Abstract
Rationale
Neonatal administration of methamphetamine (MA) to rats from postnatal day (P) 11 to 20, but not from P1 to P10, produces lasting deficits in spatial learning and memory. The preweaning period of development in the rat corresponds to human third trimester hippocampal development and because of the increased use of MA in women of childbearing age, there is a greater likelihood that fetuses will be exposed to this drug. Development of the hippocampus is dependent upon many factors, including an optimal level of corticosterone (CORT). We have demonstrated that the CORT response of animals on P11 to MA is protracted relative to administration on P15 or P20. Interestingly, the P11 animals are still in the stress hyporesponsive period.
Objectives
We postulated that because of the prolonged CORT response on P11, the effects of MA on spatial learning and memory may be confined to a shorter period of exposure.
Methods
Neonatal rats were administered MA (10 mg/kg) 4 times daily from either P11 to P15 or from P16 to P20, raised to adulthood and tested against animals only administered saline (SAL) from P11 to P20 for anxiety, swimming ability, and spatial learning and memory.
Results
Animals exposed to MA, regardless of exposure period, tended to be less anxious in the Zero maze relative to SAL animals. No differences were noted for swimming ability. Only animals exposed to MA from P11 to P15 demonstrated deficits in spatial learning and memory during acquisition as well as during a shifted platform phase where learning a new position was required.
Conclusions
The results demonstrate that spatial learning and memory deficits produced by MA administration are dependent upon when the exposure of the animal occurs and appears to be during the period of development in the rat when the response to threatening environments, stressors, is greatly reduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to some substances during gestation can have profound effects on the morphological, physiological, and/or behavioral development of the offspring. The severity of these effects is dependent upon many factors, but of particular importance is the time during development when the exposure occurs (Wilson 1973). For the central nervous system, effects depend on the timing of neurogenesis, synaptogenesis, and apoptosis as well as the availability of neurotransmitters, hormones, and the receptor systems for each pathway. Since regions of the brain mature at different rates it is possible that exposure to an exogenous substance may produce effects during one developmental time period, but not another. We have previously shown that administration of methamphetamine (MA) or 3,4-methylenedioxymethamphetamine (MDMA) to rats from postnatal days (P)11 to 20 produces lasting changes in spatial learning ability (Vorhees et al. 1994, 1998, 1999, 2000; Broening et al. 2001; Williams et al. 2002, 2003). In contrast, administration of either MA or MDMA from P1 to P10 does not produce such cognitive effects (Vorhees et al. 1994; Broening et al. 2001), although for animals treated with MA there was an increase in startle responsiveness (Vorhees et al. 1994). One region important in spatial learning and memory that is still developing during the P11–20 period is the hippocampus and this development is analogous to human hippocampal development during the third trimester (Bayer et al. 1993; Rice and Barone Jr 2000). Therefore, exposure of human fetuses to MA is of concern since women of childbearing age have increased the use of this drug during the past decade (NIH Publication no. 98-4210 1998) and little is known about the long-term effects, although several short-term effects of MA have been noted in humans (Oro and Dixon 1987; Little et al. 1988; Dixon and Bejar 1989; Struthers and Hansen 1992; Smith et al. 2001). One study suggests that amphetamine use during pregnancy in humans produces effects on the growth rate as well as cognitive performance of the children exposed in utero and these effects last well into adolescence (Cernerud et al. 1996).
In adult animals, MA has been shown to produce neurotoxicity as demonstrated by neuropathological effects such as increases in glial fibrillary acidic protein (GFAP), as well as silver and flouro-jade staining that are coupled with decreases in neurotransmitters (O'Callaghan and Miller 2000; O'Dell and Marshall 2002). Paradoxically, the developing animal appears to be resistant to the neurotoxic effects of MA that have been demonstrated in adult animals. For example, administration of MA on P20 produces no changes in GFAP, dopamine, or serotonin when measured 3 days later after four doses of MA (10 mg/kg) on a single day (Pu and Vorhees 1993; Cappon et al. 1997). Therefore, conventional neurotoxic markers used for adult animals appear to be unsatisfactory to demarcate the boundaries of the P11–20 critical period. Adult animals also demonstrate sympathetic activation and elevations in hormones of the hypothalamic-pituitary-adrenal (HPA) axis following MA administration (Morimasa et al. 1987). Exposure to MA during the neonatal period produces similar changes in the hormones of the HPA axis (Williams et al. 2000). For example, MA administration produces a sustained increase in corticosterone (CORT) and adrenocorticotropin hormone (ACTH) on P11 that lasts for at least 105 min after administration. On P15 and P20 peak levels of these hormones were observed at 30 min following drug administration; however, no differences or lower levels were found at 105 min after MA. The elevated output of the adrenal gland during the early period of drug administration (~P11–14) is during a period of development termed the stress hyporesponsive period (SHRP). The SHRP has been hypothesized to be crucial in protecting the developing nervous system from the potentially neurotoxic effects of high levels of CORT (Sapolsky and Meaney 1986). This is not to say that CORT is not required for neuronal development. On the contrary, normal hippocampal functioning and development is dependent upon glucocorticoids throughout the life of an animal, such that, concentrations that are either too high or too low produce cell death and neuronal reorganization (Gould et al. 1991a, 1991b; Conrad and Roy 1995). Restructuring of the hippocampus may therefore be a potential explanation for the MA-induced deficits observed in spatial learning and memory. The receptors for CORT located in the hippocampus, as well as other regions, are increasing in number during the P11–20 period of MA administration (Meaney et al. 1985). Interestingly, the binding capacity of these receptors in the hippocampus is very low on P3, but increases to adult-like levels by P9 and exceeds adult levels by P15.
In order to test for spatial learning and memory deficits, we have used the Morris water maze (Morris 1981). This test is known to be dependent upon the hippocampus (Morris et al. 1982), and tests the ability of an animal to locate a platform that has been submerged beneath the surface of the water in order to escape from an aversive environment. We have used this task successfully to show deficits in spatial learning and memory following MA with different strains (Vorhees et al. 1998, 1999), various concentrations and dosing regimens (Vorhees et al. 1994, 1999, 2000; Williams et al. 2003), as well as different behavioral testing protocols (Vorhees et al. 1998, 1999, 2000; Williams et al. 2002). The purpose of the present study was to determine if a smaller window of exposure within the P11–20 period of MA administration existed that would produce spatial learning and memory deficits. We postulated that because of the protracted CORT response on P11 and only transient increases were observed on P15 and 20 that the majority of the effect would be observed if MA was administered from P11 to P15 in relation to P16–20 administration. In this study, we used a more demanding version of the Morris water maze during the acquisition phase by using a smaller platform size in the hopes of optimizing the ability of this task to detect deficits in the MA-treated animals.
Materials and methods
Animals
Female Sprague-Dawley CD IGS rats (151–175 g; Charles River, Raleigh, N.C., USA) were allowed at least 2 weeks to acclimate to the housing (two females/polycarbonate cage) and lighting (14 h light: 10 h dark, lights on at 0600 hours) conditions in the laboratory prior to being mated with males of the same strain obtained from the same supplier. Females were placed with a male in a hanging wire cage for a period of 2 weeks, after which time they were singly housed in polycarbonate cages. The day a sperm plug was detected was designated embryonic day 0 (E0). Beginning on E22, litters were checked twice daily for the presence of a litter and birth was designated postnatal day 0 (P0). On P1, litters were randomly culled to eight pups with equal numbers of males and females. On P11, pups were uniquely identified with an ear punch. Dams were allowed to wean their offspring (Redman and Sweney 1976; Blass and Teicher 1980) and offspring were then separated on P28 and housed in same sex groups until P42 when the animals were randomly housed two per cage. The vivarium was temperature and humidity controlled and is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care and in compliance with all Federal animal care and use guidelines. All procedures were approved by the Institutional Animal Care and Use Committee prior to the experiments and the guidelines outlined in the "Principles of Laboratory Care" (NIH publication no. 85-23, revised 1985) were followed.
Methamphetamine administration
Either methamphetamine HCl (MA; expressed as the free base) at a dose of 10 mg/kg body weight or the saline vehicle was administered four times daily at 2-h intervals from either P11 to P15 or from P16 to P20. In order to delineate further the critical period for MA exposure, the administration of MA was subdivided so that a randomly selected male and female pair from each litter received MA from either P11 to P15 (MA-early) or from P16 to P20 (MA-late). The MA-early animals received saline from P16 to P20, whereas the MA-late animals received saline from P11 to P15. Two pairs of males and females from each litter received only saline (SAL) from P11 to P20. All injections were delivered subcutaneously in the dorsum in a volume of 3 ml/kg per injection with injection sites rotated to minimize irritation. No necrosis was observed using this procedure. Animals were weighed prior to each injection. A total of 16 litters were prepared using the split-litter design, so that all treatments were represented within each litter.
Behavioral methods
Zero maze
Beginning on approximately P50, animals were tested in the elevated zero maze for anxiety levels (Shepherd et al. 1994). The ring-shaped maze was elevated from the floor 72 cm and was 105 cm in diameter with a path width of 10 cm. The maze was partitioned in quadrants, so that adjoining quadrants either had black walls that were 28 cm in height (closed area) or a clear acrylic curb 1.3 cm in height (open area). Animals were placed in the center of one of the closed areas and their behavior recorded for 5 min with a camera that was attached over the center of the maze and connected to a video recorder. After the 5-min test, the maze was thoroughly wiped with 70% ethanol. The number of head dips, stretch-attends, and time in the open area was measured. A head dip was counted when the animal placed its head over the open area side-rail. A stretch-attend occurred when the torso and forepaws of the animal were in the closed area, but the animal stretched out into the open area, and time in the open was counted for animals that had all four paws in the open area.
Straight channel
Straight channel swimming was performed in order (1) to acclimate rats to swimming, (2) to determine if there were any motor deficits prior to maze testing, and (3) to determine if the test subjects were motivationally comparable. The trials were performed in a 15 cm wide×244 cm long water filled, gray acrylic, channel with a stainless steel ladder positioned at one end. Each rat was placed in the opposite end facing away from the ladder and received four timed trials to reach the ladder and escape the water (temperature 22±1°C). Straight channel trials were performed 1 day following the Zero maze test procedure.
Morris water maze apparatus and room
The Morris water maze apparatus was a stainless steel water tank, 210 cm in diameter, painted black, and filled with room temperature water (22±1°C). The goal platform measured 5×5 cm wide and 30.5 cm tall and was made of clear acrylic covered with a Nylon screen for traction. The platform was submerged 2±1 cm below water. Various extra-maze cues were available to the rats, including white curtains that were fastened together at opposite ends of the maze as well as large, black, unique geometric shapes placed on the three walls nearest the water tank. A camera was located above the water tank and attached to a computer and monitor so that each rat's performance could be tracked automatically using a video tracking system that identified the contrasting white color of the rat against the black background of the maze (San Diego Instruments, Polytrack System, San Diego, Calif., USA). The maze was arbitrarily divided at four cardinal points designated N, S, E, W, where N was defined as the position farthest from the experimenter. The platform was either located in the SW or NE quadrant of the apparatus, counterbalanced among the litters. Start positions were selected so that an animal did not start immediately adjacent to the quadrant that contained the hidden platform. For example, when the platform was located in the SW quadrant, the start positions were N, E, NW and SE and when located in the NE quadrant they were S, W, SE, NW. The start positions were quasi-randomized among these positions with the stipulation that no position could be used more than once a day. The Morris maze and straight channel were located in different rooms.
Morris water maze testing procedure
Morris maze training began 3 days following straight channel swimming. The procedure consisted of an acquisition phase and a shifted-platform phase. During both phases, the rat received four trials per day for 5 days with a 2-min trial limit and an ITI of 15 s spent on the platform. If a rat failed to locate the platform it was removed from the water and placed on the platform. On the day following the learning trials a 30-s probe trial was administered. During the probe trial, the platform was removed and the animal was started from a novel position, 180° from the platform and allowed 30 s to search for the platform. During the shifted platform phase, animals that originally learned the maze with the platform located in the SW quadrant had the platform moved to the NE quadrant and the converse was true for animals that originally learned the maze with the platform in the NE quadrant. The dependent measures for the learning trials during the acquisition and shifted platform phase were latency, path length, and cumulative distance from the platform. For memory (probe) trials, the dependent measures were average distance from the platform site and percent time in the target quadrant.
Statistical methods
Behavioral and body weight data were analyzed with a mixed-model split-plot analyses of variance (ANOVA) utilizing the general linear modeling procedure. Main effects were treatment group (MA dose), sex, day, time of dose (for body weights during dosing) and these were all treated as within-subjects factors. The experimental unit was the litter (n=16). Nonorthogonal planned comparisons were applied to the behavioral data, so that each MA-treated group was separately compared to the SAL group. For repeated measure factors, a test for sphericity was performed to ensure symmetry of the variance-covariance matrix. The Greenhouse-Geisser correction was used in instances in which these matrices were significantly non-spherical and significance was set at P<0.05.
Results
Body weights
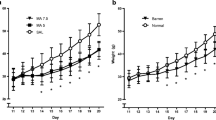
The analysis of body weights (Treatment, Sex, Day, and Time of dose) during the period of drug administration revealed that males weighed more than females, Sex [F(1,15)=33.32, P<0.0001], animals in all treatments gained weight during this period, Day [F(9,135)=202.12, P<0.0001], and larger body weights were measured after the last dose relative to the first dose of the day, Time of dose [F(1,15)=52.8, P<0.0001] (Fig. 1). The body weights of animals administered MA were decreased relative to the SAL animals, Treatment [F(2,30)=162.87, P<0.0001], and as expected this was affected by the day MA administration began as well as when during the day the weights were recorded, Treatment×Day×Time of dose [F(18,270)=5.57, P<0.0001]. Simple effects and step-down analysis of the interaction revealed that the MA-early animals had a reduction in body weights relative to the SAL animals beginning on the last dose on P11 and continuing through the last day of saline administration on P20. No differences were observed in body weight for the MA-late animals and SAL animals from P11 to P15. However, on the first day of drug administration on P16 for the MA-late animals, decreases in body weight were observed following the last dose of the day and continuing through the end of drug administration. No interactions of Treatment and Sex were observed.
Postnatal body weights of animals administered MA from P11 to P15 and SAL from P16 to P20 (MA-early), saline from P11 to P15 and MA from P16 to P20 (MA-late), or saline from P11 to P20 (SAL). Males weighed more than females. MA produced a decrease in body weights during the period of administration as well as immediately following drug exposure. *P<0.05, MA-early and MA-late versus SAL. †P<0.05 MA-early versus SAL
Following the drug administration period, the body weights prior to weaning from the mother on P28 and those following weaning were analyzed in separate ANOVAs. Body weights increased for all animals [Week F(1,15)=402.25, P<0.0001] and were greater for males than females [Sex F(1,15)=55.57, P<0.0001] on P21 and P28; however, as shown in Table 1, those animals that received MA, regardless of when the drug was administered, weighed less than the SAL-treated animals [Treatment F(1,15)=198.97, P<0.0001]. Although the interaction of Treatment×Week was significant [F(2,30)=4.82, P<0.02], differences in MA-treated animals relative to SAL-treated were observed for each week. After weaning, all animals continued to gain weight [Week F(6,90)=758.17, P<0.0001] and similar differences were still detected between males and females [Sex F(1,15)=1046.29, P<0.0001] with males weighing more than females. Although neonatal treatment of the animals with MA produced lower body weights [Treatment F(2,30)=4.73, P<0.02], this weight reduction was dependent upon when MA was administered and when the weights were measured after weaning [Treatment×Week F(12,180)=2.63, P<0.05] (Table 1). That is, the body weights of the MA-late animals did not differ from the SAL animals starting on P42, whereas no differences were detected in the MA-early animals starting on P63 relative to the SAL animals.
Zero maze
MA-early versus SAL
There were no significant treatment related effects in the Zero maze; however, several of the measures showed a trend towards significance as detailed. For example, animals exposed early to MA appeared to be less anxious since they showed a trend to enter the open areas more [F(1,15)=3.42, P=0.08] and stay in these areas longer [F(1,15)=3.61, P<0.08] when compared with SAL animals (Table 2). No treatment related differences were noted for the number of head dips or stretch-attends. Regardless of treatment, females were more likely to enter the open areas [F(1,15)=8.68, P<0.01] and stay in them longer [F(1,15)=5.01, P<0.05], and tended to have more head dips than males [F(1,15)=3.60, P<0.08], but no differences were noted for stretch-attends.
MA-late versus SAL
As with animals exposed early to MA, no treatment-related differences were noted, but again a trend towards significance was observed; however the pattern was different than for animals exposed to MA-early. For instance when comparing the animals exposed later to MA and SAL there was a tendency for treatment to interact with sex for the amount of time in the open area [F(1,15)=3.37, P<0.09] as well as the number of head dips [F(1,15)=3.37, P<0.09]. Males treated later with MA had more time in the open and head dips than females treated with MA, whereas the opposite was true for the SAL animals (Table 2). There were no treatment or sex-related effects on open area entries or stretch-attends.
Straight channel swimming
The administration of MA from either P11 to P15 or P16 to P20 did not produce any changes in swimming ability and the sex of the animal did not influence this measure (Table 2). All animals showed improvement in swimming the channel as demonstrated by a Trial main effect when comparing both the MA-early and SAL [F(3,39)=119.09, P<0.0001] and the MA-late and SAL [F(3,39)=28.13, P<0.0001].
Morris maze acquisition
MA-early versus SAL
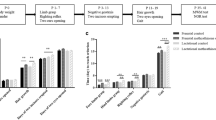
The administration of MA from P11 to P15 impaired the ability of the animals to efficiently learn the Morris water maze as demonstrated by several key indicators of performance. For example, as demonstrated in Fig. 2, the MA-early animals in comparison to SAL animals took longer to locate the submerged platform [F(1,15)=6.98, P<0.02], swam further distances [path length (cm)] in search of the platform [F(1,15)=4.77, P<0.05], and were further from the platform throughout the test as measured by the cumulative distance measure [F(1,15)=5.49, P<0.04]. All animals, regardless of treatment, demonstrated learning in the task over the 5-day test period as represented by a decrease in latency [F(4,60)=45.84, P<0.0001], path length [F(4,60)=35.06, P<0.0001], and cumulative distance [F(4,60)=43.75, P<0.0001]. Furthermore, males performed better than females on all three of these measures [F(1,15)=6.78, 14.34, and 13.20, P<0.02, 0.002, and 0.003, respectively]. Representative of this difference in the sexes, the males had a mean cumulative distance of 17,598±835 cm whereas the mean of the females was 20,918±1095 cm.
Morris water maze performance during the acquisition phase of animals administered MA from P11 to P15 and SAL from P16 to P20 (MA-early), saline from P11 to P15 and MA from P16 to P20 (MA-late), or saline from P11 to P20 (SAL). The left column represents MA-early (triangles) versus SAL (circles), whereas the right column represents MA-late (squares) versus SAL (circles). The MA-early animals performed worse on this task than the SAL animals. *P<0.05
For the memory or probe trials, the MA-early animals tended on average to be further from the former location of the platform [F(1,15)=4.05, P<0.07] and males were closer than females [F(1,15)=6.08, P<0.03], but the interaction was not significant (Table 2). No differences, however, were noted for the percentage of time in the target quadrant.
MA-late versus SAL
In contrast to the MA-early, no overall treatment differences were detected for latency, path length, or cumulative distance from the platform during the acquisition phase of testing (Fig. 2). There was, however, an interaction of treatment and sex for latency to locate the platform [F(1,15)=8.40, P<0.02], in which the males treated with MA-late had longer latencies than males treated with SAL (not shown). Nonetheless, this difference was not supported by either path length or cumulative distance, two measures thought to be more representative of spatial learning ability relative to latency (Gallagher et al. 1993; Lindner 1997; Contet et al. 2001). Decreases in latency to the platform, path length, and cumulative distance over days of testing demonstrated that animals learned the task regardless of treatment [F(4,60)=77.96, 50.61, and 62.68, P<0.0001, respectively]. Males again demonstrated better performance in the task as demonstrated by shorter latencies, shorter path lengths, and smaller cumulative distances; however, these measures were affected by the day of testing [F(4,60)=4.85, 3.81, and 5.01, P<0.007, 0.02, and 0.006, respectively]. The mean cumulative distance of males was 17,167±960 cm and the cumulative distance of females was 19,218±1013 cm.
Deficits in probe trial performance of the MA-late animals were observed for the average distance from the platform site [F(1,15)=5.73, P=0.03] and a tendency for these animals to spend less time in the target quadrant [F(1,15)=3.80, P=0.07] (Table 2). No differences between the sexes were noted for either measure.
Morris maze shifted platform
MA-early versus SAL
Following the acquisition phase, the animals were required to learn a new location of the platform. The deficits of the MA-early animals observed during the acquisition phase were still evident during this phase of testing when compared to the SAL animals. The MA-early animals took longer to locate the platform [F(1,15)=12.07, P<0.004], had longer path lengths [F(1,15)=7.97, P<0.02], and were further from the platform during learning [F(1,15)=9.42, P<0.008] (Fig. 3). All animals showed better performance over days for all measures [latency: F(4,60)=52.97, P<0.0001; path length: F(4,60)=66.69, P<0.0001; cumulative distance: F(4,60)=99.03 P<0.0001]. Males again demonstrated more rapid learning of the task than females for latency, path length, and cumulative distance (males: 10,590±712 cm; females: 15,011±871 cm) [F(1,15)=16.1, 45.33, and 35.67, P<0.001, 0.0001, and 0.0001, respectively].
Morris water maze performance during the shifted platform phase of animals administered MA from P11 to P15 and SAL from P16 to P20 (MA-early), saline from P11 to P15 and MA from P16 to P20 (MA-late), or saline from P11 to P20 (SAL). The left column represents MA-early (triangles) versus SAL (circles), whereas the right column represents MA-late (squares) versus SAL (circles). The MA-early animals performed worse on this task than the SAL animals. *P<0.05
No treatment-related differences were observed during the probe trial. As with the learning trials, males were on average closer to the platform site [F(1,15)=7.34, P<0.02] and tended to spend a greater percentage of time in the target quadrant [F(1,15)=3.44, P<0.09] relative to females.
MA-late versus SAL
Learning during the shifted platform phase was not affected by MA treatment from P16 to P20, since similar latencies, path lengths, and cumulative distances were observed (Fig. 3). All animals learned the location of the new platform and progressively decreased their search time [F(4,60)=43.70, P<0.0001], path lengths [F(4,60)=40.45, P<0.0001], and cumulative distance [F(4,60)=80.56, P<0.0001] over days. Males performed better on all parameters, latency [F(1,15)=6.18, P<0.03], path length [F(1,15)=9.66, P<0.008], and cumulative distance [males 9492±707 cm and females 13,380±904 cm, F(1,15)=11.76, P<0.004], relative to females.
For probe trials, similar to the learning trials during this phase, no treatment-related effects were observed. Males again demonstrated better performance regardless of treatment as indicated by being closer to the platform site [F(1,15)=7.15, P<0.02] and spending more time in the target quadrant [F(1,15)=7.26, P<0.02].
Discussion
The present data demonstrate that a more refined window of vulnerability exists for MA to exert lasting effects on spatial learning and memory. It is apparent that the MA-early animals had spatial navigation deficits that persisted even after the animals learned the requirements of the task; that is, the deficits were observed during the shifted-platform phase as well as the acquisition phase. By contrast, the same dose of MA administered beginning only 5 days later to the MA-late group had no effect on spatial navigation during either acquisition or shifted-platform phases.
Interestingly, the MA-late males had longer latencies in reaching the platform during the acquisition period. However, these longer latencies were not indicative of spatial learning impairment because these animals displayed no increases in path length or cumulative distance from the platform. Others have argued that path length and cumulative distance are better indicators of spatial learning ability relative to latency measures (Gallagher et al. 1993; Lindner 1997; Contet et al. 2001). No treatment differences were observed for straight channel swimming, and therefore it is difficult to determine why the MA-late males took longer to reach the platform during the acquisition phase.
Probe trial performance in the MA-late animals appeared to be deficient relative to SAL animals since the average distance from the platform was reduced. However, no differences were noted for percent time in the target quadrant. Since the probe trial represents only a 30-s sampling of behavior and the MA-late males already demonstrated that in the Morris maze they swam slower, as suggested by the longer latencies but similar path lengths, it is conceivable that the average distance measure was confounded by this factor during the acquisition phase probe trials. During the shifted-platform phase, no differences in latency were detected between the MA-late and SAL animals, and there were no differences in probe trial performance. With the MA-early animals, the learning effects were seen during both the acquisition phase and the shifted-platform phase. Therefore, one would expect that if the average distance difference between MA-late and SAL animals on the acquisition probe trial were truly a deficit in spatial learning or recall that this would be apparent in the shifted-platform phase as well, however this was not the case. Taken together, these data demonstrate that the exposure to MA after P15 has no reliable effect on spatial learning ability.
The animals exposed to MA tended to be less inhibited or fearful in the Zero maze, since they investigated the open arms more than the SAL animals. Although this trend was only suggestive for decreased anxiety, the increased time in the open would be predicted by an underactive HPA axis. In agreement with this prediction, we have recently observed in animals exposed to MA from P11 to P20, a decreased CORT response to 15 min of forced swim in adulthood (unpublished observation). For the MA-late animals only males appeared to be affected, however, both males and females were affected by the MA-early treatment. As has been demonstrated in the elevated plus maze previously, females investigated open arms more than males (Kalinichev et al. 2002).
Interestingly, the time frame for MA administration that produces the spatial deficits (i.e. P11–15) is during the stress hyporesponsive period (SHRP). This period in rats is associated with rapid growth of neurons, especially within the hippocampus, that express proteins important for the proper functioning of the HPA axis, as well as later learning and memory ability. It has been postulated that the SHRP is neuroprotective against the adverse effects of high levels of glucocorticoids that are released when there is a threat to homeostasis of an organism (Sapolsky and Meaney 1986). Compatible with this hypothesis is the finding that high concentrations of glucocorticoids produce cell death in the dentate gyrus of neonatal animals (Gould et al. 1991b). We have demonstrated previously that MA produces prolonged elevations of CORT on P11; therefore, some restructuring of the hippocampus may be involved in the spatial learning deficits we observe. Alterations either in the number of adrenocorticosteroid receptors or in the level of CORT released during learning a spatial task can influence the performance of an animal in these tasks. An optimal level of glucocorticoids is crucial for normal development of the hippocampus. For example, adrenalectomy during early adulthood produces a profound decrease in hippocampal volume that is associated with a decreased ability to navigate in the Morris water maze (Conrad and Roy 1995), whereas as previously mentioned, higher levels produce cell death (Gould et al. 1991b). Taken together, the severe changes in CORT during the SHRP produced by MA may be involved in the learning deficits observed.
Alternative explanations and pathways exist as well. Considering the number of proteins and receptor systems that are rapidly maturing during the period of MA-early administration, there may be other signaling pathways affected. For example, the number of neurons in the hippocampus that produce corticotropin-releasing factor (CRF) increases gradually from P5 to P11 and then dramatically from P11 to P18 (Chen et al. 2001). Alterations, and specifically decreases, of CRF in the hippocampus may provide a potential mechanism for the deficits in spatial learning and memory, especially since adult animals show better spatial learning in the Morris water maze following administration of either intracerebroventricular CRF or a CRF-binding protein inhibitor (Behan et al. 1995). Previously, it has been postulated that neurotrophic factors may also be potential candidates for the developmental effects of MA (Frost and Cadet 2000). The neurotrophic factors, nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), begin to increase from P1 through P14 and then decrease between P14 and P20 (Das et al. 2001). These neurotrophic factors have also been implicated in learning and memory in adult animals (Mizuno et al. 2000; Schaaf et al. 2000, 2001). Furthermore, the administration of BDNF to animals that have been exposed to hypoxia during the neonatal period showed an attenuated deficit in the Morris water maze relative to animals that did not receive BDNF, although it did not totally alleviate the effects of hypoxia (Almli et al. 2000). Therefore, changes in the neurotrophic factors during this period of development may also alter the ability of animals to learn a spatial task.
Although elevated body temperatures in adult animals are known to play a role in the neurotoxic effect of substituted amphetamines (Farfel and Seiden 1995; Cappon et al. 1997), it has been demonstrated that in pups this is not the case. Even at 21 days of age, rats do not demonstrate hyperthermia when administered MA at normal room temperature (Cappon et al. 1997). Rats begin to thermoregulate on approximately P12 and this response continues to mature until P21 (Conklin and Heggeness 1971). Therefore, it is unlikely that body temperature plays a significant role in the long-term effects of MA after early exposure. It is obvious that many different changes are occurring in the rat during the P11–15 exposure period and some of the systems undergoing rapid development are associated with spatial learning and memory and require further investigation.
Why animals exposed to MA earlier than P11 are not affected in terms of spatial navigation [as when we administered MA from P1 to P10 (Vorhees et al. 1994)] is unknown, and seemingly paradoxical, since these animals are also being administered MA during the SHRP. Several potential explanations exist: one is that the animals are able to metabolize MA more efficiently following an initial day of exposure. In support of this possibility is that animals given a bolus of CORT early in development begin to produce high levels of cytochrome P450s (Leakey and Fouts 1979), an enzyme important in the metabolism of MA. Since MA produces increases in CORT, this same mechanism is likely to be activated. A second possible explanation is that various receptor systems are not sufficiently developed until shortly after P11. Therefore, MA may not perturb the development of these systems when given earlier when only low concentrations of receptors are present. Finally, the P11–15 period we have defined in this study may not encompass all the days of the critical period, but it does appear that we can now safely exclude days immediately following P15. In any case, the identification of a mechanism for MA effects on spatial learning will have to account for the lack of effects demonstrated when MA is administered from P1 to P10 (Vorhees et al. 1994, 2000).
In this study, we used a more difficult version of the Morris water maze by reducing the size of the platform throughout the testing period in the hopes of optimizing the task to discriminate learning ability differences. In a previous study using a larger 10×10 cm platform in the same 210 cm tank during the acquisition phase (Williams et al. 2003), the cumulative distance of MA (10 mg/kg)-treated animals was increased 15.5% over SAL animals during the first 5 days of testing whereas in this study there was a 13% increase in cumulative distance for the MA-early versus SAL animals over 5 days. This suggests that performance deteriorated in both the SAL and MA-treated groups with the smaller platform. In essence, the use of a small platform during the acquisition phase may have been counterproductive, since latencies were increased and animals were therefore required to swim longer in the maze. The increased stress of swimming longer to locate the platform may have adversely affected performance. The ability of animals to learn a spatial task is dependent upon an appropriate level of glucocorticoids, otherwise performance is compromised (Conrad et al. 1999).
It is unlikely that the differences we observed in Morris water maze performance for the MA-early animals can be linked to the inability of the animals to swim, since all animals showed comparable swim times in the straight channel and decreased latencies to locate the platform in the Morris water maze. Learning the distance of the platform from the wall, however, appears to be an important aspect of learning in the Morris water maze (Maurer and Derivaz 2000). We have recently shown that training animals for the requirements of the Morris maze (swimming away from the perimeter of the tank and climbing on the platform as refuge from the water) prior to spatial learning attenuates the spatial learning deficits produced by MA exposure from P11 to P20 during the acquisition phase (Williams et al. 2003). Nonetheless, when these pretrained MA-treated animals were tested for their spatial ability in a new learning condition, using the shifted platform procedure, these animals demonstrated impairments in spatial ability. Although we did not use a pretraining method in this study, the MA-early treated animals learned the task requirements during the acquisition phase as can be seen by their asymptotic performance during the last 3 days of testing. These data suggest that the spatial deficits we see following MA administration are truly spatial deficits and are not confounded by the ability of the animal to learn other aspects of the task. This study further strengthens the previous data, refines the critical period for MA effects, and has provided a model that may help in locating the developmental processes that are critical to the long-term cognitive deficits induced by MA.
References
Almli CR, Levy TJ, Han BH, Shah AR, Gidday JM, Holtzman DM (2000) BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp Neurol 166:99–114
Bayer SA, Altman J, Russo RJ, Zhang X (1993) Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 14:83–144
Behan DP, Heinrichs SC, Troncoso JC, Liu X-J, Kawas CH, Ling N, De Souza EB (1995) Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer's disease. Nature 378:284–287
Blass EM, Teicher MH (1980) Suckling. Science 210:15–22
Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV (2001) 3,4-methylenedioxymethamphetamine (Ecstasy)-induced learning and memory impairments depend on the age of exposure during early development. J Neurosci 21:3228–3235
Cappon G, Morford LL, Vorhees CV (1997) Ontogeny of methamphetamine-induced neurotoxicity and associated hyperthermic response. Dev Brain Res 103:155–162
Cernerud L, Eriksson M, Jonsson B, Steneroth G, Zetterström R (1996) Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatr 85:204–208
Chen Y, Bender RA, Frotscher M, Baram TZ (2001) Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci 21:7171–7181
Conklin P, Heggeness FW (1971) Maturation of temperature homeostasis in the rat. Am J Physiol 220:333–336
Conrad CD, Roy EJ (1995) Dentate gyrus destruction and spatial learning impairment after corticosteroid removal in young and middle-aged rats. Hippocampus 5:1–15
Conrad CD, Lupien SJ, McEwen BS (1999) Support for a bimodal role for Type II adrenal steroid receptors in spatial memory. Neurobiol Learn Mem 72:39–46
Contet C, Rawlins JNP, Bannerman DM (2001) Faster is not surer—a comparison of C57BL/6J and 129S2/Sv mouse strains in the watermaze. Behav Brain Res 125:261–267
Das KP, Chao SL, White LD, Haines WT, Harry GJ, Tilson HA, Barone S Jr (2001) Differential patterns of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 mRNA and protein levels in developing regions of rat brain. Neuroscience 103:739–761
Dixon SD, Bejar R (1989) Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: incidence and clinical correlates. J Pediatr 115:770–778
Farfel GM, Seiden LS (1995) Role of hypothermia in the mechanism of protection against serotonergic toxicity. II. Experiments with methamphetamine, p-chloroamphetamine, fenfluramine, dizocilpine and dextromethorphan. J Pharmacol Exp Ther 272:868–875
Frost DO, Cadet JL (2000) Effects of methamphetamine-induced neurotoxicity on the development of neural circuitry: a hypothesis. Brain Res Rev 34:103–118
Gallagher M, Burwell R, Burchinal M (1993) Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci 107:618–626
Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS (1991a) Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell birth. J Comp Neurol 313:486–493
Gould E, Woolley CS, McEwen BS (1991b) Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J Comp Neurol 313:479–485
Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG (2002) Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav 73:131–140
Leakey JE, Fouts JR (1979) Precocious development of cytochrome P-450 in neonatal rat liver after glucocorticoid treatment. Biochem J 182:233–235
Lindner MD (1997) Reliability, distribution, and validity of age-related cognitive deficits in the Morris water maze. Neurobiol Learn Mem 68:203–220
Little BB, Snell LM, Gilstrap III LC (1988) Methamphetamine abuse during pregnancy: outcome and fetal effects. Obstet Gynecol 72:541–544
Maurer R, Derivaz V (2000) Rats in a transparent morris water maze use elemental and configural geometry of landmarks as well as distance to the pool wall. Spatial Cognit Computat 2:135–156
Meaney MJ, Sapolsky RM, McEwen BS (1985) The development of the glucocorticoid receptor system in the rat limbic brain. I. Ontogeny and autoregulation. Dev Brain Res 18:159–164
Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T (2000) Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci 20:7116–7121
Morimasa T, Wirz-Justice A, Kraeuchi K, Arendt J, Baumann J, Haeusler A, Degen P, Feer H (1987) Chronic methamphetamine and its withdrawl modify behavioral and neuroendocrine circadian rhythms. Physiol Behav 39:699–705
Morris RGM (1981) Spatial localization does not require the presence of local cues. Learn Motivat 12:239–260
Morris RGM, Garrud P, Rawlins JNP, O'Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683
National Institute on Drug Abuse Research Report (1998) Methamphetamine: abuse and addiction. NIH Publication No 98-4210, 1–3
O'Callaghan JP, Miller DB (2000) Neurotoxic effects of substituted amphetamines in mice and rats: challenges to the current dogma. In: Massaro E, Broderick PA (eds) Handbook of Neurotoxicity. Humana Press, New York
O'Dell SJ, Marshall JF (2002) Effects of vibrissae removal on methamphetamine-induced damage to rat somatosensory cortical neurons. Synapse 43:122–130
Oro AS, Dixon SD (1987) Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. J Pediatr 111:571–578
Pu C, Vorhees CV (1993) Developmental dissociation of methamphetamine-induced depletion of dopaminergic terminals and astrocyte reaction in rat striatum. Dev Brain Res 72:325–328
Redman RS, Sweney LR (1976) Changes in diet and patterns of feeding activity in developing rats. J Nutr 106:615–626
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108:511–533
Sapolsky RM, Meaney MJ (1986) Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res Rev 11:65–76
Schaaf MJM, de Kloet ER, Vreugdenhil E (2000) Corticosterone efffects on BDNF expression in the hippocampus: Implications for memory formation. Stress 3:201–208
Schaaf MJM, Workel JO, Lesscher HM, Vreugdenhil E, Oitzl MS, de Kloet ER (2001) Correlation between hippocampal BDNF mRNA expression and memory performance in senescent rats. Brain Res 915:227–233
Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT (1994) Behavioural and pharmacological characterisation of the elevated "zero-maze" as an animal model of anxiety. Psychopharmacology 116:56–64
Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T (2001) Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology 57:255–260
Struthers JM, Hansen RL (1992) Visual recognition memory in drug-exposed infants. J Dev Behav Pediatr 13:108–111
Vorhees CV, Ahrens KG, Acuff-Smith KD, Schilling MA, Fisher JE (1994) Methamphetamine exposure during early postnatal development in rats: I. Acoustic startle augmentaion and spatial learning deficits. Psychopharmacology 114:392–401
Vorhees CV, Reed TM, Schilling MA, Fisher JE, Moran MS, Cappon GD, Nebert DW (1998) CYP2D1 polymorphism in methamphetamine-treated rats: genetic differences in neonatal mortality and effects on spatial learning and acoustic startle. Neurotoxicol Teratol 20:265–273
Vorhees CV, Morford LL, Inman SL, Reed TM, Schilling MA, Cappon GD, Moran MS, Nebert DW (1999) Genetic differences in spatial learning between Dark Agouti and Sprague-Dawley strains: possible correlation with the CYP2D2 polymorphism in rats treated neonatally with methamphetamine. Pharmacogenetics 9:171–181
Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS (2000) Adult learning deficits after neonatal exposure to d-methamphetamine: selective effects on spatial navigation and memory. J Neurosci 20:4732–4739
Williams MT, Inman-Wood SL, Morford LL, McCrea AE, Ruttle AM, Moran MS, Rock SL, Vorhees CV (2000) Preweaning treatment with methamphetamine induces increases in both corticosterone and ACTH in rats. Neurotoxicol Teratol 22:751–759
Williams MT, Vorhees CV, Boon F, Saber AJ, Cain DP (2002) Methamphetamine exposure from postnatal day 11 to 20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Res 958:312–321
Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW, Vorhees CV (2003) Developmental d-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse 48:138–148
Wilson JG (1973) Environment and birth defects. Academic Press, New York
Acknowledgements
This study was supported by NIH research grants DA06733 (C.V.V.) and DA14269 (M.T.W.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, M.T., Moran, M.S. & Vorhees, C.V. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology 168, 329–338 (2003). https://doi.org/10.1007/s00213-003-1433-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1433-y