Abstract
Seizure is paroxysmal abnormal electrical discharges in the cerebral cortex. Inflammatory pathways and oxidative stress are involved in the pathophysiology of seizures. Stress can induce an oxidative stress state and increase the production of inflammatory mediators in the brain. We investigated the effects of acute and chronic stresses on the seizure threshold in pentylenetetrazol (PTZ)-induced seizures in mice, considering oxidative stress and inflammatory mediators in the prefrontal cortex. In this study, 30 male Naval Medical Research Institute (NMRI) mice were divided into 3 groups, including acute stress, chronic stress, and control groups. PTZ was used for the induction of seizures. The gene expression of inflammatory markers (IL-1β, TNF-α, NLRP3, and iNOS), malondialdehyde (MDA) level, nitrite level, and total antioxidant capacity (TAC) were assessed in the prefrontal cortex and serum. Our results showed that stress could increase the expression of inflammatory cytokines genes and oxidative stress in the prefrontal cortex of the brain and serum following PTZ-induced seizures, which is associated with increased seizure sensitivity and decreased the seizure threshold. The effects of chronic stress were much more significant than acute stress. We concluded that the effects of chronic stress on seizure sensitivity and enhancement of neuroinflammation and oxidative stress are much greater than acute stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A seizure is a clinical expression of abnormal, excessive, or hypersynchronous electrical discharges of neurons in the brain. Recurrent and paroxysmal unprovoked seizures are called epilepsy. Epilepsy is one of the most important causes of mortality and morbidity worldwide. In 2016, it accounted for more than 13 million disability-adjusted life years (DALYs) (Beghi et al. 2019). Despite the variety of antiepileptic drugs (AEDs), more than 40% of people with epilepsy are still resistant to AEDs, which is crucial medical trouble these days (Laxer et al. 2014; Sharma et al. 2015).
Emerging lines of research indicated that oxidative stress is involved in the pathophysiology of seizures (Verma et al. 2021). Brain tissue is rich in polyunsaturated fatty acids, so it is very vulnerable to oxidative stress. During seizure attacks, lipid peroxidation damages the cell membrane, subsequently impairing its permeability and function (Shin et al. 2011).
The brain’s antioxidant system scavenges the physiological levels of ROS, so there is always a balance between the oxidants and the antioxidants in the brain (Seminotti et al. 2020). In states where oxidants increase, they can overcome the antioxidant defense system and cause cellular and macromolecular damage (Lorente et al. 2015). Oxidative stress has been shown to reduce PTZ-induced seizure threshold and increase susceptibility to seizures (Alzoubi et al. 2018). Also, ROS results from prolonged seizures and can contribute to the development of epilepsy (Huang et al. 2018; Pearson-Smith and Patel 2017).
Nitric oxide (NO) is a small, reactive molecule that diffuses readily through tissues (Picón-Pagès et al. 2019). In the brain, NO is involved in memory formation, signal transmission, and brain blood flow regulation (Angelis et al. 2021).
However, NO derived from inducible nitric oxide synthase (iNOS) in glial cells has neurotoxic effects (Garry et al. 2015). It has been shown that NO-derived decreased seizure threshold and NOS inhibitors may increase the seizure severity (Hrnčić et al. 2010; Shafaroodi et al. 2012). Inflammatory cytokines are essential in the pathogenesis of seizures (Kegler et al. 2021). In this regard, it has been determined that systemic inflammation could exacerbate or increase the frequency of the seizure (Alyu and Dikmen 2017; Marchi et al. 2014). In this concept, Meng and colleagues showed that NLRP3 inflammasome significantly up-regulated following status epilepticus seizure in rats. Previous studies have demonstrated that NLRP3 inflammasome is involved in the activation of IL-1β which is responsible for febrile seizures (Dubé et al. 2005; Liu et al. 2020). It has been found that IL-1β resulted in the generation of recurrent unprovoked seizures, decreased seizure threshold, and promoted hyperexcitability (Ren and Torres 2009; Vezzani and Baram 2007). It has been established that TNF-α increased in the hippocampus following seizure induction in rats (Hoda et al. 2017).
Stress can cause neurohormonal and metabolic changes in the body. Stress is associated with increased inflammatory cytokine levels in plasma (Cheng et al. 2015). Acute stress can increase the expression of iNOS and cause a neuroinflammatory response in the brain (Chen et al. 2016). Prolonged stress may decrease mitochondrial membrane potential, which can cause excitotoxicity and ROS formation (Zhu et al. 2021). In addition, high levels of corticosteroids following chronic stresses may reduce the total antioxidant capacity of the central nervous system (CNS) and creates a neurotoxic environment with proinflammatory cytokines (Colpo et al. 2017; Fee et al. 2020; Kirsten et al. 2021; Qin et al. 2011). Research has shown the potential correlation of the prefrontal cortex with different types of seizures, including febrile seizures (Dubé et al. 2009). Furthermore, the prefrontal cortex has been thoroughly explored in past studies in terms of seizure activity and is a credible brain region to investigate for understanding seizure pathophysiology (Qin et al. 2021; Lisowski et al. 2013; Nikbakhsh et al. 2020).
Due to the effects of stress on the brain, it is likely that stress also affects seizures. As a result, in this study, we investigated the effects of acute and chronic stress on the seizure threshold, considering the involvement of inflammatory pathways and oxidative stress biomarkers.
Materials and methods
Animals and housing conditions
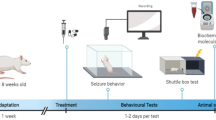
Male Naval Medical Research Institute (NMRI) mice weighing 25–35 g and 6–8 weeks old were purchased from the Pasteur Institute of Iran and used in this study. The animals were housed in standard Plexiglas cages under standard laboratory conditions (Ethical code: IR.SKUMS.REC.1398.003) as a 12-h light/dark cycle (light on from 6:00 AM), temperature 22 ± 1 °C with free access to food and water.
Study design
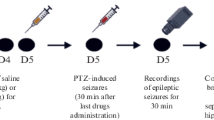
In this experiment, thirty male mice were randomly divided into three groups (n = 10). Restraint stress using falcon tubes was used to evaluate the effects of chronic and acute stresses. Group 1 was kept inside a 50-ml well-ventilated falcon tube for 30 min a day for 14 constant days (chronic stress protocol). Group 2 was kept inside the falcon tube for 1 h for 1 day (acute stress protocol). Group 3 was the control group that did not receive any stress (Hacioglu et al. 2016; Papadopoulou et al. 2015; Kurokawa et al. 2020; Fekri et al. 2021). Each animal was tested once.
Induction of seizure using PTZ
Pentylenetetrazole (PTZ) was used to induce seizure. Briefly, mice restrained for the short time in order to place the needle correctly into the vein. A winged infusion set (30 gauge) was used to infuse the PTZ (5 mg/ml) at a constant rate of 1 ml/min by a seizure pump into the tail vein of the freely moving subject. Infusion was halted when forelimb clonus followed by full clonus of the body (began with running and then loss of righting ability) was observed. The onset of a general clonus was used as the endpoint. The volume of PTZ solution required to attain the endpoint was recorded. The dose of PTZ (mg/kg of mice weight) inducing clonus seizure was calculated and considered as an index for colonic seizure threshold. The seizure threshold was calculated by using the time between injection of PTZ and onset of the seizure and calculated by the following formula (Haj-Mirzaian et al. 2019a; Amini-Khoei et al. 2015a):
time (s): the duration of PTZ administration (onset of administration until onset of clonic seizure).
PTZ was injected 60 min after restraint stress. After that seizure was observed (at the end of study), all mice were sacrificed and the prefrontal cortex and blood samples were taken for molecular and biochemical assessments.
Real-time PCR analysis for expression of inflammatory genes in the prefrontal cortex
At the end of the study, animals were sacrificed, the prefrontal cortex was isolated, and the gene expression of IL-1β, TNF-α, iNOS, and NLRP3 was examined by real-time PCR. Firstly, total RNA using TRIzol reagent (Invitrogen) was extracted from the prefrontal cortex. Alterations in the mRNA levels of genes were determined using qRT-PCR after the reverse transcription of 1 μg of RNA from each sample using a PrimeScript RT reagent kit (Takara Bio, Inc., Otsu, Japan). qRT-PCR was done on a light cycler device (Roche Diagnostics, Mannheim, Germany) using SYBR Premix Ex Taq technology (Takara Bio). Thermal cycling conditions included an initial activation step for 30 s at 95 °C afterward 45 cycles, as well as a denaturation step for 5 s at 95 °C and a combined annealing/extension step for 20 s at 60 °C. Melting curve analysis was performed to certify whether all primers yielded a single PCR product. The genes and their primers are listed in Table 1. B2m was used as a house-keeping gene (normalizer), and alterations in the expression of each target mRNA in comparison with B2m were measured based on the 2-ΔΔCt relative expression formula, as described in our previous publication (Amini-Khoei et al. 2015b).
Assessment of total antioxidant capacity in serum and prefrontal cortex
Assessment of nitrite in serum and prefrontal cortex
To determine the NO levels of serum and prefrontal cortex tissue, we measured nitrite levels as the result of the NO end product. In order to do this, 100 µg of each sample (serum and brain tissue homogenate) was poured into a 96-well plate. Then 50 µl of sulfanilamide solution (2 gr of sulfanilamide in 100 ml of hydrochloric acid 5%) was added. After 5–10 min, 50 µl of NEDD solution (0.1 gr of NEDD in 100 ml of distilled water) was added to wells and incubated at room temperature for half an hour. In the end, the Optical absorbance at 540 nm was read and determined by the standard nitrite curve in samples (Hassanipour et al. 2016; Haj-Mirzaian et al. 2018).
Assessment of malondialdehyde in serum and prefrontal cortex
Malondialdehyde (MDA) is the final form of polyunsaturated fatty acids peroxidation. The reaction of ROS with lipids is known as lipid peroxidation. MDA is generally accepted as a biomarker of oxidative stress.
To measure the level of MDA in serum and prefrontal cortex, we mixed 100 µl of the samples (serum/prefrontal cortex tissue homogenate) with 2.5 ml of 20% acetic acid, thiobarbituric acid (0.8%) and 100 µl of 8.1% SDS solution. The samples were kept in boiled water in Bain-marie for 1 h. Then, the samples were cooled and centrifuged at 4000 rpm for 10 min. At the end, the optical absorbance at 523 nm was recorded (Mahmoudi et al. 2020; Fokoua et al. 2021).
Data analysis
Data analysis was performed using the Graph Pad Prism 8 software using one-way ANOVA and Tukey’s post-test. P < 0.05 was considered statistically significant. Results were reported as mean ± SEM.
Results
Effects of acute and chronic stress on the seizure threshold
One-way ANOVA showed that there are significant different in the case of the seizure threshold among experimental groups (F (2.27) = 5.928, P < 0.05). As shown in Fig. 1, the seizure threshold was significantly decreased (P < 0.01) in the chronic stress group compared to the control group.
Evaluation of the gene expression of inflammatory cytokines in the prefrontal cortex
One-way ANOVA showed that there are significant different in the case of gene expression of TNF-α (F (2, 18) = 32/02, P < 0.001), IL-1β (F (2, 18) = 18/27, P < 0.001), NLRP3 (F (2, 18) = 19/21, P < 0.001) and iNOS (F (2, 18) = 15/24, P < 0.01) in the prefrontal cortex among experimental groups. As Fig. 2 presents, the gene expression of TNF-α in the prefrontal cortex in the chronic stress group as well as acute stress group significantly increased compared to the control group (P < 0.001). Furthermore, the gene expression of TNF-α in the prefrontal cortex in the chronic stress group significantly increased compared to the acute stress group (P < 0.05).
Gene expression of inflammatory cytokines in the prefrontal cortex in the experimental groups. Data are expressed as the mean ± SEM and were analyzed by one-way ANOVA following Tukey’s post-test. **P < 0.01 and ***P < 0.001 in comparison to the control group and #P < 0.05 an ##P < 0.01 in comparison to the acute group
The gene expression of IL-1β in the prefrontal cortex in the chronic and acute stress groups significantly increased compared to the control group (P < 0.01 and P < 0.05, respectively). Furthermore, the gene expression of Il-1β in the prefrontal cortex in the chronic stress group was significantly lower than in the acute stress group (P < 0.05).
In the case of NLRP3 gene expression, findings showed that the gene expression of NLRP3 in the prefrontal cortex in the chronic stress group significantly increased compared to the control group (P < 0.01). Furthermore, the gene expression of NLRP3 in the prefrontal cortex in the chronic stress group was significantly higher than in the acute stress group (P < 0.05).
Results showed that the gene expression of iNOS in the prefrontal cortex in the chronic stress group significantly increased compared to the control group (P < 0.001). Besides, the gene expression of iNOS in the prefrontal cortex in the chronic stress group was significantly higher than in the acute stress group (P < 0.051).
Evaluation of the total antioxidant capacity (TAC) in the serum and prefrontal cortex samples
One-way ANOVA showed that there are significant different in the case of TAC among experimental groups in the serum samples (F (2, 18) = 23/69, P < 0.001) and prefrontal samples (F (2, 18) = 47/03, P < 0.001). As shown in Fig. 3A, the TAC significantly increased in the prefrontal cortex in the chronic stress group (P < 0.001) as well as the acute stress group (P < 0.05) compared to the control group. Also, the TAC in the chronic stress group was significantly higher than in the acute stress group (P < 0.01).
Total antioxidant capacity in the prefrontal cortex (A) and serum samples (B) in the experimental groups. Data are expressed as the mean ± SEM and were analyzed by one-way ANOVA following Tukey’s post-test. *P < 0.05, **P < 0.01 and ***P < 0.001 in comparison to the control group and #P < 0.05 and ##P < 0.01 in comparison to the acute stress group
The TAC of serum samples, Fig. 3B, significantly decreased in the chronic stress group compared to the acute stress (P < 0.05) and control group (P < 0.01).
Evaluation of the MDA levels in the serum and prefrontal cortex samples
One-way ANOVA showed that there are significant different in the case of MDA level among experimental groups in the serum samples (F (2, 18) = 19/22, P < 0.001) and prefrontal samples (F (2, 18) = 27/14, P < 0.001). The results (Fig. 4A) showed that the MDA level in the prefrontal cortex of the chronic stress group significantly increased compared to the acute stress group and the control group (P < 0.01).
In the case of MDA level in the serum samples (Fig. 4B), we showed that the MDA level significantly increased in the chronic stress group in comparison to the acute stress group and the control group (P < 0.01).
Evaluation of the nitrite levels in the serum and prefrontal cortex samples
One-way ANOVA showed that there are significant different in the case of nitrite level among experimental groups in the serum samples (F (2, 18) = 15/08, P < 0.001) and prefrontal samples (F (2, 18) = 12/13, P < 0.01). According to Fig. 5A, nitrite levels in the prefrontal cortex in the acute stress group and the chronic stress group significantly increased compared to the control group (P < 0.001 and P < 0.01, respectively). Also, the nitrite level in the prefrontal cortex of the chronic stress group was significantly higher than in the acute stress group (P < 0.01).
Nitrite levels in the prefrontal cortex (A) and serum samples (B) in the experimental groups. Data are expressed as the mean ± SEM and were analyzed by one-way ANOVA following Tukey’s post-test. *P < 0.05, **P < 0.01, and ***P < 0.001 in comparison to the control group, and ##P < 0.01 in comparison to the acute stress group
According to Fig. 5B, the nitrite level in the prefrontal cortex in the chronic stress group significantly increased compared to the control group (P < 0.05). Also, nitrite level in the prefrontal cortex in the chronic stress group was significantly higher than in the acute stress group (P < 0.01).
Discussion
In the present study, the effects of the acute and chronic stresses on the PTZ-induced seizure were evaluated. We considered the role of neuroinflammation and oxidative stress. We found that chronic stress, compared to acute stress, has higher negative effects on the seizure threshold as well as neuroinflammatory and oxidative stress indicators. Findings showed that following chronic stress, the seizure threshold and antioxidant capacity decreased as well as levels of MDA, nitrite, and gene expression of some inflammatory cytokines increased in comparison with the acute stress.
Previous studies have determined that acute stress reduces the seizure threshold (Pehlivanidis et al. 2002). However, there is a controversy in the previous studies, which demonstrated that acute stress increased the seizure threshold (Shirzadian et al. 2018). In the case of chronic stress, some evidence showed that chronic stress increased the seizure threshold (Amini-Khoei et al. 2015b). In a contra verse manner, evidence determined that chronic stress could increase seizure susceptibility (Zhu et al. 2021; Nikbakhsh et al. 2020).
In regards to restraint stress, Hashemi and colleagues showed that restraint stress can decrease seizure threshold in pregnant rats. Also, repeated restraint stress decreased the seizure threshold (Hashemi et al. 2013). However, the specific effects of acute and chronic stresses on seizure sensitivity have not been precisely identified, and the particular mechanisms involved in these effects have not been understood (MacKenzie and Maguire 2015; Zhu et al. 2017). However, regarding to the abovementioned studies, our findings showed that chronic restraint stress significantly decreased the seizure threshold compared to the control group while acute restraint stress did not alter the seizure threshold compared to the control group.
Repeated restraint stress can lead to oxidative stress in the brain as an increase in glucocorticoids, ROS production, lipid peroxidation, and MDA levels (Fontella et al. 2005). Besides, antioxidants by attenuating oxidative stress have shown considerable neuroprotective effects against seizures (Fokoua et al. 2021). It has been determined that an increase in the level of MDA in the brain decreased the seizure threshold and also agents which attenuated the level of MDA exerted anticonvulsant effects (Asgharzadeh et al. 2020; Jafari and Hassanpourezatti 2022). The results of our study showed that chronic stress increased MDA levels in the serum and prefrontal cortex. But acute stress had no effects on these parameters following seizures.
Previous studies have been demonstrated that decrease in the antioxidant capacity involved in the pathophysiology of seizure. These researches showed that TAC decreased following seizure (Nieoczym et al. 2008; Menon et al. 2014). In this regard, it has been determined that some agents with potential anticonvulsant effects increased the TAC (Salimian et al. 2022; Movahed et al. 2017). Our study showed that the TAC of the prefrontal cortex increased in the chronic and acute stress groups. However, this increase was much more significant in the chronic stress group than in the acute stress group. This result may be due to the fact that the brain’s antioxidant defense system is overactive to counteract the pro-oxidative effects of chronic stress and thus increase its antioxidant capacity. In the case of serum samples, the total antioxidant capacity in the chronic stress group was significantly reduced compared to the control and acute stress groups. We suggest more studies to investigate the pathways and mediators of the brain’s antioxidant defenses following chronic stress.
Nitric oxide (NO) is considered as a neurotransmitter or neuromodulator. A high concentration of NO can induce oxidative stress-mediated events (Hassanipour et al. 2016; Haj-Mirzaian et al. 2019b). Clinical and preclinical studies have been reported that the level of nitrite increases in the serum and brain following seizure attacks (Karabiber et al. 2004; Amiri et al. 2014). Previous researches showed that the administration of nitric oxide synthase (NOS) inhibitors possessed anti-convulsion effects (Gholipour et al. 2010; Moazzami et al. 2013; Monika et al. 2011). Our results showed that nitrite levels increased in the prefrontal cortex in both acute and chronic stress groups, but it was higher in the chronic stress group than in the acute stress group. Also, serum nitrite level was much more increased in the chronic stress group than in the acute stress group, indicating that chronic stress imposes more oxidative stress conditions in the prefrontal cortex.
Additionally, iNOS gene expression in the prefrontal cortex was much more increased in the chronic stress group. Chronic stress seems to play an essential role in the development and progression of seizures by increasing iNOS gene expression and increasing prefrontal cortex and serum nitrite levels. Further studies are recommended to determine NO signaling pathways and the roles of different NOS enzymes (nNOS and eNOS) in seizures following different stressful conditions. Overall, our results showed that, unlike acute stress, chronic stress could intensify oxidative stress caused by PTZ-induced seizure and also can reduce the seizure threshold.
Previous studies have shown that the expression of the TNF-α gene in the brain tissue increased following seizures (Thompson et al. 2011; Weinberg et al. 2013). Also, inhibition of the TNF-α receptor can increase seizure tolerance (Hanak et al. 2019). The present study showed an increase in TNF-α levels in the prefrontal cortex in both acute and chronic stress groups following PTZ-induced seizure compared to the control group. This increase was much more significant in the chronic stress group.
NLRP3 promotes neuroinflammation through IL-1β production. Increased expression of NLRP3 is associated with elevated levels of IL-1β. Increased levels of NLRP3 and IL-1β are involved in seizure pathogenesis (Brito et al. 2021). The results of the present study showed that the amount of IL-1β gene expression in the prefrontal cortex tissue was higher in the stressed groups than in the control group. Moreover, the IL-1β gene expression in the acute stress group was significantly higher than in the chronic stress group. However, the level of NLRP3 in the chronic stress group was significantly higher than in the acute stress group and the control group. It seems that in the process of activating IL-1β in the acute stress group, other mechanisms are involved besides NLRP3, which is recommended to be studied in other studies. Overall, chronic stress had a more significant effect on the expression of inflammatory genes than acute stress.
Conclusion
In conclusion, the results of this study showed that stress could increase the expression of inflammatory cytokines genes and oxidative stress in the prefrontal cortex and serum following PTZ-induced seizures, which is associated with increased seizure sensitivity and decreased the seizure threshold. Meanwhile, the effects of chronic stress are much more significant than acute stress.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Alyu F, Dikmen M (2017) Inflammatory aspects of epileptogenesis: contribution of molecular inflammatory mechanisms. Acta Neuropsychiatr 29(1):1–16
Alzoubi KH, Hasan ZA, Khabour OF, Mayyas FA, Al Yacoub ON, Banihani SA et al (2018) The effect of high-fat diet on seizure threshold in rats: role of oxidative stress. Physiol Behav 196:1–7
Amini-Khoei H, Rahimi-Balaei M, Amiri S, Haj-Mirzaian A, Hassanipour M, Shirzadian A et al (2015a) Morphine modulates the effects of histamine H1 and H3 receptors on seizure susceptibility in pentylenetetrazole-induced seizure model of mice. Eur J Pharmacol 769:43–47
Amini-Khoei H, Amiri S, Shirzadian A, Haj-Mirzaian A, Alijanpour S, Rahimi-Balaei M et al (2015b) Experiencing neonatal maternal separation increased the seizure threshold in adult male mice: Involvement of the opioid system. Epilepsy Behav 52:37–41
Amiri S, Shirzadian A, Haj-Mirzaian A, Imran-Khan M, Balaei MR, Kordjazy N et al (2014) Involvement of the nitrergic system in the proconvulsant effect of social isolation stress in male mice. Epilepsy Behav 41:158–163
Angelis D, Savani R, Chalak L (2021) Nitric oxide and the brain. Part 1: Mechanisms of regulation, transport and effects on the developing brain. Pediatr Res 89(4):738–45
Asgharzadeh F, Hosseini M, Bargi R, Beheshti F, Rakhshandeh H, Mansouri S et al (2020) Effects of hydro-ethanolic extract of tanacetum parthenium and its N-butanol and aqueous fractions on brain oxidative damage in pentylenetetrazole-induced seizures in mice. Pharm Sci 26(3):252–260
Beghi E, Giussani G, Nichols E, Abd-Allah F, Abdela J, Abdelalim A et al (2019) Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(4):357–375
Chen H-JC, Spiers JG, Sernia C, Lavidis NA (2016) Acute restraint stress induces specific changes in nitric oxide production and inflammatory markers in the rat hippocampus and striatum. Free Rad Biol Med 90:219–29
Cheng Y, Jope RS, Beurel E (2015) A pre-conditioning stress accelerates increases in mouse plasma inflammatory cytokines induced by stress. BMC Neurosci 16(1):31
Colpo AC, de Lima ME, Maya-López M, Rosa H, Márquez-Curiel C, Galván-Arzate S et al (2017) Compounds from Ilex paraguariensis extracts have antioxidant effects in the brains of rats subjected to chronic immobilization stress. Appl Physiol Nutr Metab 42(11):1172–1178
Cristina de Brito Toscano E, Leandro Marciano Vieira É, Boni Rocha Dias B, Vidigal Caliari M, Paula Gonçalves A, Varela Giannetti A et al (2021) NLRP3 and NLRP1 inflammasomes are up-regulated in patients with mesial temporal lobe epilepsy and may contribute to overexpression of caspase-1 and IL-β in sclerotic hippocampi. Brain Res 1752:147230
Dubé C, Vezzani A, Behrens M, Bartfai T, Baram TZ (2005) Interleukin-1β contributes to the generation of experimental febrile seizures. Ann Neurol 57(1):152–155
Dubé CM, Zhou J-L, Hamamura M, Zhao Q, Ring A, Abrahams J et al (2009) Cognitive dysfunction after experimental febrile seizures. Exp Neurol 215(1):167–177
Fee C, Prevot T, Misquitta K, Banasr M, Sibille E (2020) Chronic stress-induced behaviors correlate with exacerbated acute stress-induced cingulate cortex and ventral hippocampus activation. Neuroscience 440:113–129
Fekri K, Mahmoudi J, Sadigh-Eteghad S, Farajdokht F, Nayebi AM (2021) Coumestrol alleviates oxidative stress, apoptosis and cognitive impairments through hippocampal estrogen receptor-beta in male mouse model of chronic restraint stress. Pharm Sci 28(2):260–274
Fokoua AR, Ajayi AM, Ben-Azu B, Chouna R, Folarin O, Olopade J et al (2021) The antioxidant and neuroprotective effects of the psychotria camptopus Verd. Hook. (Rubiaceae) stem bark methanol extract contributes to its antiepileptogenic activity against pentylenetetrazol kindling in male Wistar rats. Metab Brain Dis 36(7):2015–27
Fontella FU, Siqueira IR, Vasconcellos APS, Tabajara AS, Netto CA, Dalmaz C (2005) Repeated restraint stress induces oxidative damage in rat hippocampus. Neurochem Res 30(1):105–111
Garry PS, Ezra M, Rowland MJ, Westbrook J, Pattinson KTS (2015) The role of the nitric oxide pathway in brain injury and its treatment — from bench to bedside. Exp Neurol 263:235–243
Gholipour T, Ghasemi M, Riazi K, Ghaffarpour M, Dehpour AR (2010) Seizure susceptibility alteration through 5-HT3 receptor: modulation by nitric oxide. Seizure 19(1):17–22
Hacioglu G, Senturk A, Ince I, Alver A (2016) Assessment of oxidative stress parameters of brain-derived neurotrophic factor heterozygous mice in acute stress model. Iran J Basic Med Sci 19(4):388–393
Haj-Mirzaian A, Amiri S, Amini-Khoei H, Haj-Mirzaian A, Hashemiaghdam A, Ramezanzadeh K et al (2018) Involvement of NO/NMDA-R pathway in the behavioral despair induced by amphetamine withdrawal. Brain Res Bull 139:81–90
Haj-Mirzaian A, Nikbakhsh R, Ramezanzadeh K, Rezaee M, Amini-Khoei H, Haj-Mirzaian A et al (2019a) Involvement of opioid system in behavioral despair induced by social isolation stress in mice. Biomed Pharmacother 109:938–944
Haj-Mirzaian A, Ramezanzadeh K, Tafazolimoghadam A, Kazemi K, Nikbakhsh R, Nikbakhsh R et al (2019b) Protective effect of minocycline on LPS-induced mitochondrial dysfunction and decreased seizure threshold through nitric oxide pathway. Eur J Pharmacol 858:172446
Hanak TJ, Libbey JE, Doty DJ, Sim JT, DePaula-Silva AB, Fujinami RS (2019) Positive modulation of mGluR5 attenuates seizures and reduces TNF-α+ macrophages and microglia in the brain in a murine model of virus-induced temporal lobe epilepsy. Exp Neurol 311:194–204
Hashemi P, Ebrahimi L, Saboory E, Roshan-Milani S (2013) Effect of restraint stress during gestation on pentylenetetrazol-induced epileptic behaviors in rat offspring. Iran J Basic Med Sci 16(9):979–984
Hassanipour M, Amini-Khoei H, Shafaroodi H, Shirzadian A, Rahimi N, Imran-Khan M et al (2016) Atorvastatin attenuates the antinociceptive tolerance of morphine via nitric oxide dependent pathway in male mice. Brain Res Bull 125:173–180
Hoda U, Agarwal NB, Vohora D, Parvez S, Raisuddin S (2017) Resveratrol suppressed seizures by attenuating IL-1β, IL1-Ra, IL-6, and TNF-α in the hippocampus and cortex of kindled mice. Nutr Neurosci 20(9):497–504
Hrnčić D, Rašić-Marković A, Krstić D, Macut D, Djuric D, Stanojlović O (2010) The role of nitric oxide in homocysteine thiolactone-induced seizures in adult rats. Cell Mol Neurobiol 30(2):219–231
Huang W-Y, Lin S, Chen H-Y, Chen Y-P, Chen T-Y, Hsu K-S et al (2018) NADPH oxidases as potential pharmacological targets against increased seizure susceptibility after systemic inflammation. J Neuroinflammation 15(1):140
Jafari AM, Hassanpourezatti M (2022) Influence of methadone on the anticonvulsant efficacy of valproate sodium gabapentin against maximal electroshock seizure in mice by regulation of brain MDA TNF-α. Front Neurol 2022;13
Karabiber H, Yakinci C, Durmaz Y, Temel I, Mehmet N (2004) Serum nitrite and nitrate levels in epileptic children using valproic acid or carbamazepine. Brain Dev 26(1):15–18
Kegler A, Pascotini ET, Caprara ALF, Arend J, Gabbi P, Duarte MMMF et al (2021) Relationship between seizure type, metabolic profile, and inflammatory markers in blood samples of patients with epilepsy. Epileptic Disord 23(1):74–84
Kirsten K, Pompermaier A, Koakoski G, Mendonça-Soares S, da Costa RA, Maffi VC et al (2021) Acute and chronic stress differently alter the expression of cytokine and neuronal markers genes in zebrafish brain. Stress 24(1):107–112
Kurokawa K, Tsuji M, Takahashi K, Miyagawa K, Mochida-Saito A, Takeda H (2020) Leukemia inhibitory factor participates in the formation of stress adaptation via hippocampal myelination in mice. Neuroscience 446:1–13
Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N et al (2014) The consequences of refractory epilepsy and its treatment. Epilepsy Behav 37:59–70
Lisowski P, Wieczorek M, Goscik J, Juszczak GR, Stankiewicz AM, Zwierzchowski L et al (2013) Effects of chronic stress on prefrontal cortex transcriptome in mice displaying different genetic backgrounds. J Mol Neurosci 50(1):33–57
Liu Z, Xian H, Ye X, Chen J, Ma Y, Huang W (2020) Increased levels of NLRP3 in children with febrile seizures. Brain Dev 42(4):336–341
Lorente L, Martín MM, Almeida T, Abreu-González P, Ramos L, Argueso M et al (2015) Total antioxidant capacity is associated with mortality of patients with severe traumatic brain injury. BMC Neurol 15(1):115
MacKenzie G, Maguire J (2015) Chronic stress shifts the GABA reversal potential in the hippocampus and increases seizure susceptibility. Epilepsy Res 109:13–27
Mahmoudi T, Lorigooini Z, Rafieian-kopaei M, Arabi M, Rabiei Z, Bijad E et al (2020) Effect of curcuma zedoaria hydro-alcoholic extract on learning, memory deficits and oxidative damage of brain tissue following seizures induced by pentylenetetrazole in rat. Behav Brain Funct 16(1):7
Marchi N, Granata T, Janigro D (2014) Inflammatory pathways of seizure disorders. Trends Neurosci 37(2):55–65
Menon B, Ramalingam K, Kumar RV (2014) Low plasma antioxidant status in patients with epilepsy and the role of antiepileptic drugs on oxidative stress. Ann Indian Acad Neurol 17(4):398
Moazzami K, Emamzadeh-Fard S, Shabani M (2013) Anticonvulsive effect of atorvastatin on pentylenetetrazole-induced seizures in mice: the role of nitric oxide pathway. Fundam Clin Pharmacol 27(4):387–392
Monika B, Barbara P, Stanislaw JC, Kinga KB (2011) Nitric oxide, epileptic seizures, and action of antiepileptic drugs. CNS Neurol Disord Drug Targets 10(7):808–819
Movahed A, Ghaderi M, Daneshi A, Nabipour I, Keshavarz M (2017) Hydroalcoholic extract of Sargassum Oligocystum attenuates pentylenetetrazole-induced seizures by potentiating antioxidant activity in mice. Int J Epilepsy 4(02):159–166
Nieoczym D, Albera E, Kankofer M, Wlaź P (2008) Maximal electroshock induces changes in some markers of oxidative stress in mice. J Neural Transm 115(1):19–25
Nikbakhsh R, Nikbakhsh R, Radmard M, Tafazolimoghadam A, Haj-Mirzaian A, Pirri F et al (2020) The possible role of nitric oxide in anti-convulsant effects of Naltrindole in seizure-induced by social isolation stress in male mice. Biomed Pharmacother 129:110453
Nikbakhsh R, Nikbakhsh R, Radmard M, Tafazolimoghadam A, Haj-Mirzaian A, Pirri F et al (2020) The possible role of nitric oxide in anti-convulsant effects of Naltrindole in seizure-induced by social isolation stress in male mice. Biomed Pharmacother 129:110453
Papadopoulou A, Siamatras T, Delgado-Morales R, Amin ND, Shukla V, Zheng YL et al (2015) Acute and chronic stress differentially regulate cyclin-dependent kinase 5 in mouse brain: implications to glucocorticoid actions and major depression. Transl Psychiatry 5(6):e578-e
Pearson-Smith JN, Patel M (2017) Metabolic dysfunction and oxidative stress in epilepsy. Int J Mol Sci 18(11):2365
Pehlivanidis C, Fotoulaki M, Boucher W, Kempuraj D, Pang X, Konstantinidou A, Theoharides TC (2002) Acute stress-induced seizures and loss of consciousness in a ten-year-old boy with cutaneous mastocytosis. J Clin Psychopharmacol 22(2):221–224
Picón-Pagès P, Garcia-Buendia J, Muñoz FJ (2019) Functions and dysfunctions of nitric oxide in brain. Biochim Biophys Acta (BBA) Mol Basis Dis 1865(8):1949–67
Qin M, Xia Z, Huang T, Smith CB (2011) Effects of chronic immobilization stress on anxiety-like behavior and basolateral amygdala morphology in Fmr1 knockout mice. Neuroscience 194:282–290
Qin L, Williams JB, Tan T, Liu T, Cao Q, Ma K et al (2021) Deficiency of autism risk factor ASH1L in prefrontal cortex induces epigenetic aberrations and seizures. Nat Commun 12(1):6589
Ren K, Torres R (2009) Role of interleukin-1β during pain and inflammation. Brain Res Rev 60(1):57–64
Salimian S, Habibian-Dehkordi S, Kaboutari J, Amini-Khoei H, Barkhordari MS (2022) Anethole exerted anticonvulsant effect in pentylenetetrazolemodel of seizure in male mice: Possible antioxidant effects. Future Nat Prod 8(1):2–6
Seminotti B, da Silva JC, Ribeiro RT, Leipnitz G, Wajner M (2020) Free radical scavengers prevent argininosuccinic acid-induced oxidative stress in the brain of developing rats: a new adjuvant therapy for argininosuccinate lyase deficiency? Mol Neurobiol 57(2):1233–1244
Shafaroodi H, Moezi L, Fakhrzad A, Hassanipour M, Rezayat M, Dehpour AR (2012) The involvement of nitric oxide in the anti-seizure effect of acute atorvastatin treatment in mice. Neurol Res 34(9):847–853
Sharma AK, Rani E, Waheed A, Rajput SK (2015) Pharmacoresistant epilepsy: a current update on non-conventional pharmacological and non-pharmacological interventions. J Epilepsy Res 5(1):1–8
Shin E-J, Jeong JH, Chung YH, Kim W-K, Ko K-H, Bach J-H et al (2011) Role of oxidative stress in epileptic seizures. Neurochem Int 59(2):122–137
Shirzadian A, Ostadhadi S, Hassanipour M, Shafaroodi H, Khoshnoodi M, Haj-Mirzaian A et al (2018) Acute foot-shock stress decreased seizure susceptibility against pentylenetetrazole-induced seizures in mice: interaction between endogenous opioids and cannabinoids. Epilepsy Behav 87:25–31
Thompson SJ, Ashley MD, Stöhr S, Schindler C, Li M, McCarthy-Culpepper KA et al (2011) Suppression of TNF receptor-1 signaling in an in vitro model of epileptic tolerance. Int J Physiol Pathophysiol Pharmacol 3(2):120–132
Verma N, Maiti R, Mishra BR, Jha M, Jena M, Mishra A (2021) Effect of add-on melatonin on seizure outcome, neuronal damage, oxidative stress, and quality of life in generalized epilepsy with generalized onset motor seizures in adults: a randomized controlled trial. J Neurosci Res 99(6):1618–1631
Vezzani A, Baram TZ (2007) New roles for interleukin-1 beta in the mechanisms of epilepsy. Epilepsy Curr 7(2):45–50
Weinberg MS, Blake BL, McCown TJ (2013) Opposing actions of hippocampus TNFα receptors on limbic seizure susceptibility. Exp Neurol 247:429–437
Zhu X, Dong J, Xia Z, Zhang A, Chao J, Yao H (2017) Repeated restraint stress increases seizure susceptibility by activation of hippocampal endoplasmic reticulum stress. Neurochem Int 110:25–37
Zhu Z, Zhao X, OuYang Q, Wang Y, Xiong Y, Cong S et al (2021) Waterfall forest environment regulates chronic stress via the NOX4/ROS/NF-κB signaling pathway. Front Neurol 12:619728-
Acknowledgements
The authors are thankful to Dr. Sorayya Ghasemi and Dr. Gholam Reza Mobini for their contribution to this study.
Funding
This study was supported by a research grant (2831) from Shahrekord University of Medical Sciences, Shahrekord, Iran.
Author information
Authors and Affiliations
Contributions
HTD, EB, ES, and HA-K performed experiments and wrote the manuscript; MS-K analyzed the data; HA-K designed the study and edited the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
All steps of experimentation were performed in accordance with the regulations of the University and the Guide for the Care and Use of Laboratory Animals of National Institutes of Health (Ethics code: IR.SKUMS.REC.1398.003) and Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press). Full determinations were made to reduce the use of animals and to advance their comfort.
Consent to participate
Not applicable.
Consent for publication
All authors reviewed and approved the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dehkordi, H.T., Bijad, E., Saghaei, E. et al. Chronic stress but not acute stress decreases the seizure threshold in PTZ-induced seizure in mice: role of inflammatory response and oxidative stress. Naunyn-Schmiedeberg's Arch Pharmacol 396, 973–982 (2023). https://doi.org/10.1007/s00210-022-02364-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02364-7