Abstract
Coronavirus disease 2019 (COVID-19) is a potentially fatal disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that preferentially infects the respiratory tract. Bradykinin (BK) is a hypotensive substance that recently emerged as one of the mechanisms to explain COVID-19-related complications. Concerning this, in this review, we try to address the complex link between BK and pathophysiology of COVID-19, investigating the role of this peptide as a potential target for pharmacological modulation in the management of SARS-CoV-2. The pathology of COVID-19 may be more a result of the BK storm than the cytokine storm, and which BK imbalance is a relevant factor in the respiratory disorders caused by SARS-CoV-2 infection. Regarding this, an interesting point of intervention for this disease is to modulate BK signaling. Some drugs, such as icatibant, ecallantide, and noscapine, and even a human monoclonal antibody, lanadelumab, have been studied for their potential utility in COVID-19 by modulating BK signaling. The interaction of the BK pathway and the involvement of cytokines such as IL-6 and IL1 may be key to the use of blockers, even if only as adjuvants. In fact, reduction of BK, mainly DABK, is considered a relevant strategy to improve clinical conditions of COVID-19 patients. In this context, despite the current unproven clinical efficacy, drugs repurposing that block B1 or B2 receptor activation have gained prominence for the treatment of COVID-19 in the world.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus disease 2019 (COVID-19) is a potentially fatal disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that preferentially infects the respiratory tract and causes pneumonia in humans (Chen et al. 2020). In severe cases, patients may develop acute respiratory distress syndrome (ARDS), coagulation disturbances, septic shock, multiple organ failure, and, consequently, death (Wang et al. 2020; She et al. 2020). Severe COVID-19 has been associated with a massive release of proinflammatory cytokines and hyperactivation of innate immune cells (Lucas et al. 2020; Iwasaki et al. 2021). Recently, studies have been proposed that dysregulated bradykinin (BK) signaling may be the trigger of the cytokine storm observed in people with severe disease (van de Veerdonk et al. 2020b).
BK is a powerful hypotensive and smooth muscle stimulating polypeptide that acts as a downstream product of the kallikrein-kinin system (KSS) (Leeb-Lundberg et al. 2005). BK was discovered by three Brazilian scientists led by Dr. Maurício Rocha e Silva in a study from Bothrops jararaca snake venom. They demonstrated that trypsin-like enzymes release the pharmacological substance BK, instead of histamine, from plasma globulin precursor. The discovery of BK allowed the study of a new physio and pathological phenomenon (Hawgood 1997). The action of BK is constitutively mediated by the B2 receptor, whereas the B1 receptor is activated by the metabolites des-Arg9-BK (DABK) and Lys-des-Arg9-BK (Lys-DABK) under inflammatory conditions (Ahluwalia and Perretti 1999; Leeb-Lundberg et al. 2005).
BK is a peptide rapidly produced and degraded under physiological conditions that plays a crucial role in several processes in the endothelium (Su 2015). It acts as a regulator of tissue blood flow and vasomotricity and can be appointed as an extension member of the renin-angiotensin system (RAS) (Schmaier 2002). This molecule is involved in the induction of vasodilation, natriuresis, and hypotension, events that occur after activation of B2 receptors (Marcic et al. 1999; ERDOS 2002; Chen et al. 2005). Moreover, high concentrations of this peptide play a prominent role in the inflammatory and oxidative process (Jacox et al. 2014; Hofman et al. 2016; Ruocco et al. 2020), as well as in the sensitization of sensory nerve endings (Choi and Hwang 2018). The DABK is a biological substrate of ACE2 in lung and consequently the reduction in ACE2 function leads to impaired inactivation of DABK, resulting in activation of the B1 receptor signaling cascade and increases neutrophil recruiting and chemokine production in airway epithelial cells (Sodhi et al. 2018). Since degradation of BK is regulated by the angiotensin-converting enzyme (ACE) and the strong evidence that ACE2 can cleave DABK and Lys-DABK, it has been hypothesized that BK metabolism could be affected by SARS-CoV2 infection due to interactions between viral glycoproteins and ACE2 enzyme (Datta et al. 2020). Recently, peptide BK has emerged as one of the mechanism to explain COVID-19-related complications (Karamyan 2021).
Considering the proinflammatory, oxidative, and proliferative actions of BK and its clinical repercussion (Kempe et al. 2020), it is possible that BK has a pivotal role in the pathophysiology of COVID-19. In this review, we discuss the complex link between BK and the pathophysiology of COVID-19, investigating the role of this peptide as a potential target of pharmacological modulation in the management of SARS-CoV-2 infection.
Bradykinin pathway in the pathogenesis of COVID-19
Rameshrad et al. (2020) described the importance of BK in the SARS-CoV-2 infection by calling attention to the interplay between ACE in the renin-angiotensin system (RAS) and KSS system. The binding between SARS-CoV-2 and ACE2 unbalances the function of ACE2 by decreasing its surface expression, resulting in dysfunction in the RAS and, consequently, accumulation of angiotensin II (Ang II) (Shukla and Banerjee 2021), and increased levels of BK bioactive metabolite DABK (Colarusso et al. 2020). Therefore, the downregulation of ACE2 may lead to the increased availability of DABK, BK, and other compounds associated with hyperinflammatory response. Moreover, it was found that transmembrane protease serine 2 (TMPRSS2), a fundamental host protein used for SARS-CoV-2 to entry in the cell, has a kallikrein-like effect upon plasmatic kininogen and is involved in enhancing BK and DABK production (Nicolau et al. 2020).
Cell damage and inflammation caused by SARS-CoV-2 induce the release of DABK metabolites and activation of the B1 receptor (McLean et al. 2000). Roche and Roche (2020) reported that high levels of DABK in the extracellular environment of neighboring cells lead to a positive feedback cycle of injury and inflammation. The exposure of B1 receptor to proinflammatory cytokines during SARS-CoV-2 infection (Colarusso et al. 2020) has also been associated with leukocyte migration, oxidative stress, and increase in vascular permeability with important pulmonary repercussion (Colarusso et al. 2020; Ayres et al. 2020; Parekh et al. 2020). In addition, it has been demonstrated that activation of the B2 receptor is responsible for the release of nitric oxide (NO), prostacyclin, and may induce the hyperpolarization derived from the endothelium (Chow et al. 2020). Moreover, BK plays a key role in cardiovascular function, such as has a vasodilation profile, enhances vascular permeability, and lowers blood pressure, producing these effects through binding to its receptors, B1 and B2 (Nussberger et al. 2002).
On this context, Garvin et al. (2020) (Garvin et al. 2020), by analyzing gene expression of cells in the bronchoalveolar lavage fluid (BALF), found that both RAS and BK systems are affected during COVID-19 infection. In addition, there is a negative regulation (up to 8 times) of enzymes that degrade BK (Garvin et al. 2020). BALF samples from patients infected with COVID-19 confirmed a negative regulation of ACE. This promotes deviation in RAS, which makes it possible to sensitize the effects of BK in view of the presence of Ang 1–9. As a result of BK elevation, vasodilation occurs, which corroborates with the vascular leakage, and inflammatory cell infiltration.
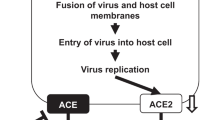
Still, Garvin et al. (2020) (Garvin et al. 2020) suggested that the pathogenesis of COVID-19 may be more a result of the BK storm than the cytokine storm. The BK storm caused by inhibition of ACE2 may be responsible for triggering the most serious symptoms from the COVID-19 since the induction of fluid leakage into the lung by BK and high levels of hyaluronic acid may generate a gelatin-like substance that makes it difficult to capture oxygen and release carbon dioxide into the lungs (Garvin et al. 2020). In addition, the ACE2 downregulation caused by SARS-CoV-2 infection increases DABK and, consequently, there is an intensification of the cytokine release. The activation of inflammatory mediators can lead to ARDS and multiple organ failure (Tolouian et al. 2020). Thus, it has been hypothesized that the elevation of BK levels plays a pivotal link between ACE2 downregulation and the severity of SARS-CoV-2 infection (Mansour et al. 2021). Therefore, ACE2, DABK, and B1 receptor are suggested as a pharmacological pathway to prevent or moderate the ARDS and complications in COVID-19 patients (Fig. 1).
Schematic representation of BK storm caused by SARS-CoV-2 infection. SARS-CoV-2 binds to ACE2 decreasing its surface expression attenuating kinin degradation and, consequently, increasing the levels of BK and DABK in plasma and tissue. Briefly, plasma kallikrein processes high-molecular-weight kininogen to BK. BK interacts with B2 receptor on the endothelial cells. DABK is formed from BK degradation by carboxypeptidase, which are ligands of the B1 receptor also present on the endothelial cells and upregulated under inflammatory conditions. DABK is degraded by ACE2, which is downregulated in COVID-19, leading to an increase in inflammatory mediators and organs failure
Furthermore, the interplay between BK and neurotensin (NT) or substance P (SP), as well as their cognate signaling pathways, has been identified as critical players in pathogenic mechanisms of COVID-19 (Karamyan 2021). The BK, NT, and SP could cause impairment of BBB under pathophysiological conditions (Al-Ahmad et al. 2021), as well as are related to inflammation-induced complications of COVID-19 pathology (Karamyan 2021). Simultaneous inhibition of BK, NT, and SP systems would be therapeutically more advantageous rather than modulation of the BK mechanism alone.
Pharmacotherapies that target BK, DABK, or B1 and B2 receptors in the treatment of COVID-19
Treatment of COVID-19 requires an understanding of underlying molecular mechanisms associated with disease progression to provide a therapeutical response with appropriate use of available drugs, including those repurposed. Recently, it was proposed that dysregulation of BK signaling could be involved in the pathogenesis of this disease. In this way, an interesting point of intervention for SARS-CoV-2 infection is to modulate BK and DABK concentrations or block B1 or B2 receptors (Vickers et al. 2002). Mansour et al. (2021) (Mansour et al. 2021) suggest that inhibition of BK signaling in severe COVID-19 patients could mitigate the lung inflammatory response with a positive impact on the disease severity, reducing mortality rates.
Ghahestani et al. (2020) (Ghahestani et al. 2020) hypothesized that blocking B2 receptors with icatibant may be a pharmacological strategy facing the BK deregulation in COVID-19 patients (Ghahestani et al. 2020). Icatibant is a safe B2 receptor antagonist (Dubois and Cohen 2010) that could be administered to reduce the BK signaling (Garvin et al. 2020). This drug is reported in the literature for its effectiveness in treating respiratory disorders, including patients who develop angioedema from the use of ACE inhibitors (Baş et al. 2015). In addition, there is a positive association between the administration of icatibant and improved oxygenation in severe COVID-19 patients, suggesting that targeting the kallikrein-kinin system might be beneficial for controlling clinical outcomes in these patients (van de Veerdonk et al. 2020a, p. 19).
According to Veerdonk et al. (2020a, b) (van de Veerdonk et al. 2020b), the pulmonary angioedema presented in COVID-19 patients may be associated with the release of kinins, resulting in a very high number of intensive care unit (ICU) admissions. Furthermore, COVID-19 severity has been associated with the upregulation of proinflammatory cytokines that could be stimulated by the BK cascade (Karamyan 2021). Therefore, blocking BK receptors might ameliorate COVID-19 complications by reducing kinin levels and, consequently, the inflammatory process (van de Veerdonk et al. 2020b). In the same context, Colarusso et al. (2020) (Colarusso et al. 2020) suggested that inhibiting the upstream signaling that leads to the BK production would be an alternative pharmacotherapeutic strategy for patients with COVID-19. The authors stated that the use of lanadelumab, a human monoclonal antibody that acts as a plasma kallikrein inhibitor, which is important for the cleavage of high-molecular-weight kininogen (HMWK) into bradykinin (Colarusso et al. 2020). It can block the upstream axis that leads to kinin production, preventing coagulation and inflammatory storm, decreasing morbidity and mortality associated with COVID-19. Furthermore, studies in sepsis experiments based on the administration of B1 receptor antagonists have shown positive results in hemodynamic disorders with reduced risk of multiple organ failure (Murugesan et al. 2016). Tolouian et al. (2020) (Tolouian et al. 2020) proposed that the inhibition of BK from the selective binding of ecallantide to plasma kallikrein (Tolouian et al. 2020), with reduction of B1 activation, could decrease the damages caused by SARS-CoV-2. In addition, B1 receptor antagonist LF22-0542, also known as safotibant, could be considered as a promising drug to treat COVID-19 due to its anti-inflammatory effects (Mahmudpour et al. 2020).
There is growing evidence on the increased risk of arterial and venous thromboembolism in patients with COVID-19. Pulmonary embolization and deep vein thrombosis have the potential to activate KKS in plasma, which leads to BK production (Schmaier 2016). In this sense, Solun et al. (2020) (Solun and Shoenfeld 2020) have suggested the administration of aprotinin—an FDA-approved monomeric polypeptide that acts as a nonspecific serine protease inhibitor—as a pharmacotherapy for severe acute lung injury. Aprotinin inhibits the intrinsic pathway of coagulation and fibrinolysis and has been used to reduce the release of proinflammatory cytokines and bleeding during surgical procedures. However, the authors emphasized a concern regarding the administration of aprotinin against ARDS and acute lung injury from COVID-19 patients affected by acute coronary syndrome, renal failure, and cerebrovascular problems, as well as, patients who have undergone coronary artery bypass surgery or use drugs from the aminoglycoside class in a synchronous manner (Solun and Shoenfeld 2020). However, aprotinin is not a specific protease inhibitor and its clinical usefulness in the disease is doubtful and needs more studies.
According to Ebrahimi (2020) (Ebrahimi 2020, p. 2), the antitussive alkaloid noscapine could also act in COVID-19 by decreasing the release of cytokines induced by BK. Noscapine is a drug used as a therapeutic resource against cough, which has been shown to be effective against cough associated with BK (Ebrahimi et al. 2003). However, its antitussive mechanism is not completely known, although it has been suggested that noscapine could act by interfering with the bradykinin cough mediation, with no involvement of μ, κ, and δ opioid receptors (Ghahestani et al. 2020). Finally, Ebrahimi 2020 suggests that the use of inhibitors of ACE in COVID-19 patients may corroborate an exacerbation of symptoms (Ebrahimi 2020).
The member from the RAS family, neprilysin (NEP), has also been postulated as a promising drug against COVID-19 due to its potential role in protecting lungs from inflammation and fibrosis (Wick et al. 2011). El Tabaa and El Tabaa (2020) reported cell signaling pathways containing NEP in the pathogenesis of COVID-19. The authors suggest that NEP can mitigate cytokine storm induced by SARS-CoV-2 invasion via inhibition of Ang II generation by neutrophil-derived cathepsin G and directing Ang I for generating Ang (1–7), which could suppress the expression of TGF-β1, as well as possess fibrinogenic actions. Moreover, NEP acts in the BK pathway by degrading BKs and consequently decreasing proinflammatory cytokine levels, which is beneficial for stabilizing endothelium and restoring its function (Pham 2006; El Tabaa and El Tabaa 2020). In the literature, it has been proposed that the administration of recombinant human neutral endopeptidase (rNEP) may mitigate lung injury by increasing the NEP concentration and, consequently, reducing the proinflammatory mediators levels, including BK (Lightner et al. 2002). Therefore, therapeutic strategies aimed to upregulated NEP expression and/or increase its activity may be a benefit for the prevention and treatment of COVID-19.
Interestingly, experimental data postulated that endotoxin-free recombinant neurolysin (rNln) contribute to the accumulation of bradykinin, substance P, and neurotensin, and could alter the progression of the disease, having similar effects that NEP. This recombinant protein did not change arterial blood pressure, heart rate, body temperature, and blood glucose levels. So, rNln could be an alternative for the treatment of COVID-19 (Wangler et al. 2016; Karamyan 2021).
Finally, another drug that could also be used in the treatment of SARS-CoV-2 infection and could modulate BK storm is heparin (Nicolau et al. 2020). Heparin can minimize the activation of KKS and, consequently, effects of inflammation and coagulation disturbances associated with COVID-19 (Nicolau et al. 2020). In fact, low-molecular-weight heparin modulates the activation of the coagulopathy pathways, mitigating coagulation disturbances and the severe acute respiratory distress syndrome (Falcone et al. 2020). In addition, an in vitro study proposed that heparin could restore vascular homeostasis by inhibiting glycocalyx disruption induced by SARS-CoV-2 infection (Potje et al. 2021). Finally, a clinical trial has tried to prove the potential of heparin in the management of SARS-CoV-2 infection. In that a randomized, placebo and controlled study, we determine if nebulized heparin may reduce the need for mechanical ventilation in hospitalized patients with COVID-19 (NCT04723563). Some studies are used the drug combination as a pharmacological option. In this context, an interventional study enrolled 308 patients to evaluate the clinical efficacy of heparin and tocilizumab in severe COVID-19 patients (NCT04600141). However, to date, the preliminary results have not yet been reported.
Additionally, the BK involvement is consistent with elevated levels of IL-6 in COVID-19 patients, actively participating in cytokine storm syndrome (Cron 2021). During the first findings in patients with COVID-19, IL-6 concentrations were noted to be elevated, and IL-6-blocking therapies were available in China, but with incipient and inconclusive results that made the therapy uninteresting for clinical purposes. However, BK also participates in the modulation of IL-1 levels, which plays a key role in the COVID-19 cytokines storm. Thus, IL-1 blockers seem to provide more benefit than IL-6 inhibition which might be related to the endotheliopathy associated with COVID-19 and the release of IL-1α or the fact that IL-1 is frequently upstream of IL-6 expression, so blocking IL-1 signaling equally indirectly blocks IL-6 (Crayne et al. 2019). Molecular docking was performed to determine the binding efficiency between the BK and the proinflammatory cytokines IL-1 or IL-6, in which it was observed that BK has a higher affinity for IL-1 (score: 85.52) than for IL-6 (score: 67.85) (Fig. 2).
Bradykinin in complex with interleukins as receptors. In A, bradykinin (BK) complexed with interleukin-1 (IL-1) (PDB ID: 2KKI); in B, BK complexed with interleukin-6 (IL-6) (PDB ID: 1ALU). All chains were colored in rainbow style for the receptors and BK as conventional atoms’ colors. Illustrations elaborated by using PyMol v.0.99 software (https://pymol.org/2/). All docking simulations were performed using Gold® v. 5.8.1 (https://www.ccdc.cam.ac.uk/solutions/csd-discovery/components/gold/), applying the ChemPLP scoring function, in which it was observed that BK has a high affinity for IL-1 (score: 85.52) than for IL-6 (score: 67.85)
Therefore, there is growing evidence that reduction of BK, DABK, and proinflammatory cytokines is a promising pharmacotherapeutic strategy to improve clinical outcomes of patients with COVID-19, especially among those with pulmonary inflammation and respiratory failure. Table 1 and Fig. 3 summarized the main molecular target of the drugs described in this article, showing the pharmacological profiles associated with COVID-19 pathophysiology.
Conclusion
BK is a peptide rapidly produced and degraded under physiological conditions that plays a crucial role in several processes, including inflammatory and oxidative events. BK emerges as a key mechanism to explain COVID-19-related complications since the dysregulated BK signaling may be the trigger of the cytokine storm observed in people with severe SARS-CoV-2 infection. Taking account that the binding between SARS-CoV-2 and ACE2 unbalances the function of ACE2, leading to increased levels DABK, drugs with potential effects in inhibiting the synthesis of BK or DABK or their action should be evaluated in high-quality randomized clinical trials for the treatment of COVID-19.
Data availability
Not applicable.
Code availability
Not applicable.
References
Ahluwalia A, Perretti M (1999) B1 receptors as a new inflammatory target. Could this B the 1? Trends Pharmacol Sci 20:100–104. https://doi.org/10.1016/s0165-6147(99)01321-8
Al-Ahmad AJ, Pervaiz I, Karamyan VT (2021) Neurolysin substrates bradykinin, neurotensin and substance P enhance brain microvascular permeability in a human in vitro model. J Neuroendocrinol 33:e12931. https://doi.org/10.1111/jne.12931
Ayres LS, Berger M, Durli ICLO, Kuhl CP, Terraciano PB, Garcez TNA, Dos Santos BG, Guimarães JA, Passos EP, Cirne-Lima EO (2020) Kallikrein-kinin system and oxidative stress in cisplatin-induced ovarian toxicity. Reprod Toxicol 93:1–9. https://doi.org/10.1016/j.reprotox.2019.12.002
Baş M, Greve J, Stelter K et al (2015) A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med 372:418–425. https://doi.org/10.1056/NEJMoa1312524
Cai G, Barber C, Kalicinsky C (2020) Review of icatibant use in the Winnipeg Regional Health Authority. Allergy Asthma Clin Immunol 16:96. https://doi.org/10.1186/s13223-020-00493-3
Chen Y, Liu Q, Guo D (2020) Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol 92:418–423. https://doi.org/10.1002/jmv.25681
Chen Z, Tan F, Erdös EG, Deddish PA (2005) Hydrolysis of angiotensin peptides by human angiotensin I–converting enzyme and the resensitization of B 2 kinin receptors. Hypertension 46:1368–1373. https://doi.org/10.1161/01.HYP.0000188905.20884.63
Choi S-I, Hwang SW (2018) Depolarizing effectors of bradykinin signaling in nociceptor excitation in pain perception. Biomol Ther (seoul) 26:255–267. https://doi.org/10.4062/biomolther.2017.127
Chow JH, Mazzeffi MA, Mccurdy MT (2020) Angiotensin II for the treatment of COVID-19-related vasodilatory shock. Anesth Analg 131:102–105. https://doi.org/10.1213/ANE.0000000000004825
Colarusso C, Terlizzi M, Pinto A, Sorrentino R (2020) A lesson from a saboteur: high-MW kininogen impact in coronavirus-induced disease 2019. Br J Pharmacol 177:4866–4872. https://doi.org/10.1111/bph.15154
Crayne CB, Albeituni S, Nichols KE, Cron RQ (2019) The immunology of macrophage activation syndrome. Front Immunol 10:119. https://doi.org/10.3389/fimmu.2019.00119
Cron RQ (2021) COVID-19 cytokine storm: targeting the appropriate cytokine. Lancet Rheumatol 3:e236–e237. https://doi.org/10.1016/S2665-9913(21)00011-4
Datta PK, Liu F, Fischer T et al (2020) SARS-CoV-2 pandemic and research gaps: understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics 10:7448–7464. https://doi.org/10.7150/thno.48076
Dubois EA, Cohen AF (2010) Icatibant. Br J Clin Pharmacol 69:425–426. https://doi.org/10.1111/j.1365-2125.2010.03642.x
Ebrahimi S, Zareie M-R, Rostami P, Mahmoudian M (2003) Interaction of noscapine with the bradykinin mediation of the cough response. Acta Physiol Hung 90:147–155. https://doi.org/10.1556/APhysiol.90.2003.2.7
Ebrahimi SA (2020) Noscapine, a possible drug candidate for attenuation of cytokine release associated with SARS-CoV-2. Drug Dev Res 81:765–767. https://doi.org/10.1002/ddr.21676
El Tabaa MM, El Tabaa MM (2020) Targeting Neprilysin (NEP) pathways: A potential new hope to defeat COVID-19 ghost. Biochem Pharmacol 178:114057. https://doi.org/10.1016/j.bcp.2020.114057
Erdos E (2002) Products of angiotensin I hydrolysis by human cardiac enzymes potentiate bradykinin. J Mol Cell Cardiol 34:1569–1576. https://doi.org/10.1006/jmcc.2002.2080
Falcone M, Tiseo G, Barbieri G et al (2020) Role of low-molecular weight heparin in hospitalized patients with SARS-CoV-2 pneumonia: a prospective observational study. Open Forum Infect Dis. https://doi.org/10.1093/ofid/ofaa563
Garvin MR, Alvarez C, Miller JI et al (2020) A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife 9:1–16. https://doi.org/10.7554/eLife.59177
Ghahestani SM, Mahmoudi J, Hajebrahimi S et al (2020) Bradykinin as a probable aspect in SARS-Cov-2 scenarios: is bradykinin sneaking out of our sight? Iran J Allergy Asthma Immunol 19:13–17. https://doi.org/10.18502/ijaai.v19i(s1.r1).2850
Hawgood BJ (1997) Maurício Rocha e Silva MD: snake venom, bradykinin and the rise of autopharmacology. Toxicon 35:1569–1580. https://doi.org/10.1016/s0041-0101(97)00008-1
Hofman Z, de Maat S, Hack CE, Maas C (2016) Bradykinin: inflammatory product of the coagulation system. Clin Rev Allergy Immunol 51:152–161. https://doi.org/10.1007/s12016-016-8540-0
Iwasaki M, Saito J, Zhao H et al (2021) Inflammation triggered by SARS-CoV-2 and ACE2 augment drives multiple organ failure of severe COVID-19: molecular mechanisms and implications. Inflammation 44:13–34. https://doi.org/10.1007/s10753-020-01337-3
Jacox L, Sindelka R, Chen J et al (2014) The extreme anterior domain is an essential craniofacial organizer acting through Kinin-Kallikrein signaling. Cell Rep 8:596–609. https://doi.org/10.1016/j.celrep.2014.06.026
Karamyan VT (2021) Between two storms, vasoactive peptides or bradykinin underlie severity of COVID-19? Physiol Rep 9:e14796. https://doi.org/10.14814/phy2.14796
Kempe S, Fois G, Brunner C et al (2020) Bradykinin signaling regulates solute permeability and cellular junction organization in lymphatic endothelial cells. Microcirculation 27:e12592. https://doi.org/10.1111/micc.12592
Leeb-Lundberg LMF, Marceau F, Müller-Esterl W et al (2005) International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev 57:27–77. https://doi.org/10.1124/pr.57.1.2
Lightner AM, Jordan TH, Bunnett NW et al (2002) Recombinant human neutral endopeptidase ameliorates pancreatic elastase-induced lung injury. Surgery 132:193–199. https://doi.org/10.1067/msy.2002.125309
Lucas C, Wong P, Klein J et al (2020) Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584:463–469. https://doi.org/10.1038/s41586-020-2588-y
Mahmudpour M, Roozbeh J, Keshavarz M et al (2020) COVID-19 cytokine storm: the anger of inflammation. Cytokine 133:155151. https://doi.org/10.1016/j.cyto.2020.155151
Mansour E, Bueno FF et al (2021) Evaluation of the efficacy and safety of icatibant and C1 esterase/kallikrein inhibitor in severe COVID-19: study protocol for a three-armed randomized controlled trial. Trials 22(1):71. https://doi.org/10.1186/s13063-021-05027-9
Marcic B, Deddish PA, Jackman HL, Erdös EG (1999) Enhancement of bradykinin and resensitization of Its B 2 Receptor. Hypertension 33:835–843. https://doi.org/10.1161/01.HYP.33.3.835
McLean PG, Perretti M, Ahluwalia A (2000) Kinin B(1) receptors and the cardiovascular system: regulation of expression and function. Cardiovasc Res 48:194–210. https://doi.org/10.1016/s0008-6363(00)00184-x
Murugesan P, Jung B, Lee D et al (2016) Kinin B1 receptor inhibition with BI113823 reduces inflammatory response, mitigates organ injury, and improves survival among rats with severe sepsis. J Infect Dis 213:532–540. https://doi.org/10.1093/infdis/jiv426
Nicolau LAD, Magalhães PJC, Vale ML (2020) What would Sérgio Ferreira say to your physician in this war against COVID-19: how about kallikrein/kinin system? Med Hypotheses 143:109886. https://doi.org/10.1016/j.mehy.2020.109886
Nussberger J, Cugno M, Cicardi M (2002) Bradykinin-mediated angioedema. N Engl J Med 347:621–622. https://doi.org/10.1056/NEJM200208223470820
Parekh RU, Robidoux J, Sriramula S (2020) Kinin B1 receptor blockade prevents angiotensin II-induced neuroinflammation and oxidative stress in primary hypothalamic neurons. Cell Mol Neurobiol 40:845–857. https://doi.org/10.1007/s10571-019-00778-1
Pham CTN (2006) Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 6:541–550. https://doi.org/10.1038/nri1841
Potje SR, Costa TJ, Fraga-Silva TFC et al (2021) Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life Sci 276:119376. https://doi.org/10.1016/j.lfs.2021.119376
Rameshrad M, Ghafoori M, Mohammadpour AH et al (2020) A comprehensive review on drug repositioning against coronavirus disease 2019 (COVID19). Naunyn Schmiedebergs Arch Pharmacol 393:1137–1152. https://doi.org/10.1007/s00210-020-01901-6
Roche JA, Roche R (2020) A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. FASEB J 34:7265–7269. https://doi.org/10.1096/fj.202000967
Ruocco G, Feola M, Palazzuoli A (2020) Hypertension prevalence in human coronavirus disease: the role of ACE system in infection spread and severity. Int J Infect Dis 95:373–375. https://doi.org/10.1016/j.ijid.2020.04.058
Schmaier AH (2002) The plasma kallikrein-kinin system counterbalances the renin-angiotensin system. J Clin Investig 109:1007–1009. https://doi.org/10.1172/JCI0215490
Schmaier AH (2016) A novel antithrombotic mechanism mediated by the receptors of the kallikrein/kinin and renin-angiotensin systems. Front Med (lausanne) 3:61. https://doi.org/10.3389/fmed.2016.00061
She J, Jiang J, Ye L et al (2020) 2019 novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. Clin Transl Med 9:19. https://doi.org/10.1186/s40169-020-00271-z
Shukla AK, Banerjee M (2021) Angiotensin-converting-enzyme 2 and renin-angiotensin system inhibitors in COVID-19: an update. High Blood Press Cardiovasc Prev 28:129–139. https://doi.org/10.1007/s40292-021-00439-9
Sodhi CP, Wohlford-Lenane C, Yamaguchi Y et al (2018) Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol 314:L17–L31. https://doi.org/10.1152/ajplung.00498.2016
Solun B, Shoenfeld Y (2020) Inhibition of metalloproteinases in therapy for severe lung injury due to COVID-19. Med Drug Discov 7:100052. https://doi.org/10.1016/j.medidd.2020.100052
Su JB (2015) Vascular endothelial dysfunction and pharmacological treatment. World J Cardiol 7:719–741. https://doi.org/10.4330/wjc.v7.i11.719
Tolouian R, Vahed SZ, Ghiyasvand S et al (2020) COVID-19 interactions with angiotensin-converting enzyme 2 (ACE2) and the kinin system; looking at a potential treatment. J Ren Inj Prev 9:e19–e19. https://doi.org/10.34172/jrip.2020.19
van de Veerdonk FL, Kouijzer IJE, de Nooijer AH et al (2020a) Outcomes associated with use of a kinin B2 receptor antagonist among patients with COVID-19. JAMA Netw Open 3:e2017708. https://doi.org/10.1001/jamanetworkopen.2020.17708
van de Veerdonk FL, Netea MG, van Deuren M et al (2020) Kallikrein-kinin blockade in patients with covid-19 to prevent acute respiratory distress syndrome. Elife 9:1–9. https://doi.org/10.7554/ELIFE.57555
Vickers C, Hales P, Kaushik V et al (2002) Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277:14838–14843. https://doi.org/10.1074/jbc.M200581200
Wang D, Hu B, Hu C et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. https://doi.org/10.1001/jama.2020.1585
Wangler NJ, Jayaraman S, Zhu R et al (2016) Preparation and preliminary characterization of recombinant neurolysin for in vivo studies. J Biotechnol 234:105–115. https://doi.org/10.1016/j.jbiotec.2016.07.007
Wick MJ, Buesing EJ, Wehling CA et al (2011) Decreased neprilysin and pulmonary vascular remodeling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 183:330–340. https://doi.org/10.1164/rccm.201002-0154OC
Acknowledgements
This work was supported by grants from FAPESE, FAPITEC-SE, CNPq, and CAPES, all agencies from Brazil. This paper is one of the technical-scientific products from the EpiSERGIPE project. We dedicate this article to all the Doctors and Front Line Health Workers who have died or are on the battlefront facing COVID-19.
Author information
Authors and Affiliations
Contributions
J.X.A.J., E.F.S., and L.J.Q.J. conceived and designed research. M.F.S. and L.H. analyzed data. L.H., J.S.S.Q., E.F.S., L.J.Q.J., and P.R.M.F. wrote the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Yes.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva, M.F., de Araújo-Júnior, J.X., da Silva-Júnior, E.F. et al. Bradykinin-target therapies in SARS-CoV-2 infection: current evidence and perspectives. Naunyn-Schmiedeberg's Arch Pharmacol 395, 275–283 (2022). https://doi.org/10.1007/s00210-022-02206-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02206-6