Abstract
Giardia duodenalis is a common cause of infection in children and travelers. The most frequent symptom is diarrhea in these patients. G. duodenalis trophozoites use a highly specialized adhesive disc to attach the host intestinal epithelium to induce intestinal damages. Pathological features of the small intestine following giardiasis include villous atrophy; infiltration of granulocytes, lymphocytes, and plasma cells into the lamina propria; and nodular lymphoid hyperplasia. The disturbed intestinal microbiota has been observed in patients with giardiasis. Therefore, a growing body of evidence has emphasized restoring the gut microbiome by probiotics in giardiasis. This study aimed to review the literature to find the pathologic features of giardiasis and its relationship with imbalanced microbiota. Then, benefits of probiotics in giardiasis and their potential molecular mechanisms were discussed. It has been illustrated that using probiotics (e.g., Lactobacillus and Saccharomyces) can reduce the time of gastrointestinal symptoms and repair the damages, particularly in giardiasis. Probiotics’ capability in restoring the composition of commensal microbiota may lead to therapeutic outcomes. According to preclinical and clinical studies, probiotics can protect against parasite-induced mucosal damages via increasing the antioxidant capacity, suppressing oxidative products, and regulating the systemic and mucosal immune responses. In addition, they can reduce the proportion of G. duodenalis load by directly targeting the parasite. They can destroy the cellular architecture of parasites and suppress the proliferation and growth of trophozoites via the production of some factors with anti-giardial features. Further researches are required to find suitable probiotics for the prevention and treatment of giardiasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Giardia duodenalis (synonymous with G. intestinalis and G. lamblia) is an enteric flagellated protozoan and a common leading cause of infection known as giardiasis in children and travelers (Dargahi et al. 2017). It infects up to ~ 28.2 million worldwide, with 500,000 new cases every year (Ryan et al. 2019). Giardia infections lead to a wide range of manifestations, from asymptomatic infection to chronic diarrhea with long-term consequences (Robertson et al. 2010). G. duodenalis has been listed as WHO’s Neglected Diseases Initiative (Savioli et al. 2006). Eight different morphologically similar but genetically distinct species (A–H) have been identified. Assemblages A and B with zoonotic potential can infect humans and a wide range of animals (Cacciò et al. 2018).
G. duodenalis has long been considered for its ability to lead to acute or chronic diarrhea and other complications, e.g., epigastric pain, nausea, vomiting, and anorexia, weight loss, malabsorption, steatorrhea, growth retardation, and development of extra-intestinal and post-infectious complications (Halliez and Buret 2013; Ratanapo et al. 2008; Wensaas et al. 2012). The common symptoms can be observed typically 2 weeks after infection, which are usually mild (Buret 2008). Malabsorptive diarrheal disease is the main result of pathologic features induced by G. duodenalis trophozoites (Cotton et al. 2011). The altered composition of intestinal microbiota observed in patients with immunological disorders (e.g., common variable immunodeficiency) increase the susceptibility to Giardia infection (Deng et al. 2001). Giardiasis can be controlled by inactivation of cysts (found in the environment and swallowed from mouth) and trophozoites (released from cysts) that are able to attach the intestinal epithelium and invade the host (Adam 2001; Escobedo and Cimerman 2007). G. duodenalis uses the ventral disc to bind to the host intestinal epithelium and apply its effects via mechanical and inflammatory factors (Hanevik et al. 2007).
Up to now, no specific vaccines have been introduced against Giardia infection. Using hygiene and sanitation can help to control the infection (Olson et al. 2000). There are some specific medications for treatment of giardiasis, such as 5-nitro derivatives of imidazole (tinidazole and metronidazole), furan, and thiazole, (Watkins and Eckmann 2014). However, they are accompanied by several unpleasant side effects (e.g., metallic taste) and can increase the level of treatment failure and drug resistance in many poor patients (Upcroft 1998). These results have encouraged researchers to examine the effects of alternative therapeutic approaches, such as probiotics (Shukla et al. 2008), plant extracts (Ponce-Macotela et al. 1994), active components of plants (Said et al. 2012), and bee products (Freitas et al. 2006) that are safe, inexpensive, and an effective choice to protect and treat the intestinal parasitosis (Upcroft and Upcroft 2001).
It was shown that infection with G. duodenalis might induce alterations in species diversity and composition of commensal microbiota with an essential role in gut homeostasis (Fekete et al. 2021). Disturbed microbiota in giardiasis contributes to dysregulated lipid metabolism, and decreased adipose tissue and body weight, leading to growth impairment (Riba et al. 2020). Also, interactions between the microbiome and immune system are an important factor in giardiasis (Al-Megrin et al. 2021). The therapeutic strategies to restore the normal gut microbiota via probiotics administration may prevent or treat giardiasis via several molecular mechanisms (Ventura et al. 2018). Findings of clinical studies indicated beneficial effects of probiotics on gastrointestinal (GI) diseases (e.g., diarrheas, GI disorders, elimination of Helicobacter, irritable bowel syndrome, and inflammatory bowel disease) (Markowiak and Śliżewska 2017). Probiotics offer a number of potential health benefits for GI diseases by surrogating normal gut microbiota, leading to increasing the secretion of antimicrobial agents, neutralizing toxins, repairing mucus layer, interfering with attachment of microorganism, regulating immune responses, or a combination of these mechanisms (Goyal et al. 2011; Schroeder 2019).
In the present study, we aimed to review pathological features of giardiasis with focusing on the gut microbiota impairment. Recently, preclinical and clinical studies have shown that probiotics can be prescribed for the prevention and treatment of Giardia infection. Several probiotic microbes have been illustrated to have anti-giardiasis properties. Therefore, beneficial effects of probiotics against giardiasis were reviewed by emphasizing the underlying molecular mechanisms.
The pathogenesis of Giardia duodenalis
Direct contact of G. duodenalis with the villi in the small intestine and subsequent tissue damages induces symptoms in patients (Ankarklev et al. 2010). The pathogenesis of Giardia can be understood by an ecological perspective on giardiasis (Frank and Pace 2008). For initiation of giardiasis in humans, ten environmentally resistant cysts are enough. During the GI transition of cysts, they are changed as replicative and motile forms, so-called trophozoïtes (Cotton et al. 2011). These forms of parasite proliferate in the gut lumen, firmly attach to the epithelium with a highly specialized adhesive disc, and mainly induce symptoms including abdominal pain, diarrhea, nausea, vomiting, and weight loss. At the same time, half of the infections are asymptomatic (Lamireau and Enaud 2017). Raising the number of trophozoites to 106/cm in the intestine can increase its permeability. The higher amount of parasitic material may promote pro-inflammatory responses of intestine via translation to subepithelial regions (Cotton et al. 2015). Moreover, the ability of G. duodenalis isolates in invasion of host tissues was recently investigated in an animal model of the gerbil. In this study, G. duodenalis belonged to assemblage A, genetic group A, harvested in the log phase from duodenal fluid of a case with intraepithelial Giardia infection (see Martínez-Gordillo et al. 2014), and subsequently introduced into the gerbil (Reynoso-Robles et al. 2015). Attachment of these pathogens to the lumen of the intestine is responsible for a wide range of histopathological features via mechanical and inflammatory mechanisms (Farthing 1997). Early definitions of intestinal malabsorption was related to the Giardia trophozoites competing for host nutrients, or they act as a mechanical barrier and inhibit absorption (Katelaris and Farthing 1992).
Oxidative stress also plays a significant role in the pathogenesis of G. duodenalis (Dargahi et al. 2017). As a defense mechanism, invading the host tissues by pathogens leads to the generation of free radicals, e.g., reactive oxygen species (ROS). Increasing ROS results in lipid peroxidation and oxidative stress (Argüello-García et al. 2015). The serum level of malondialdehyde (MDA, a final product of lipid peroxidation as a result of oxidative stress) might be considered as a main biomarker of the acute phase of giardiasis (Kadhim and Al-Naemy 2020). Nitrosative stress and associated reactive species in the human intestine induce cytotoxicity in G. duodenalis (Lloyd et al. 2003).
The GI mucosal barrier plays a vital role in the absorption of nutrient, regulation of the immune system, and limiting the transportation of harmful exogenous and endogenous antigens and microorganisms (Helander and Fändriks 2014; Salvo Romero et al. 2015). This barrier is composed of two main components, including a produced mucus layer with highly glycosylated mucin proteins overlying the second component, the intestinal epithelium (Vancamelbeke and Vermeire 2017). The severe structural damage of mucosa is related to a variety of GI diseases (Meddings 2008). Furthermore, GI barrier dysfunction has been recorded seen during the infections induced by GI pathogens (Berkes et al. 2003; Chin et al. 2002). Giardiasis also is related to GI barrier dysfunction (Buret 2007). GI barrier dysfunction, along with increased intestinal permeability, during giardiasis is induced by several mechanisms, such as activated myosin‐light‐chain kinase and enhanced rates of apoptosis in intestinal enterocytes (Troeger et al. 2007). Giardia infection children and adults is accompanied by a broad range of pathological alterations in the small intestine, e.g., intraepithelial lymphocytosis, villous atrophy, infiltration of granulocytes, lymphocytes, and plasma cells into the lamina propria, and nodular lymphoid hyperplasia (Koot et al. 2009). Following Giardia infection, the immune cells of Peyer’s patches (PP), especially their resident macrophages, play a vital role in the initial phases of effective immune responses (Carlson et al. 1986).
Increased intestinal permeability and epithelial barrier dysfunction after giardiasis are associated with disrupted cellular tight junctional ZO-1 and F‐actin. It seems that this event can be regulated in part through the caspase‐3 and myosin‐light‐chain kinase actions (Chin et al. 2002; Scott et al. 2002). The epithelial barrier dysfunction allows luminal antigens to stimulate host immune‐dependent signaling pathways (Fink and Singer 2017). Infiltration of different types of immune cells (including macrophages, T cells, and neutrophils) and secretion of antibodies (e.g., IgA, IgG, and IgM) are essential for the resolution of Giardia infection (Hawrelak 2003). In addition, the secretion of α-defensins by Paneth cells in the gut act as anti-giardial agent (Klotz and Aebischer 2015). Histopathological findings confirmed that there were a significant inflammation in mucosal tissues of the small intestine in infected individuals and G. muris-infected animal models (Campbell et al. 2004; Craven et al. 2012; Scott et al. 2004). Accordingly, an increased number of intra-epithelial lymphocytes and mast cell hyperplasia have been observed in the post-infection period (Hardin et al. 1997).
Furthermore, increased levels of pro-inflammatory cytokines and chemokines have been reported. The upregulation of interlukin-17A (IL-17A) was observed during Giardia infections in both cattle and mice (Dann et al. 2015; Dreesen et al. 2014; Grit et al. 2014). It has been revealed that restimulation of CD4+ T cells isolated from human patients infected with Giardia can enhance the regulation of IL-177. Subsequently, IL-17A induces the secretion of complement factors and antimicrobial peptides and also regulates the production of specific IgA in the intestine (Dann et al. 2015; Paerewijck et al. 2017). In the neonatal mice model of giardiasis, the initiation of protective immune response in the intestine has been featured by the upregulation of IL-17A and mannose-binding lectin-2 (MBL-2), and the production of parasite-specific IgA (Paerewijck et al. 2019). Thereby, the attachment of these components to the Giardia trophozoites is essential to clear the infection from the intestine.
It has been confirmed that mutant mice models lacking mature B cells, IgA production, the functional polymeric immunoglobulin receptor A (pIgR), or IL‐17A failed to modulate the infection induced by G. duodenalis or the murine species G. muris. These findings highlighted the prominence of humoral immunity and Th17 cells for the regulation of giardiasis (Dann et al. 2015; Dreesen et al. 2014; Singer 2016). It has been recently reported that differential activity of Th17 cells, production of IgA, and regulatory responses of T cells attributed to variations in susceptibility of inbred mouse lines toward infection with G. muris (Yordanova et al. 2019). Over the past decade, it has been reported that both IgA production and Th17 cell activity are regulated by eosinophils during the parasite infection (Shah et al. 2020; Strandmark et al. 2016). The healthy small intestine contains a high proportion of eosinophils, which stimulate the development of PP and secretion of intestinal mucus, induce the homeostatic IgA class switching (Jung et al. 2015), and also limit the function of intestinal Th17 cells (Sugawara et al. 2016). The evidence from in vitro studies proved that the chemokine profile induced by G. duodenalis is unlike the host responses commonly found within other GI pathogens, whereby parasites significantly enhanced the gene expression of CCL20, CCL2, and CXCL1-3 (Roxström-Lindquist et al. 2005). Besides, tumor necrosis factor-alpha (TNF-α) and IL-6 contribute to protection against G. duodenalis infection and determine the parasite burden (Zhou et al. 2007). The host immune response against parasitic infections is directly affected by cytokines. Increased levels of IL-2, 6, 17, and 23 in patients with Giardia infection are the results of immune response and local intestinal inflammation (Mitra et al. 2012). Determining the relationship between cytokines (including TNF-α and ILs (IL-2, IL-4, IL-10)) and giardiasis in 42 patients infected by G. duodenalis confirmed the important role of IL-4 as an inflammatory regulator. However, TNF-α was not detected in these patients (Baqai et al. 2000). In another study on patients with giardiasis, it was demonstrated that the levels of blood serum IL-5 and Ig E (2 times), IL-6 (2.5 times), and interferon-gamma (IFN-γ) (4 times) were higher than healthy controls (Matowicka-Karna et al. 2009). In an animal study, it was shown that the exposure of lamina propria of mouse small intestine to G. duodenalis trophozoites increased the levels of IL-1β, IL-17A, IL-17F, and IFN-γ, whereas levels of IL-13, IL-5, and IL22 were not changed or decreased (Lee et al. 2019).
Gut homeostasis and healthy function are related to the role of term intestinal microbiota, as it is frequently changed during GI diseases. In the intestine microenvironment, trophozoites compete with the commensal microbiome for ecological and nutrient niches (Singer and Nash 2000). During the Giardia infections, compositional and functional alterations in the intestinal microbiota have been verified, including disturbance of the microbial biofilm structure, and virulence difference in commensal species diversity and abundance (Beatty et al. 2013). The intestinal microbiome interacts with Giardia via both direct and indirect mechanisms. These interactions can control host immune responses, mucus barrier function, metabolism, and pain signaling, even after parasite clearance. In contrast, the microbiota direction exhausts the Giardia pathogenesis via colonization resistance, uncontrolled immune responses, and villus atrophy (Fekete et al. 2021).
Taken together, a better understanding of the mechanisms underlying giardiasis may help to develop novel, effective therapeutic strategies. In addition, preclinical and clinical studies have emphasized the critical role of the microbiota and the possible implications of probiotics in Giardia infection.
Giardia, gut microbiome, and probiotics
It is well known that the gut microbiota has a significant role in human health through fermenting non-soluble fibers, impeding colonization by pathogenic components, and stimulating immune responses (Ding et al. 2019; Sharma et al. 2010). The association between gut microbiota and body health leads to a complex ecosystem, where changes in one side can lead to a reaction in the other (Berrilli et al. 2012). It was suggested that the composition of gut microbiota could affect the process of G. duodenalis infection. The post-infectious outcomes of Giardia infection might be due to microbiota dysbiosis (alterations of composition and enhanced pathogenic bacteria) induced by the parasite following the acute phase of disease (Buret et al. 2015). In another study, it was demonstrated that infection with G. duodenalis disrupted the gut microbiota and bile homeostasis in a mice model, leading to metabolic dysregulation and growth impairment (Riba et al. 2020). As offered by previous researches, specific compositions of the microbiome may impact resistance and susceptibility to the colonization of G. duodenalis (67).
In the GI tract of a healthy human, the intestinal mucosa has close contact with multispecies biofilms encompassing the microbiota. These communities may influence intestinal homoeostasis and disease (Kleessen and Blaut 2005; von Rosenvinge et al. 2013). It has been demonstrated that bacterial biofilm covering the gut mucin phylogenetically and metabolically differ from those growing in a planktonic phase (Macfarlane et al. 2005). Findings from a study indicated that G. duodenalis infection disrupted intestinal microbiota and promoted bacterial invasion. These alterations resulted in the disruption of tight junction in intestinal epithelial cells, apoptosis, and facilitating bacterial translocation through the epithelial barrier (Beatty et al. 2017). It was demonstrated that the distribution of gut microbiota composition via antibiotics (without affecting the parasite) limited the efficiency of disaccharidases, inhibited the activation of CD8+ T cell, and also did not alter the proportion of lamina propria CD4+ T cells and T cell receptor–expressing lymphocytes observed in the mouse model of giardiasis. These findings indicated that commensal bacteria might contribute to activation of CD8 + T lymphocyte during the acute phase of infection (Keselman et al. 2016). These findings confirm the importance of intestinal microbiota composition and the possible use of probiotic therapy for the prevention and treatment of G. duodenalis infections. Treatment resistance in Giardia has been recorded for most currently available drugs (Busatti et al. 2009). In this regard, new treatment alternatives with higher efficiency and fewer side effects are needed.
There is a growing body of evidence documenting that using probiotics in nutrition promotes human health. Probiotics have been defined as non-pathogenic viable microbes (yeasts or bacteria) that exert beneficial impacts on the host wellbeing, when consumed in adequate amounts (Fuller 1989). The known consumed probiotic strains belong to the genus Lactobacillus (characterized by the production of lactic acid), Bifidobacterium, Enterococcus, and Saccharomyces (yeast) (Fekete et al. 2021). Probiotic microbes can improve the host health via directly targeting pathogens, regulating gastric acids and bile acid toxicity, modulating systemic and mucosal immune responses, covering the intestinal mucosa, strengthening mucosal barrier function, reinforcing epithelial integrity, and suppressing the transportation of microorganisms and their metabolites into the subepithelial regions (Conlon and Bird 2015; Markowiak and Śliżewska 2017). Stimulation of mucosal immune response through increasing the IgA production was seen after treatment with probiotics containing Lactobacillus (L.) and Bifidobacterium. These bacteria can alter the cytokine milieu in the intestinal mucosa through upregulation of IL-6, IL-10, and TGFβ in epithelial cells, leading to potentiate IgA production (Hardy et al. 2013). We reviewed findings of preclinical and clinical investigations to understand the beneficial effects of probiotics against giardiasis and their potential molecular mechanisms.
Probiotics in preclinical studies for the treatment of giardiasis
Several scientific researches on the probiotics administration for the prevention and treatment of intestinal parasites (e.g., G. duodenalis) have been conducted. It was demonstrated that treatment of infected gerbils with L. johnsonii La1 (108 CFU) for 1 week before trophozoite inoculation did not affect the morphological impairment observed in intestinal epithelium, but reduced quantity of active trophozoite and infection length (Humen et al. 2005). In a similar study, daily treatment of gerbils with L. casei and L. rhamnosus (109 CFU for 30 days) prior to Giardia infection and also when simultaneously infected with Giardia reduced both duration and severity of infection, decreased the amount of active intestinal trophozoites, protected against mucosal damage, and resulted in the resolution of Giardia infection (Shukla et al. 2008). In another study, administration of L. casei restored the membrane integrity of microvillus, improved the quantity of goblet cells, promoted edematous and vacuolated epithelial cells, and reduced ileitis in the mouse model of giardiasis (Shukla et al. 2012). The efficiency of four bacterial strains (109 CFU daily for 13 days via orogastric gavage), including L. rhamnosus GG (LGG), L. plantarum, L. acidophilus, and L. casei, was evaluated in the modulation of G. murine infection. Among these strains, LGG extorted more advantages in reduction of G. duodenalis cycle duration and active intestinal trophozoites, leading to effective suppression of infective disease (Goyal et al. 2011). In in vitro and in vivo studies, therapeutic effects of bacteriocins from newly isolated Egyptian strains of Lactobacilli (including L. acidophilus [P106] and L. plantarum [P164]) against G. duodenalis were evaluated. In vitro findings presented that L. acidophilus bacteriocin (50 µg) decreased the attachment and the quantity of trophozoites (by ~ 58.5%). Oral administration of L. acidophilus bacteriocin (50 µg/day for five successive days) decreased the gut density of parasite and ameliorated intestinal pathology of infected mice. Therefore, L. acidophilus (P106) showed great promise as a potential anti-giardial therapeutic (Amer et al. 2014). It was revealed that oral gavage with S. boulardii 15 days before infection and 22 days after could significantly reduce the proportion of parasite load (reduction of 70%), enhance the height of the intestinal villi and depth of crypt, improve the production of mucus, and increase the number of goblet cells and intraepithelial lymphocytes (Ribeiro et al. 2018). In another study, probiotic bacteria, including L. acidophilus, Bifidobacterium bifidum, and L. helveticus, showed preventive effects administered before infection in the mouse model. In addition, these therapeutics significantly reduced infection intensity (87.5% after 20 days) and intestinal changes (Al-Megrin et al. 2021). The beneficial effect of B. longum 51A and Weissella paramesenteroides was evaluated in another research. Findings indicated that oral administration of bacteria 10 days before induction of giardiasis could protect the intestine against infection (Fonseca et al. 2019). It was also demonstrated that there is a therapeutic effect of probiotic L. casei in combination with albendazole on the outcome of Giardia infection in a murine model. This combination restored crypts and villi to normal morphology, and diminished the trophozoite proportion in the intestinal fluid and excretion of cysts in feces (Shukla et al. 2013). Moreover, intraperitoneal injection of heat-inactivated (killed) probiotics and probiotic protein of LGG exerted anti-giardial effects. Their administration declined the severity and duration of disease mainly by restoration of the intestinal microbiome and the mucosal epithelium to the normal status, along with modulation of mucosal immune responses, in the mice model of infection. The probiotic protein was more effective than killed probiotic, suggesting that this component is a potential preventive vaccine candidate for Giardia infection (Shukla et al. 2020). In a mice model of G. duodenalis infection, effects of kefir-fermented milk (composed of bacteria and yeasts in a complex symbiotic association) were evaluated and showed that feeding mice with kefir declined giardiasis severity and stimulated the humoral and cellular immunity against infection (Franco et al. 2013). According to the literature, probiotics can modulate the toxic effects of G. duodenalis via several molecular mechanisms. It was shown that probiotic protects against parasite-induced mucosal damage through suppressing the parasite growth, increasing the antioxidant capacity, reducing oxidants, stimulating systemic humoral and cellular immunity, and modulating the inflammatory status of the intestinal mucosa (Franco et al. 2013; Goyal et al. 2013; Travers et al. 2016). Here, we reviewed the results of clinical investigation to highlight potential effects of probiotics in the patients with giardiasis.
Probiotics in clinical studies for the treatment of giardiasis
Probiotics have been used in the prevention and treatment of several GI inflammatory conditions, such as inflammatory bowel disease (pouchitis and ulcerative colitis), constipation, irritable bowel, and liver infectious disease (Olveira and González-Molero 2016). According to the literature, few clinical studies have suggested the consumption of probiotics or their related products as an alternative therapy for Giardia infection. Most studies have focused on the benefits of such microorganisms for the prevention and treatment of symptoms such as diarrhea observed in GI diseases.
Regarding the health benefits of S. boulardii, the effects of oral S. boulardii (250 mg/ three times a day/for 10 days) on acute diarrhea were investigated in patients following amebiasis. This approach could significantly improve the duration of symptoms and reduce the amount of excreted cysts after 4 weeks. The yeast could restore the normal gut microbiota, while the exact molecular mechanism has not been clarified (Mansour-Ghanaei et al. 2003). In a small clinical trial, beneficial effects of S. boulardii in amelioration of symptoms and reduction of parasite proportion were proven in children with symptomatic Blastocystis hominis infection (Dinleyici et al. 2011). In a double-blind, placebo-controlled study, S. boulardii capsules (250 mg b.i.d. orally) in combination with metronidazole (750 mg/3 times a day for 10 days) showed effective anti-giardial impacts as an adjunctive therapy in adult patients. This combination could increase levels of enteric disaccharidases, stimulate immune responses, activate intestinal enzymes, and induce a trophic effect on the intestine (Besirbellioglu et al. 2006). Numerous studies have indicated that probiotics have health-restoring benefits with lower risk of infection. However, some groups of people, including cases under neonatal stages and/or those with medical conditions (e.g., the leaky gut, malignancies, diabetes mellitus, and post-organ transplant convalescence) should use these products with caution (Kothari et al. 2019). Nevertheless, further clinical researches are necessary to investigate the beneficial effects of probiotics in human. To understand how probiotics can prevent or treat giardiasis, we described the potential molecular mechanism of probiotics.
Potential mechanisms of probiotics in the treatment of giardiasis
Despite the beneficial effects of probiotics, their underlying mechanisms in prevention and treatment of giardiasis are still unclear. Potential antioxidant properties of probiotics have proven in several in vitro and in vivo studies. Consumption of these alternatives may decrease oxidative damage, improve the free radical scavenging rate, and modify the activity of antioxidant enzymes in human cells (Mishra et al. 2015). Antioxidant features of probiotics were investigated in several studies. It was shown that feeding Giardia-infected mice with LGG increased enzymatic antioxidant levels (superoxide dismutase and glutathione) and intestinal disaccharidases (lactase and sucrase), and reduced levels of oxidants. According to histopathological findings, normal cellular morphology of the small intestine and reduced infiltration of lymphocytes were seen in the probiotic receiving group compared with infected mice (Goyal et al. 2013).
Immunomodulatory features of probiotics have been demonstrated. It was illustrated that probiotics are able to modulate the systemic and mucosal immune response in the fight against giardiasis. Oral administration of the probiotic LGG (109/0.1 mL) to Giardia-infected mice (106/0.1 mL) could restore the normal gut microbiota and modulate the mucosal immune response via regulating anti-inflammatory (e.g., IL-6 and IL-10) and pro-inflammatory (e.g., IFN-γ) cytokines, increasing the secretion of IgA antibody, enhancing the number of IgA+ cells and CD4+ T cells, and reducing the number of cytotoxic CD8+ T cells in the lamina propria (Goyal and Shukla 2013). It was demonstrated that Enterococcus faecium SF68 had specific anti-giardial immune responses with a progressive elevation in levels of intestinal IgA and serum IgG, as well as a decline in antigenic overload and the enteric parasitic in the mice model. In addition, a higher percentage of CD4+ T cells in the spleen and PP were recorded (Benyacoub et al. 2005). In contrast, it was reported that short-term administration of probiotic E. faecium SF68 (5 × 108 CFU) could not be effective on cyst shedding, fecal levels of IgA, fecal antigen shedding, or circulating leukocyte phagocytic function in dogs with giardiasis (Simpson et al. 2009).
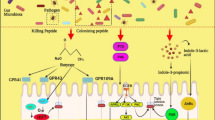
Probiotics may alleviate giardiasis via directly targeting parasites. L. acidophilus (P106) could directly alter the cellular architecture of the trophozoites via disorganization of the cytoplasmic components, cell membrane, and adhesive disc (Amer et al. 2014). In an in vitro study, it was reported that Lactobacillus johnsonii La1 could secrete some low molecular mass factors that suppress the proliferation of G. duodenalis trophozoites in G1 phase of cell cycle without detecting necrosis nor apoptosis (Pérez et al. 2001). In addition, the activity of bile-salt hydrolase (BSH) results in deconjugated bile salts which is one of most important mechanisms involving in the suppression of trophozoite growth. It was reported that L. johnsonii La1 prevented in vitro growth of G. duodenalis through deconjugated bile salts produced by extracellular BSH-like activities (Travers et al. 2016). In another study, BSH like activities of L. johnsonii La1 and L. gasseri CNCM I-4884 were evaluated. Their results confirmed that these two strains might contribute to the anti-Giardia features via expression of BSH47 and BSH56 genes (Allain et al. 2018). These findings suggest that probiotics alone or in combination with antiprotozoal drugs are safe and effective approaches in the prevention and treatment of Giardia infection via direct effects on parasite, along with antioxidant and immunomodulatory properties. All discussed mechanism are summarized in Fig. 1.
Potential mechanisms of probiotics in the treatment of giardiasis. ROS: reactive oxygen species, Ig: immunoglobulin, IECs: intestinal epithelial cells, IL: interferon (created by https://biorender.com/)
Conclusion
In summary, G. duodenalis targets the gut via several mechanical and chemical mechanisms and induces several symptoms. It seems that the composition of the gut microbiota is important for defense against the microorganisms, e.g., G. duodenalis. The disturbed microbiota during the giardiasis may exhaust the symptoms of diseases. Therefore, restoring the normal microbiota and morphology of intestinal mucosa and directly targeting the parasite can attenuate the severity of disease. It has been reported that probiotics are safe and effective agents in the treatment of Giardia infection. Probiotics with different available forms show antioxidant, anti-inflammatory, immunomodulatory, and extracellular BSH-like activities in treatment of giardiasis. They are able to re-establish the intestinal microbiota, repair the mucosal barrier of intestine, promote the number of epithelial and goblet cells, and restore the microstructure of the intestine. These alternative therapeutic strategies can destroy the cellular architecture of parasites and modulate the immune response. Despite the beneficial effects of probiotics, further researches are required to find the suitable probiotics in prevention and treatment of giardiasis.
Availability of data and materials
No datasets were generated or analyzed during the current study.
References
Adam RD (2001) Biology of Giardia lamblia. Clin Microbiol Rev 14:447–475
Al-Megrin WA, Mohamed SH, Saleh MM, Yehia HM (2021) Preventive role of probiotic bacteria against gastrointestinal diseases in mice caused by Giardia lamblia. Biosci Rep 41:BSR20204114
Allain T et al (2018) Bile salt hydrolase activities: a novel target to screen anti-Giardia Lactobacilli? Front Microbiol 9:89
Amer EI, Mossallam SF, Mahrous H (2014) Therapeutic enhancement of newly derived bacteriocins against Giardia lamblia. Exp Parasitol 146:52–63
Ankarklev J, Jerlström-Hultqvist J, Ringqvist E, Troell K, Svärd SG (2010) Behind the smile: cell biology and disease mechanisms of Giardia species. Nat Rev Microbiol 8:413–422. https://doi.org/10.1038/nrmicro2317
Argüello-García R, Cruz-Soto M, González-Trejo R, Paz-Maldonado LMT, Bazán-Tejeda ML, Mendoza-Hernández G, Ortega-Pierres MG (2015) An antioxidant response is involved in resistance of Giardia duodenalis to albendazole. Front Microbiol 6:286
Baqai R, Kazmi SU, Qureshi H (2000) Role of cytokines in giardiasis. J Pak Med Assoc 50:113–115
Beatty J et al (2013) Gut microbiota biofilm disruptions by Giardia: pathology in human enterocytes and germ‐free mice. Wiley Online Library
Beatty JK et al (2017) Giardia duodenalis induces pathogenic dysbiosis of human intestinal microbiota biofilms International. J Parasitol 47:311–326. https://doi.org/10.1016/j.ijpara.2016.11.010
Benyacoub J et al (2005) Enterococcus faecium SF68 enhances the immune response to Giardia intestinalis in mice. J Nutr 135:1171–1176
Berkes J, Viswanathan VK, Savkovic SD, Hecht G (2003) Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 52:439–451. https://doi.org/10.1136/gut.52.3.439
Berrilli F, Di Cave D, Cavallero S, D’Amelio S (2012) Interactions between parasites and microbial communities in the human gut. Front Cell Infect Microbiol 2:141
Besirbellioglu BA et al (2006) Saccharomyces boulardii and infection due to Giardia lamblia. Scand J Infect Dis 38:479–481
Buret AG (2007) Mechanisms of epithelial dysfunction in giardiasis. Gut 56(316):317. https://doi.org/10.1136/gut.2006.107771
Buret AG (2008) Pathophysiology of enteric infections with Giardia duodenalius. Parasite 15:261–265. https://doi.org/10.1051/parasite/2008153261
Buret AG et al (2015) Giardia duodenalis: new research developments in pathophysiology, pathogenesis, and virulence factors. Curr Trop Med Rep 2:110–118
Busatti HG, Santos JF, Gomes MA (2009) The old and new therapeutic approaches to the treatment of giardiasis: where are we? Biol Targets Ther 3:273
Cacciò SM, Lalle M, Svärd SG (2018) Host specificity in the Giardia duodenalis species complex. Infect Genet Evol 66:335–345
Campbell DI, McPhail G, Lunn PG, Elia M, Jeffries DJ (2004) Intestinal inflammation measured by fecal neopterin in Gambian children with enteropathy: association with growth failure, Giardia lamblia, and intestinal permeability. J Pediatr Gastroenterol Nutr 39:153–157. https://doi.org/10.1097/00005176-200408000-00005
Carlson JR, Heyworth MF, Owen RL (1986) Response of Peyer’s patch lymphocyte subsets to Giardia muris infection in BALB/c mice: I. T-cell subsets. Cell Immunol 97:44–50
Chin AC, Teoh DA, Scott KG, Meddings JB, Macnaughton WK, Buret AG (2002) Strain-dependent induction of enterocyte apoptosis by Giardia lamblia disrupts epithelial barrier function in a caspase-3-dependent manner. Infect Immunol 70:3673–3680. https://doi.org/10.1128/iai.70.7.3673-3680.2002
Conlon MA, Bird AR (2015) The impact of diet and lifestyle on gut microbiota and human health. Nutrients 7:17–44
Cotton JA, Amat CB, Buret AG (2015) Disruptions of host immunity and inflammation by Giardia duodenalis: potential consequences for co-infections in the gastro-intestinal tract. Pathogens 4:764–792
Cotton JA, Beatty JK, Buret AG (2011) Host parasite interactions and pathophysiology in Giardia infections. Int J Parasitol 41:925–933. https://doi.org/10.1016/j.ijpara.2011.05.002
Craven M et al (2012) Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn’s disease. PLoS ONE 7:e41594. https://doi.org/10.1371/journal.pone.0041594
Dann SM et al (2015) IL-17A promotes protective IgA responses and expression of other potential effectors against the lumen-dwelling enteric parasite Giardia. Exp Parasitol 156:68–78
Dargahi D, Zare Bavani M, Einollahi N, Dashti N, Rezaeian M, Abbasi S (2017) Prevalence of Giardia lamblia among food handlers and day-care workers in Tehran. J Payavard Salamat 10:402–408
Deng M, Nuanualsuwan S, Cliver DO (2001) Inactivation of Cryptosporidium parvum oocysts by bacterial strains. J Eukaryot Microbiol Suppl:37s-39s. https://doi.org/10.1111/j.1550-7408.2001.tb00446.x
Ding R-x et al (2019) Revisit gut microbiota and its impact on human health and disease. J Food Drug Anal 27:623–631. https://doi.org/10.1016/j.jfda.2018.12.012
Dinleyici EC, Eren M, Dogan N, Reyhanioglu S, Yargic ZA, Vandenplas Y (2011) Clinical efficacy of Saccharomyces boulardii or metronidazole in symptomatic children with Blastocystis hominis infection. Parasitol Res 108:541–545. https://doi.org/10.1007/s00436-010-2095-4
Dreesen L, De Bosscher K, Grit G, Staels B, Lubberts E, Bauge E, Geldhof P (2014) Giardia muris infection in mice is associated with a protective interleukin 17A response and induction of peroxisome proliferator-activated receptor alpha. Infect Immunol 82:3333–3340
Escobedo AA, Cimerman S (2007) Giardiasis: a pharmacotherapy review. Expert Opin Pharmacother 8:1885–1902
Farthing MJ (1997) The molecular pathogenesis of giardiasis. J Pediatr Gastroenterol Nutr 24:79–88
Fekete E, Allain T, Siddiq A, Sosnowski O, Buret AG (2021) Giardia spp. and the gut microbiota: dangerous liaisons. Front Microbiol 11:618106–618106. https://doi.org/10.3389/fmicb.2020.618106
Fink MY, Singer SM (2017) The intersection of immune responses, microbiota, and pathogenesis in giardiasis. Trends Parasitol 33:901–913. https://doi.org/10.1016/j.pt.2017.08.001
Fonseca J et al (2019) Probiotic effect of Bifidobacterium longum 51A and Weissella paramesenteroides WpK4 on gerbils infected with Giardia lamblia. J Appl Microbiol 127:1184–1191
Franco MC, Golowczyc MA, de Antoni GL, Perez PF, Humen M, de los Angeles Serradell M (2013) Administration of kefir-fermented milk protects mice against Giardia intestinalis infection. J Med Microbiol 62:1815–1822
Frank DN, Pace NR (2008) Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol 24:4–10
Freitas S, Shinohara L, Sforcin J, Guimarães S (2006) In vitro effects of propolis on Giardia duodenalis trophozoites. Phytomedicine 13:170–175
Fuller R (1989) Probiotics in man and animals. J Appl Bacteriol 66:365–378
Goyal N, Tiwari RP, Shukla G (2011) Lactobacillus rhamnosus GG as an effective probiotic for murine giardiasis. Interdiscip Perspect Infect Dis 2011
Goyal N, Shukla G (2013) Probiotic Lactobacillus rhamnosus GG modulates the mucosal immune response in Giardia intestinalis-infected BALB/c mice. Dig Dis Sci 58:1218–1225
Goyal N, Rishi P, Shukla G (2013) Lactobacillus rhamnosus GG antagonizes Giardia intestinalis induced oxidative stress and intestinal disaccharidases: an experimental study. World J Microbiol Biotechnol 29:1049–1057
Grit G et al (2014) Evaluation of cellular and humoral systemic immune response against Giardia duodenalis infection in cattle. Vet Parasitol 202:145–155
Halliez MC, Buret AG (2013) Extra-intestinal and long term consequences of Giardia duodenalis infections. World J Gastroenterol 19:8974–8985. https://doi.org/10.3748/wjg.v19.i47.8974
Hanevik K et al (2007) Persisting symptoms and duodenal inflammation related to Giardia duodenalis infection. J Infect 55:524–530
Hardin JA, Buret AG, Olson ME, Kimm MH, Gall DG (1997) Mast cell hyperplasia and increased macromolecular uptake in an animal model of giardiasis. J Parasitol 83:908–912
Hardy H, Harris J, Lyon E, Beal J, Foey AD (2013) Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology. Nutrients 5:1869–1912
Hawrelak J (2003) Giardiasis: pathophysiology and management. Altern Med Rev 8:129–142
Helander HF, Fändriks L (2014) Surface area of the digestive tract – revisited. Scand J Gastroenterol 49:681–689. https://doi.org/10.3109/00365521.2014.898326
Humen MA et al (2005) Lactobacillus johnsonii La1 antagonizes Giardia intestinalis in vivo. Infect Immunol 73:1265–1269
Jung Y et al (2015) IL-1β in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol 8:930–942. https://doi.org/10.1038/mi.2014.123
Kadhim RA, Al-Naemy NZ (2020) Giardiasis and oxidative stress: a case–control study. Eurasian J Biosci 14:5679–5683
Katelaris PH, Farthing MJ (1992) Diarrhoea and malabsorption in giardiasis: a multifactorial process? Gut 33:295–297. https://doi.org/10.1136/gut.33.3.295
Keselman A, Li E, Maloney J, Singer SM (2016) The microbiota contributes to CD8+ T cell activation and nutrient malabsorption following intestinal infection with Giardia duodenalis. Infect Immunol 84:2853–2860
Kleessen B, Blaut M (2005) Modulation of gut mucosal biofilms. Br J Nutr 93:S35–S40
Klotz C, Aebischer T (2015) The immunological enigma of human giardiasis. Curr Trop Med Rep 2:119–127
Koot BG, ten Kate FJ, Juffrie M, Rosalina I, Taminiau JJ, Benninga MA (2009) Does Giardia lamblia cause villous atrophy in children?: A retrospective cohort study of the histological abnormalities in giardiasis. J Pediatr Gastroenterol Nutr 49:304–308
Kothari D, Patel S, Kim S-K (2019) Probiotic supplements might not be universally-effective and safe: a review. Biomed Pharmacother 111:537–547. https://doi.org/10.1016/j.biopha.2018.12.104
Lamireau T, Enaud R (2017) Differential diagnosis of inflammatory bowel disease. In: Pediatric inflammatory bowel disease. Springer, pp 199–209
Lee HY, Park EA, Lee KJ, Lee KH, Park SJ (2019) Increased innate lymphoid cell 3 and IL-17 production in mouse lamina propria stimulated with Giardia lamblia. Korean J Parasitol 57:225–232. https://doi.org/10.3347/kjp.2019.57.3.225
Lloyd D, Harris J, Maroulis S, Mitchell A, Hughes M, Wadley R, Edwards M (2003) Nitrosative stress induced cytotoxicity in Giardia intestinalis. J Appl Microbiol 95:576–583
Macfarlane S, Woodmansey EJ, Macfarlane GT (2005) Colonization of mucin by human intestinal bacteria and establishment of biofilm communities in a two-stage continuous culture system. Appl Environ Microbiol 71:7483–7492
Mansour-Ghanaei F, Dehbashi N, Yazdanparast K, Shafaghi A (2003) Efficacy of Saccharomyces boulardii with antibiotics in acute amoebiasis. World J Gastroenterol 9:1832
Markowiak P, Śliżewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9. https://doi.org/10.3390/nu9091021
Martínez-Gordillo MN, González-Maciel A, Reynoso-Robles R, Montijo-Barrios E, Ponce-Macotela M (2014) Intraepithelial giardia intestinalis: a case report and literature review. Medicine (Baltimore) 93:e277. https://doi.org/10.1097/md.0000000000000277
Matowicka-Karna J, Dymicka-Piekarska V, Kemona H (2009) IFN-gamma, IL-5, IL-6 and IgE in patients infected with Giardia intestinalis. Folia Histochem Cytobiol 47:93–97
Meddings J (2008) The significance of the gut barrier in disease. Gut 57:438–440. https://doi.org/10.1136/gut.2007.143172
Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J (2015) Probiotics as potential antioxidants: a systematic review. J Agric Food Chem 63:3615–3626
Mitra Z, Nasrin D, Nahid E, Arezoo J (2012) Evaluation of cytokines changes in patients infected with Giardia lamblia in comparison with healthy subjects. Payavard Salamat 6
Olson M, Ceri H, Morck D (2000) Giardia vaccination. Parasitol Today 16(213):217
Olveira G, González-Molero I (2016) An update on probiotics, prebiotics and symbiotics in clinical nutrition. Endocrinol Nutr (Engl Ed) 63:482–494. https://doi.org/10.1016/j.endoen.2016.10.011
Paerewijck O et al (2017) Interleukin-17 receptor A (IL-17RA) as a central regulator of the protective immune response against Giardia. Sci Rep 7:1–14
Paerewijck O, Maertens B, Gagnaire A, De Bosscher K, Geldhof P (2019) Delayed development of the protective IL-17A response following a Giardia muris infection in neonatal mice. Sci Rep 9:1–7
Pérez PF, Minnaard J, Rouvet M, Knabenhans C, Brassart D, De Antoni GL, Schiffrin EJ (2001) Inhibition of Giardia intestinalis by extracellular factors from Lactobacilli: an in vitro study. Appl Environ Microbiol 67:5037–5042
Ponce-Macotela M, Navarro-Alegria I, Martinez-Gordillo M, Alvarez-Chacon R (1994) In vitro effect against Giardia of 14 plant extracts. Rev Investig Clin 46:343–347
Ratanapo S et al (2008) Multiple modes of transmission of giardiasis in primary schoolchildren of a rural community, Thailand. Am J Trop Med Hyg 78:611–615
Reynoso-Robles R, Ponce-Macotela M, Rosas-López LE, Ramos-Morales A, Martínez-Gordillo MN, González-Maciel A (2015) The invasive potential of Giardia intestinalis in an in vivo model. Sci Rep 5:15168. https://doi.org/10.1038/srep15168
Riba A et al (2020) Disturbed gut microbiota and bile homeostasis in Giardia-infected mice contributes to metabolic dysregulation and growth impairment. Sci Transl Med 12
Ribeiro M et al (2018) Effect of probiotic Saccharomyces boulardii in experimental giardiasis. Benef Microbes 9:789–797
Robertson LJ, Hanevik K, Escobedo AA, Mørch K, Langeland N (2010) Giardiasis—why do the symptoms sometimes never stop? Trends Parasitol 26:75–82. https://doi.org/10.1016/j.pt.2009.11.010
Roxström-Lindquist K, Ringqvist E, Palm D, Svärd S (2005) Giardia lamblia-induced changes in gene expression in differentiated Caco-2 human intestinal epithelial cells. Infect Immunol 73:8204–8208. https://doi.org/10.1128/iai.73.12.8204-8208.2005
Ryan U, Hijjawi N, Feng Y, Xiao L (2019) Giardia: an under-reported foodborne parasite. Int J Parasitol 49:1–11
Said D, Elsamad L, Gohar Y (2012) Validity of silver, chitosan, and curcumin nanoparticles as anti-Giardia agents. Parasitol Res 111:545–554
Salvo Romero E, Alonso Cotoner C, Pardo Camacho C, Casado Bedmar M, Vicario M (2015) The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig 107:686–696. https://doi.org/10.17235/reed.2015.3846/2015
Savioli L, Smith H, Thompson A (2006) Giardia and Cryptosporidium join the ‘neglected diseases initiative.’ Trends Parasitol 22:203–208
Schroeder BO (2019) Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterol Rep 7:3–12
Scott KG, Meddings JB, Kirk DR, Lees-Miller SP, Buret AG (2002) Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology 123:1179–1190. https://doi.org/10.1053/gast.2002.36002
Scott KG, Yu LC, Buret AG (2004) Role of CD8+ and CD4+ T lymphocytes in jejunal mucosal injury during murine giardiasis. Infect Immunol 72:3536–3542. https://doi.org/10.1128/iai.72.6.3536-3542.2004
Shah K, Ignacio A, McCoy KD, Harris NL (2020) The emerging roles of eosinophils in mucosal homeostasis. Mucosal Immunol 13:574–583. https://doi.org/10.1038/s41385-020-0281-y
Sharma R, Young C, Neu J (2010) Molecular modulation of intestinal epithelial barrier: contribution of microbiota. J Biomed Biotechnol 2010
Shukla G, Devi P, Sehgal R (2008) Effect of Lactobacillus casei as a probiotic on modulation of giardiasis. Dig Dis Sci 53:2671–2679
Shukla G, Sidhu RK, Verma A (2012) Restoration of anthropometric, biochemical and histopathological alterations by Lactobacillus casei supplementation in Giardia intestinalis infected renourished BALB/c mice. Antonie Van Leeuwenhoek 102:61–72
Shukla G, Kaur H, Sharma L (2013) Comparative therapeutic effect of probiotic Lactobacillus casei alone and in conjunction with antiprotozoal drugs in murine giardiasis. Parasitol Res 112:2143–2149
Shukla G, Kamboj S, Sharma B (2020) Comparative analysis of antigiardial potential of heat inactivated and probiotic protein of probiotic Lactobacillus rhamnosus GG in murine giardiasis. Probiot Antimicrobial Proteins 12:271–279
Simpson K et al (2009) Influence of Enterococcus faecium SF68 probiotic on giardiasis in dogs. J Vet Intern Med 23:476–481
Singer SM (2016) Control of giardiasis by interleukin-17 in humans and mice—are the questions all answered? Clin Vaccine Immunol 23:2–5
Singer SM, Nash TE (2000) The role of normal flora in Giardia lamblia infections in mice. J Infect Dis 181:1510–1512. https://doi.org/10.1086/315409
Strandmark J, Rausch S, Hartmann S (2016) Eosinophils in homeostasis and their contrasting roles during inflammation and helminth infections. Crit Rev Immunol 36:193–238. https://doi.org/10.1615/CritRevImmunol.2016018726
Sugawara R et al (2016) Small intestinal eosinophils regulate Th17 cells by producing IL-1 receptor antagonist. J Exp Med 213:555–567. https://doi.org/10.1084/jem.20141388
Travers M-A et al (2016) Deconjugated bile salts produced by extracellular bile-salt hydrolase-like activities from the probiotic Lactobacillus johnsonii La1 inhibit Giardia duodenalis in vitro growth. Front Microbiol 7:1453
Troeger H et al (2007) Effect of chronic Giardia lamblia infection on epithelial transport and barrier function in human duodenum. Gut 56:328–335. https://doi.org/10.1136/gut.2006.100198
Upcroft P (1998) Drug resistance in Giardia: clinical versus laboratory isolates. Drug Resist Updates 1:166–168
Upcroft P, Upcroft JA (2001) Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev 14:150–164
Vancamelbeke M, Vermeire S (2017) The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol 11:821–834. https://doi.org/10.1080/17474124.2017.1343143
Ventura LLA, Oliveira DRd, Gomes MA, Torres MRF (2018) Effect of probiotics on giardiasis. Where are we? Braz J Pharm Sci 54
von Rosenvinge EC, O’May GA, Macfarlane S, Macfarlane GT, Shirtliff ME (2013) Microbial biofilms and gastrointestinal diseases. Pathog Dis 67:25–38
Watkins RR, Eckmann L (2014) Treatment of giardiasis: current status and future directions. Curr Infect Dis Rep 16:396. https://doi.org/10.1007/s11908-014-0396-y
Wensaas KA, Langeland N, Hanevik K, Mørch K, Eide GE, Rortveit G (2012) Irritable bowel syndrome and chronic fatigue 3 years after acute giardiasis: historic cohort study. Gut 61:214–219. https://doi.org/10.1136/gutjnl-2011-300220
Yordanova IA et al (2019) RORγt+ Treg to Th17 ratios correlate with susceptibility to Giardia infection. Sci Rep 9:1–16
Zhou P, Li E, Shea-Donohue T, Singer SM (2007) Tumour necrosis factor alpha contributes to protection against Giardia lamblia infection in mice. Parasite Immunol 29:367–374. https://doi.org/10.1111/j.1365-3024.2007.00953.x
Author information
Authors and Affiliations
Contributions
M.Z. and N.D. conceived of the presented idea. M.Z. and N.D. equally participated in drafting the article. M.Z. participated in revising it critically for important intellectual content. M.Z. and N.D. gave final approval of the version to be submitted and any revised version.
Corresponding author
Ethics declarations
Ethical approval
N/A
Consent to participate
N/A
Consent to publish
N/A
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dashti, N., Zarebavani, M. Probiotics in the management of Giardia duodenalis: an update on potential mechanisms and outcomes. Naunyn-Schmiedeberg's Arch Pharmacol 394, 1869–1878 (2021). https://doi.org/10.1007/s00210-021-02124-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-021-02124-z