activity” is a term that is often used to describe the functional nature of BDZ ligands (Jackson and Nutt 1995; Sanger and Cohen 1995). For example, when a ligand fails to alter behavior or is functionally inactive in a behavioral test, it is said to “lack” intrinsic activity. In contrast, when it enhances or decreases behavior compared with placebo, it is believed to “possess” intrinsic activity. The concept of intrinsic activity is fundamentally important when attempting to evaluate the interactions of negative GABAergic modulators with alcohol since these agents typically enhance neuronal activity due to their proconvulsant/convulsant nature, and could consequently reduce alcohol actions by a subtractive mechanism, rather than by activation at specific GABA receptors (for an excellent review, see Jackson and Nutt 1995). Unfortunately, the relative magnitude of intrinsic activity observed following administration of a BDZ has been shown to be highly dependent on the behavioral paradigm employed (Nutt and Lister 1988; Jackson and Nutt 1995). Thus, to more accurately evaluate the intrinsic nature of novel BDZs, and their interaction with alcohol, it is important to employ multiple behavioral measures and species.
The objective of the present study was to test the hypothesis that an α5 subtype inverse agonist might be functionally relevant in regulating the reinforcing, motor impairing, and sedative effects of EtOH following systemic injections in Long-Evans rats. To enhance our capacity to observe intrinsic activity the interaction of the α5 inverse agonist with EtOH was evaluated on two behavioral measures (e.g. oscillating bar, open field).
Materials and methods
Animals
Male outbred Long-Evans rats (n=29) were obtained from Harlan Industries (Indianapolis, Ind., USA). Rats were approximately 4–5 months of age and weighed between 323 and 395 g at the beginning of the experiment. Animals in the EtOH self-administration and EtOH intoxicating studies were housed individually in wire-mesh stainless steel cages or plastic tubs. The vivarium was maintained at an ambient temperature of 21°C and was on a 12:12 reversed light:dark cycle (lights off at 0700 hours). All rats were provided ad libitum access to food and water. However, rats in the EtOH self-administration study were fluid deprived for 23 h daily during the initial 2 days of the training phase. Thereafter, rats were maintained on ad libitum food and water in a manner similar to the rats in the EtOH sedation studies. All training and experimental sessions took place between 10 a.m. and 3 p.m. All procedures were conducted in adherence with the NIH Guide for the Care and Use of Laboratory Animals (refer to the 1996 edition of the guide).
Drugs and solutions
RY024 was synthesized by two of the authors (X.H., J.M.C.) using previously published procedures (Lui et al. 1995, 1996). All RY024 drug treatments were prepared as an emulsion in 1% Tween-20 vehicle (Sigma Chemical Co., St Louis, Mo., USA) and mixed with a 0.90% sodium chloride solution to a volume of 1 ml/kg body weight. When necessary, some drug solutions were sonicated. For the EtOH self-administration study, EtOH (10% v/v) solutions were prepared daily by mixing 95% pure EtOH (USPA) with deionized water. For the oscillating bar and open field studies, EtOH (10% v/v) was prepared by mixing 95% pure EtOH with a 0.90% sodium chloride solution. Injection volumes were administered in volumes (2.7–4.3 ml) sufficient to produce 0.75 and 1.25 g/kg doses. All drug injections were given IP.
Apparatus
EtOH-maintained responding
Behavioral testing was conducted in 15 standard operant conditioning chambers (Coulbourn Instruments) equipped with two removable levers and two dipper fluid delivery systems enclosed in sound-attenuated cubicles (see June et al. 1998a, 1998b, 1998c). A green cue light above each lever was used to indicate the presence of a reinforcer. The light was illuminated for 2.5 s. Each reinforced response delivered 0.1 ml of the reinforcer (EtOH or water). The reinforcer was presented for a duration of 3 s. Operant conditioning sessions were 60 min. The operant conditioning chamber parameters used to assess the various reinforcers are well established in our laboratory (see June 2002).
Oscillating bar task
The oscillating bar test has been demonstrated to be a reliable measure of sensitivity and tolerance to the motor-impairing effects of injected EtOH (0.5–1.5 g/kg IP) (Lê and Israel 1994). The test measured the latency of a rat to fall from an elevated rectangular bar that moved in a 120° angle at a frequency of 10–60 oscillations over a 2-min period. For example, if a rat remained on the bar for 2 min it was removed and given a score of 120 s. At the beginning of each session, rats were placed in the middle of the bar and they were required to maintain their balance to avoid falling to the electrified grid floor (0.5 mA). The motor that controlled the bar was activated immediately following the placement of the animal on the bar. The apparatus was identical to that previously reported (Lê and Israel 1994).
Open-field arena
Locomotor activity in the open field was determined by two Digiscan Activity Monitors (Acuscan Electronics, Columbus, Ohio, USA). Each monitor consisted of a clear Plexiglas cage measuring 42 cm×42 cm×30 cm. Movement was detected by two sets of four infrared perpendicular photobeams in the walls of the chamber with 16 beams along each axis. Each interruption of a photobeam constituted a count. Data were collected and analyzed by an automated Digiscan Analyzer, which interfaced the two test chambers and a Macintosh computer. All experiments were conducted under dim lighting (25 W) conditions. The floor of the open field was cleaned after each subject to eliminate any traces of the previous rat’s path. The following activity parameters were evaluated in the open field: ambulatory counts; total distance traveled in cm.
General procedures
EtOH-maintained responding
The concurrent schedule procedure was used to investigate the capacity of RY024 to reduce EtOH-maintained responding. The procedures have been described previously (for review, see June et al. 1998a, 1998c; June 2002). After a period of stabilization on the concurrent FR1 schedule for pure EtOH (10% v/v) on both levers, water was then substituted in the dipper delivery system for one of the two levers. Following stabilization (i.e. +20% of the average responses for 5 consecutive days) on the concurrent FR1 schedule for EtOH and water, the drug treatment phase began (for specific details, see June 2002).
Blood alcohol concentration (BAC) measurement
To ensure animals were consuming pharmacologically relevant amounts of EtOH during operant sessions, BACs were collected in all animals on days they did not receive any drug treatment. After the first 20 min of the operant session, approximately 100 μl of whole blood was collected from the rats tail tip into a heparin coated microsample tube (for specific details of these procedures, see June 2002). This time period was selected since the rising phase of the blood alcohol curve have been shown to correlate significantly with the euphoric effects of EtOH (Lewis and June 1990). After collection, the whole blood was immediately centrifuged for 5 min at 1100 rpm. Plasma samples of 5 μl were collected with a Gilson Microman M-25 Pipette and injected directly into a GL-5 Analyzer (Analox Instruments, Luxenburg, Mass., USA). Results were calculated in units of mg/dl and printed within 20 s of each trial.
Experimental treatment phase
Following the baseline stabilization phase, animals (n=15) were pretreated with RY024 (0 0.125 0.50 1.5 and 3.5 mg/kg) 5 min prior to placing them in the operant conditioning chamber. All rats received their drug treatment in a randomized design, to control for order and sequence effects. To control for residual carryover effects, each drug pretreatment was separated by at least 3–4 days and subsequent pretreatments were never administered until both the EtOH and water-maintained responding had returned to baseline levels for 3 consecutive days.
Training and acclimation phase
Oscillating bar
Prior to any exposure to EtOH, each rat was trained daily for 14 days at a speed of 20 oscillations/min (Lê and Israel 1994). During the initial week, rats were given two or three daily training sessions, while during the second week they were given two daily training sessions. Each training session consisted of two 2-min trials for a total of four trials per day. The dependent variable measure was time spent on the bar in seconds per trial. A baseline control condition for the acclimation phase was determined by averaging the two 2-min trials during the second week.
Open-field activity
To habituate the rats to the activity monitor prior to any drug treatment, rats were given five daily 10-min sessions (June et al. 1995a, 1995b, 1998b). These sessions thoroughly habituated the animals to the open field arena. As noted above, the dependent variable measures were horizontal count (i.e. ambulatory counts) and the total distance traveled in cm.
Experimental treatment phase
Oscillating bar
Similar to the acclimation phase, two daily training sessions (a total of four trials) were given on the experimental drug-treatment days. The first two trials evaluated the performance on the oscillating bar in the absence of any drug treatment. These data were combined and a mean was calculated on the two baseline trials. These data represented the baseline control condition. The second two trials assessed the effects of the drug treatment. The average of the two 2-min trials for the drug treatment data was also combined and a mean calculated for the drug treatment trials. The combined drug treatment data were then compared against the combined baseline control condition data. All rats participating in the oscillating bar (n=10) received their drug treatment in a randomized design to control for order and sequence effects. RY024 (3.0–7.5 mg/kg) was administered alone or in combination with EtOH. When given as a combination treatment, RY024 was given 5 min prior to a placebo saline injection. Five minutes after the saline injections, animals were placed on the oscillating bar for the two 2-min trials. Only the 7.5 mg dose of RY024 was tested alone on the oscillating bar task due to the limited quantity of the test agent. A minimum of 3 and a maximum of 5 days were allotted between each drug treatment days. The maximum times followed the 1.25 g/kg treatments.
Open-field activity
In the open field tests, a separate group of rats (n=10) were used to evaluate the effects of the drug treatments. All rats participating in the open-field activity (n=10) studies received their drug treatment in a randomized design to control for order and sequence effects. RY024 (3–15 mg/kg) was administered alone, or in combination with EtOH. When given as a combination treatment RY024 was given 5 min prior to the EtOH treatment (e.g. 0.75 or 1.25 g/kg). Five minutes after the EtOH injections, animals were placed in the open field for a 10-min test. When given alone, RY024 was given 5 min prior to a placebo saline injection. Five minutes after the saline injections, animals were placed in the open field for a 10-min test. Between drug injection days, activity measurements were collected and evaluated to determine if any baseline shifting occurred during the testing phase. Only the 7.5 mg dose of RY024 was tested alone in the open field due to the limited quantity of the test agent. Similar to the oscillating bar study, a minimum of 3 and a maximum of 5 days were allotted between each drug treatment days. Again, the maximum periods followed the 1.25 g/kg treatments.
Statistical analysis
The operant response data were analyzed by a single factor repeated measures ANOVA with drug treatment (i.e. dose) as the independent factor. The dependent variables were EtOH and water-maintained responding. Each dependent variable was analyzed separately. Post-hoc comparisons between individual drug treatments were made using the Newman-Keuls test in all experiments. In the oscillating bar and locomotor activity studies, a single factor repeated measures ANOVA was conducted to examine the effects of the treatment conditions. The duration of time spent on the oscillating bar (e.g. seconds), and the number of beam breaks were the dependent measures for the two behavioral tasks. For locomotor activity, separate analyses were conducted on each of the locomotor activity parameters (e.g. ambulatory counts total distance traveled in cm).
Results
Blood EtOH content (BAC)
EtOH responding in the Wistar rats yielded intakes of 0.56–1.1 g/kg absolute EtOH. BACs ranged from 21 to 36 mg/dl with a mean of 29±6.5 mg/dl.
Effects of RY024 on EtOH and water-maintained responding
Figure 1 shows the effect of RY024 on EtOH and water-maintained responding under the concurrent FR1 schedule. Except for the lowest dose (0.125 mg/kg), RY024 (0.50–3.5 mg/kg) produced a marked suppression on EtOH-maintained responding with all doses tested. Compared with the control condition, responding was reduced by 72–76% of control levels. However, the effects were not dose related. A dose of 3.5 mg/kg was no more effective in suppressing EtOH-maintained responding than a 0.50 mg dose. A highly significant main effect of drug treatment emerged from these data [F(4,56)=38.53, P<0.0001]. Newman-Keuls post-hoc tests revealed that the 0.50–3.5 mg/kg RY023 doses significantly suppressed EtOH-maintained responding compared with the control condition (P<0.01).
Figure 1 (lower panel) shows rates of responding maintained by water following injection of the 0.125–3.5 mg/kg dose of RY023. Compared with the control condition, RY024 was without effect on water responding with the 0.125–1.5 mg/kg doses, however, the highest dose reduced water maintained responding. These data yielded a highly significant main effect of drug treatment [F(4,56)=11.16, P<0.0001]. Newman-Keuls post-hoc tests revealed that only the 3.5 mg/kg dose of RY023 significantly suppressed water-maintained responding compared with the control condition (P<0.01).
Effects of RY024, EtOH, and RY024 in combination with EtOH on the oscillating bar
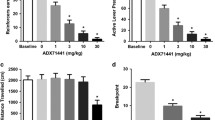
Figure 2 shows the control baseline condition (i.e. average of the two trials before the drug treatments) (BL) compared with the EtOH (0.75 g/kg and 1.25 g/kg), RY024 (7.5 mg/kg), and three combination treatment conditions [i.e. RY024 (3.0 mg/kg)+EtOH (0.75 g/kg); RY024 (7.5 mg/kg)+EtOH (0.75 g/kg) and RY024 (7.5 mg/kg)+EtOH (1.25 g/kg]. As noted above, because of the limited quantity of the α5 selective ligand, only the 7.5 mg/kg RY024 dose was tested alone. Compared with the control condition, the 0.75 and 1.25 g/kg EtOH doses reduced the duration of time spent on the bar by 48% and 93% of control levels, respectively. However, when the 3.0 and 7.5 mg doses of RY024 were given prior to EtOH, both completely reversed the EtOH-induced suppression produced by the 0.75 g/kg EtOH dose. The 7.5 mg/kg dose was also observed to attenuate the motor impairing effects produced by the 1.25 g/kg EtOH dose, but did not completely reverse it. Time spent on the bar was reduced only to about 30% of control levels with the 7.5 mg/kg+1.25 g/kg EtOH dose, compared with 93% with the 1.25 g/kg EtOH dose alone (see Fig. 2). When the 7.5 mg/kg dose of RY024 was given alone, it failed to alter the time spent on the oscillating bar. These data profiles yielded a significant main effect of drug treatment [F(6,72)=47.63], (P<0.001). Newman-Keuls post-hoc test confirmed that the EtOH alone doses significantly reduced the time spent on the oscillating bar relative to the control condition (P<0.01). In addition, RY024 reversed the sedation of the 0.75 g/kg dose with 3.0 and 7.5 mg/kg doses (P<0.001), while the 7.5 mg/kg dose attenuated the sedation produced by the 1.25 g/kg doses (P<0.001). A post-hoc test also confirmed that the 7.5 mg RY024 treatment did not alter the number of oscillations relative to the control condition (P>0.05). Animals given the RY024 treatment were indistinguishable from control levels. Finally, post-hoc analyses showed that the baseline control condition was similar to the 0.75 g/kg EtOH combination conditions [3.0 7.5 mg/kg RY024], confirming the complete reversal of the 0.75 g/kg EtOH dose condition (P>0.05).
Effects of IP injections of RY024 (3.0 mg 7.5 mg) on the motor-impairing effects produced by the 0.75 and 1.25 g/kg EtOH doses on an oscillating bar task in Long-Evans rats. Bars are ±SEM (n=14 rats). *P=0.05, baseline condition is significantly greater than the EtOH treatment. † P=0.05, the combination treatment is significantly different from the EtOH treatment
Effects of RY024, EtOH, and RY024 in combination with EtOH in the open field
The two locomotor activity parameters revealed identical effects of the drug treatments; hence to avoid redundancy, we present only one of the activity parameters. Figure 3 shows the control baseline condition (i.e. pooled average of the three trials before the drug treatments) (BL) compared with the EtOH (0.75 g/kg 1.25 g/kg), RY024 (7.5 mg/kg), and three combination treatment conditions [i.e. RY024 (3.0 mg/kg)+EtOH (0.75 g/kg); RY024 (7.5 mg/kg)+EtOH (0.75 g/kg); RY024 (7.5 mg/kg)+EtOH (1.25 g/kg)] on the ambulatory count parameter. A highly significant main effect of drug treatment emerged from these data [F(6,72)=84.87], (P<0.001). When the 3.0 mg RY024 dose was given prior to the 0.75 g/kg EtOH dose, it potentiated the EtOH-induced sedation (P<0.01). Similarly, the 7.5 mg/kg RY024 treatment also potentiated the suppression of the 0.75 g/kg EtOH dose (P<0.01), albeit the magnitude of sedation was not as great as the 3.0 mg/kg treatment. In contrast, the 7.5 mg/kg RY024 treatment attenuated the profound sedation produced by the 1.25 g/kg EtOH dose (P<0.01). However, given alone, the 7.5 mg/kg dose produced marked intrinsic effects (i.e. suppression) on locomotion (P<0.01) (see Fig. 3). As noted above, because of the limited quantity of the α5 selective ligand, only the 7.5 mg/kg RY024 dose was tested alone in the open field.
Effects of IP injections of RY024 (7.5 15 mg) on the sedation produced by the 0.75 and 1.25 g/kg EtOH doses in the open field in Long-Evans rats. Bars are ±SEM (n=14 rats). *P=0.05, baseline condition is significantly greater than the EtOH treatments and RY024 treatment alone. † P=0.05, the combination treatment is significantly different from the EtOH treatment
Discussion
The data from the present study on EtOH-maintained responding with RY024 are in agreement with our recent finding in P rats (June et al. 2001). That study demonstrated that RY023, a structurally related α5 selective ligand, was highly selective in suppressing EtOH-maintained responding following direct microinfusion into the hippocampus. Several prior reports have shown that the hippocampus contain the greatest concentration of α5 receptors in the CNS (Wisden et al. 1992; Turner et al. 1991). It is possible that the hippocampal α5 receptors may also regulate alcohol-motivated responding following systemic administrations of an α5 selective ligand. In any event, the present data extend those in P rats by demonstrating that a structurally similar α5 selective ligand can suppress EtOH-maintained responding even in outbred rats following systemic administration. Moreover, both studies add credence to our hypothesis that the hippocampus may represent an extension of the mesolimbic circuitry that regulates EtOH-seeking behaviors (see June et al. 2001).
On the oscillating bar task, RY024 completely antagonized the motor impairing effects produced by a moderate dose of EtOH (e.g. 0.75 g/kg). RY024 also markedly attenuated the profound motor impairing effects produced by the 1.25 g/kg EtOH dose. The higher doses of RY024 (7.5 mg/kg) given alone did not alter performance on the oscillating bar. Thus, the RY024-induced antagonism of EtOH’s motor impairing effects occurred independent of intrinsic effects.
In the open-field, neither RY024 dose (e.g. 3.0 and 7.5 mg) attenuated the mild sedation produced by the 0.75 g/kg EtOH dose. Rather, RY024 appeared to potentiate the suppression of the 0.75 g/kg EtOH dose. In contrast, RY024 attenuated the sedation of the 1.25 g/kg EtOH dose. Given alone, the 7.5 mg dose of RY024 produced profound intrinsic effects. Moreover, the magnitude of intrinsic activity seen with the 7.5 mg dose of RY024 was similar to the suppression of the higher EtOH dose (1.25 g/kg), and markedly lower than the reduction of the 0.75 g/kg EtOH dose. Thus, an alternative hypothesis is that the 0.75 g/kg EtOH dose may have antagonized the inhibitory/intrinsic actions of the 7.5 mg dose of RY024. Unfortunately, to resolve this interactional conundrum, additional studies will have to be conducted.
The data from the present study showing that RY024 did not produce any apparent intrinsic effect on the oscillating bar are in agreement with previous research evaluating the capacity of local injections of the imidazobenzodiazepine inverse agonists (e.g. Ro15-4513, RY008) to antagonize the sedative effects of EtOH on the rotorod (Dar 1992; Meng and Dar 1994), and open field (June et al. 1998a). In our previous study, systemic injections of RY008 (5 mg and 10 mg) (a structurally related inverse agonist) also antagonized the sedation produced by the 1.25 g/kg EtOH doses (June et al. 1998d). However, as in the present study, RY008 was not effective in blocking the sedation produced by the 0.75 g/kg dose. Also, similar to the present study, when the 5 and 10 mg doses of RY008 were given alone, both produced intrinsic effects (i.e. reduction in performance). Taken together, the data of the present study along with our previous research allow several interpretations to be made regarding the capacity of selective and non-selective ligands to antagonize moderate to high doses of EtOH.
First, in agreement with Jackson and Nutt (1995), the present study confirms that the degree to which intrinsic activity is observed with BDZ inverse agonists may be related to the behavioral paradigm employed. It is important to note, however, that the intrinsic actions of RY024 in the current study were in the same direction as EtOH. Nevertheless, RY024 was still capable of attenuating the behavioral actions of EtOH. Thus, we propose this precludes a “subtractive hypothesis” in explaining the attenuation of alcohol’s action in the open field. Second, systemic injections of BDZ inverse agonists appear to be more effective in attenuating, but not reversing the EtOH sedation produce by doses >1.25 g/kg (June et al. 1998d). Third, in the open field, partial and full BDZ inverse agonists (e.g. RY024 CGS8216) both appear to augment the EtOH-induced sedation produce by the 0.75 g/kg dose (the current study; also see June et al. 1998e). We have also observed a similar interactional profile with Ro15-4513 and the 0.75 g/kg EtOH dose (unpublished observation). The rationale for this is not exactly clear, however, it may reflect the capacity of a ligand to enhance GABA’s action at specific GABAA receptor subtypes, and in turn potentiate EtOH’s sedative effects. For example, to our surprise, we (June et al. 1998d) previously observed that RY008 and Ro15-4513 potentiated rather than reduced GABA current at α6β2γ2 receptors expressed in Xenopus oocytes (33% and 48%, respectively). Wafford et al. (1993) reported a similar augmentation by Ro15-4513 at both the α4 and α6 receptor subtypes. Recall that the diazepam insensitive (DI) receptors have been suggested to play an important role in the sedative effects of moderate to large doses of EtOH (Turner et al. 1991; Korpi et al. 1993). In further support of the hypothesis that inverse agonist may produce agonist effects at selected αreceptors, Wong et al. (1993), using cortical and cerebellar membranes, demonstrated that ZG-63 (an inverse agonist) displayed positive, negative, and neutral effects at cortical diazepam sensitive, cerebellar diazepam sensitive, and cerebellar diazepam insensitive receptors, respectively, under the “GABA shift assay” (Skolnick et al. 1982). Finally, that the α5 ligand interacted with the lower and higher EtOH doses in a qualitatively different manner in the open field compared with the oscillating bar task could suggest that the two tasks are exploiting different, but overlapping neuromechanism(s) of action. This remains to be tested.
In summary, the present results demonstrate that RY024, the α5 selective ligand is capable of antagonizing the reinforcing, motor impairing, and the sedative effects of EtOH in Long-Evans rats. The exact neuromechanisms regulating the actions of the α5 ligand in these behaviors are not clearly understood. We postulate that the hippocampus (which contains the highest concentration of α5 receptors in the CNS) (see Wisden et al. 1992) to be a pivotal substrate regulating efferent and afferent projection pathways modulating alcohol’s neurobehavioral effects. The role played by intrinsic efficacy in the capacity of RY024 to antagonize alcohol’s action cannot unequivocally be determined in the present study; however, our data do not strongly support a direct role for the modulatory influences of intrinsic efficacy in the behaviors examined in the present study. Nevertheless, the identification of the GABA α5 receptor subtype ligands affords alcohol researchers a unique opportunity to explore the role of this GABA receptor in alcohol’s neurobehavioral effects.
References
Barnard EA, Skolnick P, Olsen RW, Möhler W, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ (1998) International union of pharmacology. XV. Subtypes of γ-aminobutyric acid receptors: classification on the basic of subunit structure and receptor function. Pharmacol Rev 50:291–313
Bell R L, Stewart R B, Woods II J E, Lumeng L, Li T K, Murphy J M, McBride W J (2001) Responsivity and development of tolerance to the motor impairing effects of moderate doses of ethanol in alcohol-preferring (P) and –nonpreferring (NP) rat lines. Alcohol Clin Exp Res 25:644–650
Dar MS (1992) Selective antagonism of acute ethanol-induced motor disturbances by centrally administered Ro15-4513 in mice. Pharmacol Biochem Behav 42:473–479
Draski LJ, Deitrich RA (1996) Initial effects of ethanol on the central nervous system. In: Deitrich RA, Erwin VG (eds) Pharmacological effects of ethanol on the nervous system. CRC Press, Boca Raton, pp 244–277
Jackson HC, Nutt DJ (1995) Inverse agonists and alcohol. In: Sarter M, Nutt DJ, Lister RG (eds) Benzodiazepine receptor inverse agonists. Wiley-Liss, New York, pp 113–118
June HL (2002) Preclinical models to evaluate potential pharmacotherapeutic agents in treating alcoholism and studying the neuropharmacological bases of ethanol-seeking behaviors in rats. In: Crawley J, Gerfen C, Mc Kay R, Rogawski M, Sibley D, Skolnick P (eds) Current protocols in neuroscience. Wiley, New York, pp 1–23
June HL, Lin M, Greene TL, Lewis MJ, Murphy JM (1995a) Effects of negative modulators of GABAergic efficacy on ethanol intake: correlation of biochemical changes with pharmacological effect using a behavioral paradigm. J Exp Clin Psychopharmacol 3:252–258
June HL, Duemler S, Greene TL,Williams J, Lin M, Chen SHA, Lewis MJ, Murphy JM (1995b) Effects of RO19-4603 on the maintenance of tolerance to a single dose of ethanol. J Pharmacol Exp Ther 247:1105–1112
June HL, Torres L, Cason CR, Hwang BH, Braun MR, Murphy JM (1998a) The novel benzodiazepine inverse agonist RO19-4603 antagonizes ethanol motivated behaviors: neuropharmacological studies. Brain Res 784: 256–275
June HL, Zucarelli D, Craig KS, DeLong J, Cason CR, Torres L, Murphy JM (1998b) High affinity benzodiazepine antagonists reduce responding maintained by EtOH presentation in ethanol-preferring (p) rats. J Pharmacol Exp Ther 284:1006–1014
June HL, Eggers MW, Warren-Reese C, Ricks A, Cason CR (1998c) The effects of the novel benzodiazepine receptor inverse agonist Ru34000 on ethanol-maintained responding. Eur J Pharmacol 350:151–158
June H L, Cason C R, Cheatham G, Ruiyan L, Gan T, Cook JM (1998d) GABAA-benzodiazepine receptors in the striatum are involved in the sedation produced by a moderate, but not an intoxicating ethanol dose in outbred Wistar rats. Brain Res 794:103–118
June HL, Dejaravu SL, Williams J, Cason CR, Eggers MW, Greene TL, Leviege T, Torres LB, Murphy JM (1998e) GABAergic modulation of the behavioral actions of in alcohol-preferring (P) and nonpreferring (NP) rats. Eur J Pharmacol 42:139–151
June HL, Harvey SC, Foster KL, McKay PF, Cummings R, Garcia M, Mason D, Grey C, McCane SL, Williams L, Durr LF, Johnson TB, Xiaohui H, Rock S, Cook JM (2001) GABAA-receptors containing α5 subunits in the CA1 and CA3 hippocampal fields regulate ethanol-motivated behaviors: an extended ethanol reward circuitry. J Neurosci 21:2166–2177
Koob GF, Braestrup C, Britton KT (1986) The effects of Ro15-4513 on the release of punished responding produced by chlordiazepoxide and ethanol in the rat. Psychopharmacology 90:173–178
Korpi ER, Kleingoor C, Ketterman H, Seeburg PH (1993) Benzodiazepine-induced motor impairment linked to point mutation in cerebellar GABAA receptor. Nature 361:356
Lê AD, Israel Y (1994) A simple technique for quantifying intoxication-induced by low doses of ethanol. Pharmacol Biochem Behav 48:229–234
Lewis MJ, June HL (1990) Neurobehavioral studies of ethanol reward and activation. Alcohol 7:213–219
Liljequist S, Engel J (1982) Effects of GABAergic agonists and antagonists on various ethanol-induced behavioral changes. Psychopharmacology 64:61–65
Lui RY, Zhang PW, McKernan R, Wafford K, Cook J M (1995) Synthesis of a novel imidazobenzodiazepine ligands for the α5β2γ2 Bz5 GABAA receptor subtype. Med Chem Res 5:700–709
Lui RY, Hu RJ, Zhang PW, Skolnick P, Cook J M (1996) Synthesis and pharmacological properties of novel 8-substituted imidazobenzodiazepines: high affinity, selective probes for α5 containing GABAA receptors. J Med Chem 39:1928–1934
McBride WJ, Li TK (1998) Animals models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol 12:339–369
Meng Z, Dar MS (1994) Intrastriatal Ro15-4513 functionally antagonizes ethanol-induced motor incoordination and striatal adenosinergic modulation of ethanol-induced motor incoordination in rats. J Pharmacol Exp Ther 271:524–534
Nutt DJ, Lister RG (1989) Antagonizing the anticonvulsant effects of ethanol using drugs acting at the benzodiazepine/GABA receptor complex. Pharmacol Biochem Behav 31:751–755
Rudolf U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H (1999) Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature 401:796–800
Sanger DJ, Cohen C (1995) Fear and anxiety induced by inverse agonists. In: Sarter M, Nutt DJ, Lister RG (eds) Benzodiazepine receptor inverse agonists. Wiley, New York, pp 185–213
Skolnick P, Schweri M, Williams E, Moncada V, Paul S (1982) An in vitro test which differentiates benzodiazepines “ agonist” and “antagonist”. Eur J Pharmacol 78:133–136
Skolnick P, Hu R J, Cook CM, Hurt S D, Trometer JD, Lui R, Huang Q, Cook JM (1997) RY80: A high affinity, selective ligand for γ-aminobutyric acidA receptors containing alpha-5 subunits. J Pharmacol Exp Ther 283:488–493
Suzdak P, Glowa JR, Crawley JN, Schwartz RD, Skolnick P, Paul SM (1986) A selective imidazodiazepine antagonist of ethanol in the rat. Science 234:1243–1247
Turner D, M Sapp, DW, Olsen RW (1991) The benzodiazepine/alcohol antagonist RO15-4513: binding to a GABAA receptor subtype that is insensitive to diazepam. J Pharmacol Exp Ther 257:1236–1242
Wafford KA, Bain CJ, Whiting PJ, Kemp JA (1993) Functional comparison of the role of γ subunits in recombinant human γ-aminobutyric acidA/benzodiazepine receptors. Mol Pharmacol 44:437–442
Wisden H, Laurie DJ, Monyer H, Seeburg PH (1992) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain: telencephalon, diencephalon, mesencephalon. J Neurosci 12:1040–1062
Wong et al. (1993)
Acknowledgements
This research was supported in part by grants AA10406, AA11555, and AA12407 (H.L.J.) from the National Institute of Alcohol Abuse and Alcoholism (NIAAA) and MH 46851 (J.M.C.) from the National Institute of Mental Health (NIH). Pete McKay was supported in part by a UROP stipend from the School of Science at IUPUI. Katrina Foster and Marin Garcia were supported in part by grant T35 M from the National Heart Lung and Blood Institute of the NIH (Short Term Training Program for Minority Students in Biomedical Research). Katrina Foster was also supported in part by a Minority Neuroscience Fellowship from the American Psychological Association and the Alcohol Research Center Training Grant from NIAAA (AA07462).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McKay, P.F., Foster, K.L., Mason, D. et al. A high affinity ligand for GABAA-receptor containing α5 subunit antagonizes ethanol’s neurobehavioral effects in Long-Evans rats. Psychopharmacology 172, 455–462 (2004). https://doi.org/10.1007/s00213-003-1671-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1671-z