Abstract
Rationale
Hypericum perforatum is used as a natural antidepressant, and other antidepressants have been marketed to aid in smoking cessation.
Objective
We investigated the effects of an extract of Hypericum perforatum (Ph-50) on withdrawal signs produced by nicotine abstinence in mice.
Methods
Nicotine (2 mg/kg, four injections daily) was administered for 14 days to mice. Different doses of Ph-50 (125–500 mg/kg) were administered orally immediately after the last nicotine injection. In another experiment, Ph-50 (500 mg/kg) was orally administered in combination with nicotine, i) starting from day 8 until the end of the nicotine treatment period, or ii) during nicotine treatment and after nicotine withdrawal, or iii) immediately after the last nicotine injection. On withdrawal from nicotine, all animals were evaluated for locomotor activity and abstinence signs.
Results
The locomotor activity reduction induced by nicotine withdrawal was abolished by Ph-50, which also significantly and dose-dependently reduced the total nicotine abstinence score when injected after nicotine withdrawal.
Conclusions
These data show that treatment with Hypericum perforatum attenuates nicotine withdrawal signs in mice. Further studies are necessary to test the possibility that it may be used for smoking cessation treatment in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tobacco addiction has a considerable health and economic impact on society and has become one of the largest health problems worldwide (Murray and Lopez 1997), making it necessary to develop strategies for reducing tobacco use and treating nicotine dependence.
Nicotine plays a key role in maintaining the smoking of tobacco and, if not the only reason for maintaining the tobacco habit, is the major component responsible for addiction (Stolerman and Jarvis 1995). Smoking cessation precipitates the appearance of an aversive withdrawal syndrome in humans. The aversive aspects of nicotine withdrawal syndrome are thought to be powerful motivational factors contributing to the maintenance of smoking (Kenny and Markou 2001). Different therapeutic approaches based on nicotine replacement, including gums and nasal sprays, have been used as aids to smoking cessation (Rose et al. 2001), but these forms of therapy have not produced a significant increase in the numbers of smokers quitting, with subsequent reductions in smoking-related morbidity and mortality (Peters and Morgan 2002; Sutherland 2002).
Evidence regarding the association between depression and tobacco smoking is emerging (Carton et al. 2002; Dierker et al. 2002; Scarinci et al. 2002). In particular, it has been postulated that nicotine withdrawal in smokers may elicit a state in which they are more sensitive to the adverse effects of stress (Balfour and Ridley 2000). Moreover, following observation of spontaneous smoking cessation in depressed smokers treated with the antidepressant bupropion (Ferry et al. 1992), this drug has been recently marketed as an aid for smoking cessation (Sutherland 2002). Recently, the phytomedicine Hypericum perforatum (St John's Wort) has become popular as a natural antidepressant effective for treating mild to moderate depression. The efficacy of Hypericum extracts is comparable to that of tricyclic antidepressants, but it has superior patient compliance (Philipp et al. 1999). In the light of these findings, we investigated the effects of treatment with an extract of Hypericum perforatum on somatic signs of nicotine withdrawal in the mouse.
Materials and methods
Male Swiss 8-week-old mice, housed at a constant temperature (22±2°C) and a light cycle of 12/12 h (7.00 a.m./7.00 p.m.) with free access to food and drinking water, were used. Adaptation and experiments were carried out in accordance with the internationally accepted principles and the national laws concerning the care and the use of laboratory animals and with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Animals were randomly allocated to different groups. For the first experiment, two groups of mice were injected with saline (controls) or nicotine hydrogen tartrate salt (Sigma-Aldrich) 2 mg/kg SC, respectively, four injections daily, 4 h apart, starting at 0800 hours for 14 days. This intermittent nicotine treatment more closely resembles human use (Isola et al. 1999). Three other groups of mice, treated with nicotine for 14 days as previously described, received oral acute administration of Hypericum perforatum extract (Ph-50 125–500 mg/kg) immediately after the last nicotine injection. A further group of mice, not treated previously with nicotine, received the highest dose of Ph-50 (500 mg/kg) orally. In the second experiment, two groups of mice were treated with saline (controls) or nicotine (14 days) and three other groups were treated as follows: i) one with Ph-50 (500 mg/kg) only during nicotine withdrawal (group 1, During withdrawal; DW), ii) another group with Ph-50 (500 mg/kg) during nicotine treatment beginning from day 8 of nicotine treatment and after the last nicotine injection (group 2, Continual treatment; CT) and iii) oral acute administration of Ph-50 (500 mg/kg) following nicotine withdrawal (group 3, Following withdrawal; FW). On completion of the nicotine treatment, all animals were evaluated for locomotor activity and abstinence signs at 24 h following the last saline or nicotine injection. All groups of mice comprised six animals. Ph-50 is a solid (powder) extract of Hypericum perforatum containing 50% flavonoids, 0.3% hypericin and 4.5% hyperforin (Calapai et al. 2001a) (the remaining part is composed of polysaccharides represented by maltodextrins), purchased from Pharmalife-Research (Italy) and dissolved in saline before administration.
Locomotor activity was evaluated in all groups 24 h after the last injection of saline or nicotine in an open field apparatus subdivided into nine communicating squares. The animals were placed in the apparatus and the number of squares crossed in 6 min was determined (Calapai et al. 1995). Locomotor activity was evaluated by observers blind to drug treatment.
Abstinence signs were evaluated by four experienced observers, blind to group treatments, at 24 h into withdrawal. Prior to each observation session, nicotine- and saline-treated mice were placed in transparent cages for habituation for 30 min, and they were returned after 60 min (adaptation: 30 min, observation: 30 min) to their home cage upon completion of the behavioral evaluation. For the evaluation of behavioural signs of withdrawal, a Nicotine Abstinence Scale was compiled. This scored the frequency of the following signs: rearing, jumping, body shakes, head shakes, forelimb shakes, scratching, chewing, abdominal constrictions and facial tremor during 30 min of observation (Isola et al. 1999). The severity of the abstinence syndrome was evaluated by computing the total abstinence signs score.
All statistical procedures were performed using the SPSS statistical software package release 6.1.3. (SPSS, Chicago, Ill., USA). Data analysis was performed using one-way analysis of variance (ANOVA) with the Scheffé post hoc test for multiple comparisons. The data are expressed as means±SE. Statistical significance was set at P<0.05.
Results
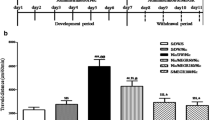
Discontinuing intermittent administration of nicotine caused a reduction of locomotor activity. Control (saline) animals crossed a mean (±SEM) of 72.7±3.28 squares. Animals treated only with nicotine crossed 39.5±3.4 squares (F=47.91; P<0.001). Mice receiving the highest dose of Ph-50 (500 mg/kg) in groups 1 (DW), 2 (CT) and 3 (FW) crossed a significantly increased number of squares (65±2.98, F=31.81; 66±5.4, F=17.25 and 62±6.6, F=9.18, respectively; P<0.01 versus mice treated only with nicotine) with respect to mice that received only nicotine. A group of mice not treated with nicotine but only with Ph-50 (500 mg/kg) did not show changes in locomotor activity (73.2±3.5) with respect to control animals (Fig. 1).
Locomotor activity in mice (number of squares crossed in 6 min) after discontinuing of repeated administration of nicotine (2 mg/kg daily SC; 14 days). N treated only with nicotine; DW Ph-50 (500 mg/kg) given only during nicotine withdrawal; CT Ph-50 (500 mg/kg) during nicotine treatment (beginning from day 8 of nicotine treatment) and after the last nicotine injection; FW Ph-50 (500 mg/kg) following nicotine withdrawal. Each column represents the mean±SE of six animals. *P<0.01 vs N
Ph-50 given alone (score: 0.71±0.19) did not modify the total abstinence score when compared to controls (score: 0.67±0.21). The total abstinence signs score was significantly reduced compared to that of animals treated only with nicotine (score: 5.83±0.17) in all the groups of mice that received nicotine and were treated with Ph-50. This effect was dose-dependent in group 3 (DW score 3.25±0.23, F=81.37; P<0.001) (Fig. 2) and was more pronounced in group 2 (CT score 2.31±0.25, F=25.23; P<0.001) (Fig. 3).
Effects of different oral doses of an Hypericum perforatum extract (Ph-50 125–500 mg/kg) on total abstinence score during nicotine withdrawal after 14 days of nicotine treatment (2 mg/kg daily SC). Ph-50 was administered after the last saline or nicotine injection. Each column represents the mean±SE of six animals. *P<0.01 vs nicotine; P<0.05 vs nicotine+Ph-50 (250)
Total abstinence score during nicotine withdrawal after 14 days of nicotine treatment (2 mg/kg daily SC). N treated only with nicotine; DW Ph-50 (500 mg/kg) given only during nicotine withdrawal; CT Ph-50 (500 mg/kg) during nicotine treatment (beginning from day 8 of nicotine treatment) and after the last nicotine injection; FW Ph-50 (500 mg/kg) following nicotine withdrawal. Each column represents the mean±SE of six animals. *P<0.01 vs N; °P<0.05 vs DW; #P<0.01 vs CT
Discussion
The nicotine withdrawal syndrome comprises both physical or "somatic" and affective components. A somatic nicotine withdrawal syndrome analogous to that observed in humans can be induced in mice (Isola et al. 1999). This experimental model was used in the present study and our data provide valuable confirmation of the work of Isola et al., showing abstinence signs after discontinuing chronic nicotine administration in mice. Moreover, our results indicate that administration of Hypericum perforatum extracts can attenuate somatic symptoms of nicotine withdrawal.
In considering the mechanism of action of Hypericum, it is pertinent that the rationale leading to the use of bupropion for aiding smoking cessation is based on its ability to inhibit reuptake of dopamine and noradrenaline (Holm and Spencer 2000). Hypericum perforatum extracts are widely used for therapy of depression and the mechanism of its antidepressant action seems to imply, as with bupropion, an increase in deficient neurotransmitter activity associated with the pathogenesis of this disorder. Acute administration of Hypericum extracts produces an increase in brain content of neurotransmitters such as noradrenaline, dopamine and serotonin (Calapai et al. 1999), and deficits in these neurochemicals have been all considered to play a role in the expression of drug abstinence syndromes (Quattrocki et al. 2000). Thus, it is possible to speculate that Hypericum extracts attenuate the somatic aspects of nicotine abstinence in mice by enhancing the functional tone of these neurotransmitters. However, if is not clear what is the active moiety responsible for the antidepressant action of Hypericum perforatum, the compounds hypericin, hyperforin and flavonoids contained in the plant all having been suggested (Calapai et al. 1999, 2001b).
We observed that all the somatic signs produced by nicotine withdrawal were significantly attenuated by administration of Hypericum extracts. A reduction in nicotine abstinence signs was significantly more apparent when Hypericum was administered either during nicotine treatment or immediately after the last nicotine injection. However, mice receiving Hypericum only after nicotine treatment also showed a significant reduction in total abstinence score. This indicates not only that administration of Hypericum after discontinuing nicotine may reduce abstinence suffering but also that the co-administration of Hypericum and nicotine can influence the development of nicotine dependence. Hypolocomotion induced by nicotine withdrawal was also influenced by Hypericum administered after the last nicotine injection. When Hypericum was given alone at the highest dose, it did not affect locomotor activity in animals not treated with nicotine, thus demonstrating that its effects on specific somatic signs of abstinence are not simply the expression of a general motor facilitatory action.
In conclusion, this is the first time that a beneficial effect of Hypericum perforatum on nicotine abstinence has been shown. It is also evident that Hypericum treatment in the period preceding nicotine withdrawal can influence the development of nicotine dependence. However, further studies are needed to clarify whether an approach involving the use of Hypericum perforatum can be considered to help humans in smoking cessation.
References
Balfour DJ, Redley DL (2000) The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav 66:79–85
Calapai G, Squadrito F, Rizzo A, Marciano MC, Campo GM, Caputi AP (1995) Multiple actions of the coumarine derivative cloricromene and its protective effects on ischemic brain injury. Naunyn-Schmiedeberg's Arch Pharmacol 351:209–215
Calapai G, Crupi A, Firenzuoli F, Costantino G, Inferreara G, Campo GM, Caputi AP (1999) Effects of Hypericum perforatum on levels of 5-hydroxytryptamine, noradrenaline and dopamine in the cortex, diencephalon and brainstem of the rat. J Pharm Pharmacol 51:723–728
Calapai G, Crupi A, Firenzuoli F, Inferrera G, Ciliberto G, Parisi A, De Sarro G, Caputi AP (2001a) Interleukin-6 involvement in antidepressant action of Hypericum perforatum. Pharmacopsychiatry 34:S8–S10
Calapai G, Crupi A, Firenzuoli F, Inferrera G, Squadrito F, Parisi A, De Sarro G, Caputi AP (2001b) Serotonin, norepinephrine and dopamine involvement in the antidepressant action of Hypericum perforatum. Pharmacopsychiatry 34:45–49
Carton S, Le Houezec J, Lagrue G, Jouvent R (2002) Early emotional disturbances during nicotine patch therapy in subjects with and without a history of depression. J Affect Disord 72:195–199
Dierker LC, Avenevoli S, Stolar M, Merikangas KR (2002) Smoking and depression: an examination of mechanisms of comorbidity. Am J Psychiatry 159:947–953
Ferry LH (1999) Non-nicotine pharmacotherapy for smoking cessation. Prim Care 26:653–669
Holm KJ, Spencer CM (2000) Bupropion: a review of its use in the management of smoking cessation. Drugs 59:1007–1024
Isola R, Vogelsgerg V, Wemlinger TA, Neff NH, Hafjiconstantinou M (1999) Nicotine abstinence in mouse. Brain Res 850:189–196
Kenny PJ, Markou A (2001) Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav 70:531–549
Murray CJ, Lopez AD (1997) Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 349:1436–1442
Peters MJ, Morgan LC (2002) The pharmacotherapy of smoking cessation. Med J Aust 176:486–490
Phillipp M, Kohnen R, Hiller K (1999) Hypericum extract versus imipramine or placebo in patients with moderate depression: randomised multicentre study of treatment for eight weeks. BMJ 319:1534–1538
Quattrocki E, Baird A, Yurgelun-Todd D (2000) Biological aspects of the link between smoking and depression. Harv Rev Psychiatry 8:99–110
Rose JE, Behm FM, Westman EC (2001) Acute effects of nicotine and mecamylamine on tobacco withdrawal symptoms, cigarette reward and ad lib smoking. Pharmacol Biochem Behav 68:187–197
Scarinci IC, Thomas J, Brantley PJ, Jones GN (2002) Examination of the temporal relationship between smoking and major depressive disorder among low-income women in public primary care clinics. Am J Health Promot 16:323–330
Stolerman IP, Jarvis MJ (1995) The scientific case that nicotine is addictive. Psychopharmacology 117:2–10
Sutherland G (2002) Current approaches to the management of smoking cessation. Drugs 62:53–61
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Catania, M.A., Firenzuoli, F., Crupi, A. et al. Hypericum perforatum attenuates nicotine withdrawal signs in mice. Psychopharmacology 169, 186–189 (2003). https://doi.org/10.1007/s00213-003-1492-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1492-0