Abstract
Purpose
Cyclophosphamide is an alkylating agent with nephrotoxicity that constrains its clinical application. Berberine is an isoquinoline derivative alkaloid with biological functions like antioxidant and anti-inflammatory. The current research intended to examine the nephroprotective impacts of berberine against cyclophosphamide-stimulated nephrotoxicity.
Methods
Forty animal subjects were randomly separated into five categories of control (Group I), cyclophosphamide (200 mg/kg, i.p., on 7th day) (Group II), and groups III and IV that received berberine 50 and 100 mg/kg orally for seven days and a single injection of cyclophosphamide on 7th day. Group V as berberine (100 mg/kg, alone). On day 8, blood samples were drawn from the retro-orbital sinus to determine serum levels of blood urea nitrogen (BUN), creatinine (Cr), neutrophil gelatinase-associated lipocalin (NGAL), and kidney injury molecule-1 (KIM-1) as biomarkers for kidney injury. Nitric oxide (NO), malondialdehyde (MDA) and glutathione (GSH) levels, catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) activities as oxidative stress factors, tumor necrosis factor-α (TNF-α) and interleukin 1 beta (IL-1β) levels as inflammatory mediators were assessed in kidney tissue.
Results
The results of this study demonstrated that berberine was able to protect remarkably the kidney from CP-induced injury through decreasing the level of BUN, Cr, NGAL, KIM-1, NO, MDA TNF-α, IL-1β and increasing the level of GSH, CAT, SOD, and GPx activities.
Conclusion
Berberine may be employed as a natural agent to prevent cyclophosphamide-induced nephrotoxicity through anti-oxidant and anti-inflammatory effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclophosphamide (CP) is an alkylating agent that has been in clinical use for more than 60 years (Lawson et al. 2008). It is extensively used to treat various forms of malignancies such as lymphoma and breast cancer. Also, CP is widely used in organ transplantation, rheumatoid arthritis, multiple sclerosis, etc., as an immunosuppressant (Crivellari et al. 2000; Perini et al. 2007). It is well-known that cyclophosphamide and its reactive metabolites such as phosphoramide mustard and acrolein result in bladder acute inflammation as well as nephrotoxicity or hepatotoxicity, which limits long-term using of CP in clinical practice (Rodriguez-Antona and Ingelman-Sundberg 2006; Wahlang et al. 2015). There are evidence indicating the possible role of inflammatory pathways in negative consequences of cyclophosphamide and a number of researchers reported that CP enhances inflammatory markers including factor-α (TNF-α) and interleukin 1 beta (IL-1β), and IL-6 in the organs (Hamsa and Kuttan 2011). By contrast, recent studies have shown that oxidative stress (OS) also has a crucial impact on CP-induced nephrotoxicity (Stankiewicz et al. 2002). CP depletes reduced glutathione (GSH) as a protective antioxidant and increases OS factors like malondialdehyde (MDA) and nitric oxide (NO); CP also inhibits the activities of powerful antioxidant enzymes like glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) (Kuo et al. 2004; Li et al. 2014). It seems both inflammatory and stress oxidative pathways play a role in CP-nephrotoxicity and several studies suggested that antioxidant and anti-inflammatory agents can be an important therapeutic approach to reduce CP-nephrotoxicity (Stankiewicz et al. 2002).
Berberine (BBR) is an isoquinoline derivative alkaloid isolated from many plant species like Cortex phellodendron (Huang bai), Hydrastis canadensis (goldenseal), and Rhizoma coptidis (Huanglian) (Kuo et al. 2004). Recently, an increase number of studies have revealed this compound has an extensive spectrum of biological functions such as antioxidant and anti-inflammatory (Li et al. 2014), anticancer (Sun et al. 2009), and anti-hyperglycemic (Mahmoud et al. 2017) effects. Studies have been shown that BBR attenuates the output of inflammatory mediators like TNF-α, IL-1β, IL-6, and IL-8 (Lou et al. 2011; Zhang et al. 2011, 2016b). In addition, BBR seems to increase the cellular antioxidant defense machinery including increasing the activity of CAT, SOD, and GPx and decrease OS factors like protein carbonyl (PC) content, MDA and NO levels (Zhou and Zhou 2011).
In this study, we utilized cyclophosphamide as an experimental model to study the possible impact of berberine as an antioxidant and anti-inflammatory agent on CP-induced nephrotoxicity.
Materials and method
Drug and chemicals
Cyclophosphamide was purchased from Roche chemical company (Grenzach, Germany). Berberine, reduced glutathione, tris–hydrochloric acid, bovine serum albumin (BSA), 2,2′-dinitro-5,5′-dithiodibenzoic acid (DTNB), oxidized glutathione, trichloroacetic acid (TCA), and 2-thiobarbituric acid (TBA) were obtained from Sigma St. Louis.
Animal and study design
Forty male NMRI mice (25 ± 2 g) were purchased from the Experimental Animal Center Laboratory of Ahvaz Jundishapur University of Medical Sciences. The mice were kept in a room with 25 ± 2 °C temperature in polycarbonate cages with a 12/12 cycle. In addition, food and water were available without any limitation. The investigation complies with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 1996). The Animal Ethics Committee of the Ahvaz Jundishapur University of Medical Sciences approved our research protocol (Ethic code: IR.BHN.REC.1397.033).
Five experimental groups consisting of 8 randomly selected mice each were established:
-
Control group (I): 0.1 ml of normal saline (vehicle of BBR, p.o.) was administered for a week and one dose of 0.9% normal saline was injected (vehicle of CP, i.p.) on 7th day.
-
CP group (II): 0.1 ml of normal saline was administered for a week and one dose of CP (200 mg/kg, i.p.) was injected on the 7 h day.
-
Group III (CP + BBR 50): BBR (50 mg/kg, p.o.) was administered for a week and one dose of CP (200 mg/kg, i.p.) was injected on the 7th day.
-
Group IV (CP + BBR 100): BBR (100 mg/kg, p.o.) was administered for a week and one dose of CP (200 mg/kg, i.p.) was injected on the 7th day.
-
Group V (BBR group): BBR (100 mg/kg, p.o.) was administered for a week and one dose of normal saline 0.9% was injected on the 7th day.
The dose of CP and BBR was selected based on our previous studies and other studies (El-Naggar et al. 2015; Goudarzi et al. 2017). On 8th day, ketamine/xylazine (60/6 mg/kg, i.p) was used for performing anesthetized and then were sacrificed by fast decapitation. The retro-orbital sinus was selected for taking blood samples. All blood samples were centrifuged at 3000 g for 10 min and kept at − 20 °C until investigations. After removing and washing the kidneys with normal saline, the right kidney was fixed in 10% phosphate-buffer formalin for histological analysis. The left kidney was used for biochemical assays.
Tissue homogenization and protein measurement
The left kidney weighed and homogenized (1/10 w/v) with a tissue homogenizer (Wisetis HG-150) for 60 min in ice-cold phosphate-buffered saline (PBS) solution (10 mM Na2HPO4, 10 mM KH2PO4, 0.9 g NaCl/100 mL, pH 7.4) and the clear supernatant was kept in − 70 °C for further investigations. The total protein content in the clear supernatant of the kidney was estimated following the technique of Bradford (Bradford 1976).
Analysis of serum biochemical parameters
The level of serum biochemical marker blood urea nitrogen (BUN) and creatinine (Cr) was measured using enzyme-linked immunosorbent assay (ELISA) kit according to the protocol of the manufacturer (Pars Azmun. Co. Iran), and using an auto-analyzer (BT-3000 TARGA-model, Biotecnica Instruments) and Jaffe method (Najafi et al. 2015). BUN and Cr levels were expressed as mg/dL serum. Assessment of KIM-1 and NGAL levels was done using commercial kits (IBL-America (Immuno-Biological Laboratories)).
Assay of MDA level
MDA in the kidney as a marker of lipid peroxidation was assayed via the TBA color reaction by Aust’s technique by an ELISA reader (Biotech 808, USA) at 532 nm (Goudarzi et al. 2021; Moore and Roberts 1998). MDA content was expressed as nmol/mg protein.
Assay of GSH level
GSH in the kidney was measured by a modified version of Ellman’s technique following the observation of a yellow complex with Ellman’s (2,2′-dinitro-5, 5′-dithiodibenzoic acid Reagent) (Kalantar et al. 2016a; Riddles et al. 1979). The obtained yellow color mix was read at 412 nm by an ELISA reader (Biotech 808, USA).
Assay of CAT activity
CAT function in the kidney was measured using Abebi’s method (Aebi 1984; Goudarzi et al. 2020) using H2O2 as a substrate. Briefly, H2O2 was mixed and decomposition rate of H2O2 was evaluated through estimating the absorbance alternations at 240 nm for 60 s. A unit of catalase (CAT) function was considered 1 μM of H2O2 that is decomposed in 1 min and the function of this enzyme was expressed as U/mg protein.
Assay of NO level
Griess’s technique was applied to measure the NO in the kidney along with Griess diazotization reaction following conversion of the nitrate to nitrite by nitrate reductase in the supernatant (Oktem et al. 2012). The quantification was based on the absorbance at 540 nm by an ELISA reader (Biotech 808, USA). NO level was expressed as nmol/mg protein.
Assay of SOD and GPx activity
The function of SOD and GPx enzymes was measured by the kits following the protocol of the producer (ZellBio GmbH, Germany), and the function of these enzymes was expressed as U/mg protein.
TNF-α and IL-1β assay
Levels of TNF-α and IL-1β cytokines in the kidney were evaluated using ELISA kits, following the guideline published by the producer (R&D Systems, Inc., Minneapolis, MN, USA).
Histopathological assays
In order to perform histological examination, the left kidney from all groups was fixed in formalin (10%) for at least 24 h and following preparing the tissue, all tissues were put into paraffin. Routine hematoxylin and eosin (H&E stain) were applied to stain 5 μm sections (Kalantar et al. 2016b; Wadie et al. 2021). A light microscope in a blind manner (Nikon Labophot, Japan) was used to study six microscopy slides per animal and light microscopic assessment was conducted by authors in a blinded manner for assessment of histological alternations like congestion of RBC and inflammatory cell infiltration.
Statistical analysis
The GraphPad Prism version 6.01 (GraphPad Software, USA) was used to conduct all statistical analyses. Parameters within groups were statistically analyzed using the ANOVA, followed by Tukey’s post hoc and p value less than 0.05 was considered significant. Results were reported as mean ± standard deviations (SD) of number of experiments (n = 8).
Results
Effects of CP and BBR on serum biochemical parameters
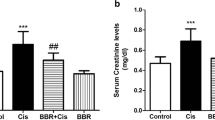
Results indicated the serum levels of BUN, Cr, NGAL, and KIM-1 were significantly elevated after CP administration in comparison to the control group (all p < 0.05), and these increases in the groups received BBR were significantly attenuated than CP group (all p < 0.05). The serum levels of serum biochemical parameters in the group treated with berberine alone were similar to controls and there were no significant changes (Fig. 1a–d).
Effects of CP and BBR on serum biochemical parameters. Values are mean ± SD (n = 8). ANOVA and Tukey’s tests were used for comparisons. *Significant difference in comparison with the control group (p < 0.05). #Significant difference in comparison with the CP group (p < 0.05). CP: cyclophosphamide, BBR: berberine, BUN: blood urea nitrogen, Cr: creatinine, KIM-1: kidney injury molecule-1, NGAL: neutrophil gelatinase-associated lipocalin
Effects of CP and BBR on OS parameters
The impacts of CP and BBR on NO and MDA levels as oxidative parameters are illustrated in Fig. 2(a, b). CP treatment led to a significant increase in the level of NO and MDA in comparison to controls (p < 0.05). Although BBR treatment at the dose of 50 mg/kg reduced slightly MDA levels (no significant) but 100 mg/kg of BBR significantly decreased the MDA levels compared with the CP group. Also, BBR treatment (50 and 100 mg/kg) considerable declined NO than the CP group (p < 0.05), Furthermore, there were not any considerable differences among the group received BBR (alone) and the control group.
Effects of CP and BBR on oxidative stress parameters. Values are mean ± SD (n = 8). ANOVA and Tukey’s tests were used for comparisons. *Significant difference in comparison with the control group (p < 0.05). #Significant difference in comparison with the CP group (p < 0.05). CP: cyclophosphamide, BBR: berberine, MDA: malondialdehyde, NO: nitric oxide
Effects of CP and BBR on antioxidant markers
The impacts of BBR on the function of antioxidant enzymes and the GSH content are provided in Fig. 3(a–d). Results indicated that a single dose of CP administration decreased activity of antioxidant enzymes and GSH level (all p < 0.05). Pre-treatment with BBR at the dose of 100 mg/kg significantly increased GPx activity and GSH content compared with the CP group (p < 0.05). Moreover, BBR (50 and 100 mg/kg) slightly enhanced CP-stimulated decreased of CAT and SOD activity but there were not any significantly changes. In addition, the BBR (alone) group did not show any significant change in comparison with control group.
Effects of CP and BBR on antioxidant markers. Values are mean ± SD (n = 8). ANOVA and Tukey’s tests were used for comparisons. *Significant difference in comparison with the control group (p < 0.05). #Significant difference in comparison with the sham group (CP group) (p < 0.05). CP: cyclophosphamide, BBR: berberine GSH: glutathione, GPx: glutathione peroxidase, SOD: superoxide dismutase: CAT: catalase
Effects of CP and BBR on inflammatory parameters
As shown in Fig. 4(a, b), CP treatment significantly increased IL-1β and TNF-α levels as inflammatory parameters than controls. Pretreatment with BBR at the dose of 100 mg/kg significantly decreased IL-1β and TNF-α levels than the CP. Also, the BBR (alone) group did not show any significant change in comparison with control group.
Effects of CP and BBR on inflammatory parameters. Values are mean ± SD (n = 8). ANOVA and Tukey’s tests were used for comparisons. *Significant difference in comparison with the control group (p < 0.05). #significant difference in comparison with the CP group (p < 0.05). CP: cyclophosphamide, BBR: berberine, TNF-α: tumor necrosis factor-α, IL-1β: interleukin 1 beta
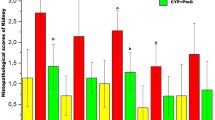
Effects of CP and BBR on histopathological alternations
Nephrotoxicity induced by CP in mice was further assessed using H&E stained sections. Representative examples of the histological appearance in the experimental groups are shown in Fig. 5a–e. The kidney sections from the control and BBR (100 mg/kg, alone) groups had a usual structure of the kidney cells (Fig. 5a and e), but on the other hand, in the CP group, the kidney exhibited notable histopathological changes. CP induced extensive injuries such as degeneration of the renal tubules, glomerular atrophy, hemorrhage, and inflammatory cell infiltration (Fig. 5b), while pretreatment with BBR (50 and 100 mg/kg) for seven days declined the occurrence and intensity of histopathological lesions compared to CPs (Fig. 5c and d). Furthermore, the renal tubules and glomerulus have a nearly normal structure.
Histopathological observations (kidney sections stained with hematoxylin and eosin, magnification × 200) showing effects of berberine on cyclophosphamide-induced renal toxicity. a Control group, b cyclophosphamide group, c berberine (50 mg/kg) + cyclophosphamide group, d berberine (100 mg/kg) + cyclophosphamide group, e berberine (100 mg/kg, alone). C: congestion of RBC, l: infiltration of inflammatory cells
Discussion
In this research, the effects of BBR on CP-stimulated nephrotoxicity in mice were investigated. Drug-stimulated nephrotoxicity is a major medical problem and there is a growing number of hospitalized patients who develop a drug-induced renal problem (Hoitsma et al. 1991). Detection of acute kidney injury often is according to serum levels of BUN and Cr that illustrates the filtration capacity of the glomerulus and these are usually the first steps to the diagnosis of renal injury (Salazar 2014). However, these factors are not very sensitive or specific and can be affected by nonrenal variables independent from kidney injury or malfunction. Some biomarkers such as KIM-1 and NGAL are made predominantly by the injured kidney and seems to be more specific and sensitive (Edelstein 2017). Our findings confirmed that administration of CP (200 mg/kg) cause nephrotoxicity significantly as indicated by enhanced levels of BUN, Cr, KIM-1, and NGAL. On the other hand, pretreatment with BBR reduced the level of serum markers than CP group. Free radicals are generated in a wide variety of chemicals and medications such as cyclophosphamide and depletes mitochondria enzymatic and non-enzymatic antioxidant defense systems(Halliwell 2001). In addition, pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) are a series of immunoregulatory molecules that control the mucosal immune system. In addition, it worth noting that neutrophils and macrophages intervene epithelial uniformity and are known for their impact on pathogenesis of several health problems (Neurath 2014). It seems both free radicals and pro-inflammatory cytokines play a crucial role in the toxicity of cyclophosphamide and several studies previously demonstrated that cyclophosphamide in animals and humans induced several kinds of damage such as nephrotoxicity, hepatotoxicity, ovarian toxicity, and testicular toxicity (Fraiser et al. 1991). Previous studies have shown that cyclophosphamide caused nephrotoxicity and hepatotoxicity through increasing inflammatory responses, OS markers such as MDA, NO, and depletion in glutathione content and antioxidant enzyme activities (such as GPx, SOD, CAT, GSH, quinone reductase). Our findings showed that CP increases levels of oxidative and inflammatory parameters including MDA, NO, TNF-α, IL-1β and GSH, GPx, SOD and CAT activities were declined, which is in line with the literature (Goudarzi et al. 2018; Temel et al. 2020). Also, pretreatment with the antioxidant, BBR, prior to CP results in a decrease in the renal MDA, NO TNF-α, IL-1β and an elevation in GSH levels and GPx activity. The obtained findings are consistent with other indicating the antioxidant and anti-inflammtory effects caused by BBR (Chen et al. 2016; Hasanein et al. 2017; Wang et al. 2018; Yu et al. 2019).
The exact mechanism underlying the effects of berberine is unclear. However, many studies have shown the beneficial effects of berberine on inflammation and oxidative stress. Previous reports have shown protective effects of BBR against nephrotoxicity by inhibition of TGF-β/Smad/EMT pathway and activating Nrf2 pathway (Zhang et al. 2016a). The results of this study are consistent with these previous reports and indicated BBR administration significantly affected CP-induced nephrotoxicity in mice via regulation kidney injury biomarkers in serum, oxidative stress, and inflammatory factors in tissue. Kidney histopathological results also were consistent with biochemical assessment and demonstrated the beneficial effect of berberine in structural changes of CP-induced nephrotoxicity in mice.
Conclusion
In summary, present study demonstrated that BBR alongside with CP in mice can protect renal tissue from CP-induced nephrotoxicity through significantly decreases the level of BUN, Cr, NGAL, and KIM-1 in serum and modification oxidative, inflammatory parameters and improving histological changes of kidney tissue.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126
Bradford N (1976) A rapid and sensitive method for the quantitation microgram quantities of a protein isolated from red cell membranes. Anal Biochem 72:e254
Chen X, Zhang Y, Zhu Z, Liu H, Guo H, Xiong C, Xie K, Zhang X, Su S (2016) Protective effect of berberine on doxorubicin-induced acute hepatorenal toxicity in rats. Mol Med Rep 13:3953–3960
Crivellari D, Bonetti M, Castiglione-Gertsch M, Gelber RD, Rudenstam C-M, Thürlimann B, Price KN, Coates AS, Hürny C, Bernhard J (2000) Burdens and benefits of adjuvant cyclophosphamide, methotrexate, and fluorouracil and tamoxifen for elderly patients with breast cancer: the International Breast Cancer Study Group Trial VII. J Clin Oncol 18:1412–1422
Edelstein CL (2017) Biomarkers in acute kidney injury. Biomarkers of Kidney Disease 241–315
El-Naggar SA, Alm-Eldeen AA, Germoush MO, El-Boray KF, Elgebaly HA (2015) Ameliorative effect of propolis against cyclophosphamide-induced toxicity in mice. Pharm Biol 53:235–241
Fraiser LH, Kanekal S, Kehrer JP (1991) Cyclophosphamide toxicity. Drugs 42:781–795
Goudarzi M, Khodayar MJ, Hosseini Tabatabaei SMT, Ghaznavi H, Fatemi I, Mehrzadi S (2017) Pretreatment with melatonin protects against cyclophosphamide-induced oxidative stress and renal damage in mice. Fundam Clin Pharmacol 31:625–635
Goudarzi M, Kalantar M, Sadeghi E, Karamallah MH, Kalantar H (2021) Protective effects of apigenin on altered lipid peroxidation, inflammation, and antioxidant factors in methotrexate-induced hepatotoxicity. Naunyn Schmiedebergs Arch Pharmacol 394:523–531
Goudarzi M, Esmaeilizadeh M, Dolatshahi M, Kalantar H, Frouzandeh H, Kalantar M (2018) Protective effect of elaeagnus angustifolia L. Fruit hydroalcoholic extract on cyclophosphamide-induced nephrotoxicity in mice. Shiraz E Med J 19
Goudarzi M, Karamallah MH, Malayeri A, Kalantar M, Mansouri E, Kalantar H (2020) Protective effect of alpha-lipoic acid on di-(2-ethylhexyl) phthalate-induced testicular toxicity in mice. Environ Sci Pollut Res 1–9
Halliwell B (2001) Free radicals and other reactive species in disease. e LS
Hamsa T, Kuttan G (2011) Protective role of Ipomoea obscura (L.) on cyclophosphamide-induced uro-and nephrotoxicities by modulating antioxidant status and pro-inflammatory cytokine levels. Inflammopharmacology 19:155–167
Hasanein P, Ghafari-Vahed M, Khodadadi I (2017) Effects of isoquinoline alkaloid berberine on lipid peroxidation, antioxidant defense system, and liver damage induced by lead acetate in rats. Redox Rep 22:42–50
Hoitsma AJ, Wetzels JF, Koene RA (1991) Drug-induced nephrotoxicity. Drug Safety 6:131–147
Kalantar M, Houshmand G, Kalantar H, Asadi M, Goudarzi M (2016b) Protective effect of hydroalcoholic extract of Lavandula officinalis L. on gentamicin induced nephrotoxicity in rats. J Babol Univ Med Sci 18:62–67
Kalantar H, Sabetkasaei M, Shahriari A, Haj Molla Hoseini M, Mansouri S, Kalantar M, Kalantari A, Khazaei Poul Y, Labibi F, Moini-Zanjani T (2016a) The effect of rapamycin on oxidative stress in MCF-7 and MDA MB-231 human breast cancer cell lines. Jundishapur J Nat Pharm Prod 11
Kuo C-L, Chi C-W, Liu T-Y (2004) The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett 203:127–137
Lawson M, Vasilaras A, De Vries A, Mactaggart P, Nicol D (2008) Urological implications of cyclophosphamide and ifosfamide. Scand J Urol Nephrol 42:309–317
Li Z, Geng Y-N, Jiang J-D, Kong W-J (2014) Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid-Based Complement Alternat Med 2014
Lou T, Zhang Z, Xi Z, Liu K, Li L, Liu B, Huang F (2011) Berberine inhibits inflammatory response and ameliorates insulin resistance in hepatocytes. Inflammation 34:659–667
Mahmoud AM, Abdel-Rahman MM, Bastawy NA, Eissa HM (2017) Modulatory effect of berberine on adipose tissue PPARγ, adipocytokines and oxidative stress in high fat diet/streptozotocin-induced diabetic rats. J Appl Pharm Sci 7:1–10
Moore K, Roberts LJ (1998) Measurement of lipid peroxidation. Free Radical Res 28:659–671
Najafi H, Ashtiyani SC, Sayedzadeh SA (2015) Therapeutic effects of curcumin on the functional disturbances and oxidative stress induced by renal ischemia/reperfusion in rats. Avicenna J Phytomed 5:576
Neurath MF (2014) Cytokines in inflammatory bowel disease. Nat Rev Immunol 14:329–342
Oktem G, Uysal A, Oral O, Sezer ED, Olukman M, Erol A, Akgur SA, Bilir A (2012) Resveratrol attenuates doxorubicin-induced cellular damage by modulating nitric oxide and apoptosis. Exp Toxicol Pathol 64:471–479
Perini P, Calabrese M, Rinaldi L, Gallo P (2007) The safety profile of cyclophosphamide in multiple sclerosis therapy. Expert Opin Drug Saf 6:183–190
Riddles PW, Blakeley RL, Zerner B (1979) Ellman’s reagent: 5, 5′-dithiobis (2-nitrobenzoic acid)—a reexamination. Anal Biochem 94:75–81
Rodriguez-Antona C, Ingelman-Sundberg M (2006) Cytochrome P 450 pharmacogenetics and cancer. Oncogene 25:1679–1691
Salazar JH (2014) Overview of urea and creatinine. Lab Med 45:e19–e20
Stankiewicz A, Skrzydlewska E, Makiela M (2002) Effects of amifostine on liver oxidative stress caused by cyclophosphamide administration to rats. Drug Metab Drug Interact 19:67–82
Sun Y, Xun K, Wang Y, Chen X (2009) A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anticancer Drugs 20:757–769
Temel Y, Kucukler S, Yıldırım S, Caglayan C, Kandemir FM (2020) Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn Schmiedebergs Arch Pharmacol 393:325–337
Wadie W, Mohamed AH, Masoud MA, Rizk HA, Sayed HM (2021) Protective impact of lycopene on ethinylestradiol-induced cholestasis in rats. Naunyn Schmiedebergs Arch Pharmacol 394:447–455
Wahlang B, Falkner KC, Cave MC, Prough RA (2015) Role of cytochrome P450 monooxygenase in carcinogen and chemotherapeutic drug metabolism. Adv Pharmacol 74:1–33
Wang X, Feng S, Ding N, He Y, Li C, Li M, Ding X, Ding H, Li J, Wu J (2018) Anti-inflammatory effects of berberine hydrochloride in an LPS-induced murine model of mastitis. Evid-Based Complement Alternat Med 2018
Yu X, Wang Y, Dai F, Zhao J, Li P (2019) The protective effects of Berberine and Hesperidin on inflammatory factor-stimulating cardiac fibroblasts. Eur Rev Med Pharmacol Sci 23:5468–5476
Zhang S, Zhang B, Dai W, Zhang X (2011) Oxidative damage and antioxidant responses in Microcystis aeruginosa exposed to the allelochemical berberine isolated from golden thread. J Plant Physiol 168:639–643
Zhang X, He H, Liang D, Jiang Y, Liang W, Chi Z-H, Ma J (2016a) Protective effects of berberine on renal injury in streptozotocin (STZ)-induced diabetic mice. Int J Mol Sci 17:1327
Zhang Z, Li X, Li F, An L (2016b) Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int Immunopharmacol 38:426–433
Zhou J-Y, Zhou S-W (2011) Protective effect of berberine on antioxidant enzymes and positive transcription elongation factor b expression in diabetic rat liver. Fitoterapia 82:184–189
Funding
This work was supported by Deputy of Research of Shoushtar University of Medical Sciences, Shoushtar, Iran (Grant number: TRC-9803) and Vice-Chancellor of Research, Toxicology Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Author information
Authors and Affiliations
Contributions
MK and MAM conceived and designed the study. MAM, HK, and ES performed experiments. MAM, MG, and ES analyzed data. HK and HRK wrote the manuscript. All authors read and approved the manuscript and all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The investigation complies with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 1996). The investigation complies with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 1996). The Animal Ethics Committee of the Ahvaz Jundishapur University of Medical Sciences approved our research protocol (Ethic code: IR.BHN.REC.1397.033).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mombeini, M.A., Kalantar, H., Sadeghi, E. et al. Protective effects of berberine as a natural antioxidant and anti-inflammatory agent against nephrotoxicity induced by cyclophosphamide in mice. Naunyn-Schmiedeberg's Arch Pharmacol 395, 187–194 (2022). https://doi.org/10.1007/s00210-021-02182-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-021-02182-3