Abstract
We previously reported that hypothalamic tumor necrosis factor-alpha (TNF-α) mRNA expression via histamine H4 receptors contributes to the development of cisplatin-induced anorexia; however, its precise mechanisms remain unclear. It has been reported that chemotherapeutic agents induce the suppression of orexin neuron activity, and the administration of orexin inhibits chemotherapeutic agent-induced gastric discomfort. Other studies demonstrated that the central administration of TNF-α impairs the orexinergic system, and that orexin excites the histaminergic system. We investigated the involvement of orexinergic and histaminergic systems in the therapeutic effect of an H4 receptor antagonist against cisplatin-induced anorexia. Cisplatin decreased the expression of prepro-orexin mRNA, which encodes precursors of orexin, in the hypothalamus of mice. The period of expression decreased in parallel with the onset of anorexia, and treatment with an H4 receptor antagonist (JNJ7777120, 10 mg/kg) inhibited the decrease in expression. The effect of the H4 receptor antagonist on cisplatin-induced anorexia in mice was antagonized by an orexin OX2 receptor antagonist (JNJ10397049, 5 mg/kg) rather than an orexin OX1 receptor antagonist (SB408124, 30 mg/kg). Although an OX2 receptor agonist (YNT-185, 20 mg/kg) or a histamine H3 receptor inverse agonist (ciproxifan, 1 mg/kg) inhibited the cisplatin-induced anorexia, the inhibitory effect of the OX2 receptor agonist was antagonized by an H3 receptor silent antagonist (VUF5681, 5 mg/kg). The combination of JNJ7777120 (10 mg/kg) and ciproxifan (0.5 mg/kg) completely resolved the cisplatin-induced anorexia. These results suggest that activation of the orexinergic and histaminergic pathway is involved in the therapeutic effect of an H4 receptor antagonist against cisplatin-induced anorexia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients often develop anorexia during the course of cancer chemotherapy (Zhou et al. 2017). Insufficient control of such eating disorders may induce not only impairment of their quality of life but also refusal of further therapy. Although chemotherapeutic agent-induced anorexia is frequently associated with nausea and vomiting, it is not completely inhibited even with the use of anti-emetic drugs (Taguchi et al. 2009). Several studies have shown that the mechanism of appetite loss may be related to the production of inflammatory cytokines such as interleukin-1β (Plata-Salamán 1991) and tumor necrosis factor (TNF)-α (Wood and Weymann 2013; Smith et al. 2014). We recently reported that hypothalamic TNF-α mRNA expression via histamine H4 receptors may contribute to the development of cisplatin-induced anorexia in mice; thus, we considered that inflammatory cytokines and subsequent signal transduction pathways are involved in the development of cisplatin-induced anorexia (Yamamoto et al. 2018). However, the precise mechanisms are still unclear.
Previous studies reported that the central administration of orexin inhibits chemotherapeutic agent-induced fatigue-like behavior in rats (Weymann et al. 2014; Guo et al. 2018). Other studies demonstrated that TNF-α impairs the function of the orexinergic system via the suppression of prepro-orexin (PPO) mRNA, which encodes precursors of orexin, and orexin OX2 receptor mRNA expression (Zhan et al. 2011), and that the enhancement of orexinergic system through OX2 receptors excites the histaminergic system (Eriksson et al. 2011). Based on these findings, we hypothesized that activation of the orexinergic system via OX2 receptors is involved in the therapeutic effect of an H4 receptor antagonist against cisplatin-induced anorexia in mice. In this study, we investigated the expression of PPO mRNA in the hypothalamus of cisplatin-treated mice with or without an H4 receptor antagonist and the involvement of OX2 receptors in the development of cisplatin-induced anorexia.
Among the histamine receptors except for H4 receptors, histamine H3 receptors are expressed in the central nervous system and act as autoreceptors in presynaptic histaminergic neurons (van der Werf and Timmerman 1989), and H3 receptor inverse agonists are known to enhance the activity of histaminergic neurons (Morisset et al. 2000); therefore, we also investigated the effects of H3 receptor ligands on the prevention of cisplatin-induced anorexia in mice with or without an H4 receptor antagonist, and elucidated the underlying mechanisms of its therapeutic effects.
Materials and methods
Animals
All experimental protocols were approved in accordance with the Animal Experimental Committee of Osaka University (name of committee: Animal Care Committee of the School of Allied Health Sciences, Faculty of Medicine, Osaka University, approval number: 28-01-00), and all experiments were conducted in accordance with the Animal Experiment Guidelines of Osaka University.
Male DBA/2 mice aged 6 to 7 weeks old (20–25 g) were purchased from Japan SLC (Shizuoka, Japan), and were housed in home cages in a room with a regular light/dark cycle (lights on 5:00–17:00) at a constant temperature (approximately 25 °C) and humidity (approximately 50%). Throughout the experiment, all mice had free access to standard laboratory chow pellets (CE-2, CLEA Japan, Tokyo, Japan) and tap water.
RT-PCR experiment
Effects of cisplatin on the expression of PPO mRNA
Experiments were conducted according to our previous reports (Yamamoto et al. 2018). Briefly, mice received cisplatin (7.5 mg/kg, i.p (intraperitoneally)) or saline (1 mL/100 g of body weight) at 17:00 and then their brains were removed at 2, 26, and 50 h after administration. Total RNA in the hypothalamus was extracted using the Total RNA Extraction System (Viogene, New Taipei, Taiwan), according to the manufacturer’s instructions. RNA was converted into first-stranded cDNA with the reverse transcriptase enzyme kit (ReverTra Ace®, TOYOBO Life Science, Osaka, Japan), which was then used as a template for the reverse transcription polymerase chain reaction (RT-PCR) with PPO and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific primers. The sequences of primers and thermal cycler (GeneAtras G02, Astec, Co., Ltd., Fukuoka, Japan) conditions for RT-PCR are listed in Table 1. PCR products were separated on 3% agarose gels (Nippon GENE, Tokyo, Japan) by electrophoresis and stained with a 1/10,000 dilution of GelGreen TM Nucleic AcidGel Stain (Biotium, Hayward, CA, USA). Gels were captured with E-graph (AE-900, ATTO, Tokyo, Japan) and their band densities were analyzed for quantification using the ATTO CS Analyzer version 3.0. There were five mice in each of the experimental groups.
Effects of an H4 receptor antagonist on cisplatin-induced expression of PPO mRNA
The method was almost identical to that for the detection of cisplatin-induced PPO mRNA, except that an H4 receptor antagonist, JNJ7777120 (10 mg/kg), was subcutaneously (s.c.) injected 5 min before and 24 and 48 h after cisplatin administration. Control animals subcutaneously received saline containing 0.5% dimethyl sulfoxide (DMSO). There were five mice in the group.
Behavioral experiment
General procedure
Experiments were conducted according to our previous reports (Yamamoto et al. 2018; Yamamoto and Yamatodani 2018). Briefly, mice were adapted to the experimental environment for at least 7 days. On the day of the experiment, mice i.p. received cisplatin (7.5 mg/kg) at 17:00. Control animals were i.p. injected with saline. Their daily food intakes for 3 days before and after receiving cisplatin were recorded and used in analysis. Food consumption was measured using an automatic measurement system for mice (FDM300SW, Melquest, Toyama, Japan). The mean food consumption for 3 days before cisplatin administration for each mouse was defined as the baseline intake, and subsequent daily consumption was expressed as the percent of the baseline intake. Since animals were not used more than once, they were sacrificed by i.p. injection of excess sodium pentobarbital (100 mg/kg) at the end of the experiment.
Effects of an OX2 receptor agonist on cisplatin-induced anorexia
Mice were s.c. injected with a nonpeptide OX2 receptor selective agonist, YNT-185 (20 or 40 mg/kg; Irukayama-Tomobe et al. 2017), 5 min before cisplatin (7.5 mg/kg) injection. YNT-185 was then administered every 24 h throughout the observation period, and daily food consumption was measured. There were five to eight mice in each experimental group. As YNT-185 was dissolved or diluted with hydrochloric acid (HCl)-acidified saline (pH 2.4), control animals received acidified saline (pH 2.4).
Effects of an H3 receptor silent antagonist on OX2 receptor agonist-induced improvement of anorexia
The experimental method was almost identical to that described in the “Effects of an OX2 receptor agonist on cisplatin-induced anorexia” section, except that a histamine H3 silent antagonist, VUF5681 (1 or 5 mg/kg, s.c.), which is considered to inhibit the constitutive activity of the H3 receptor (Moreno-Delgado et al. 2006), was injected simultaneously with YNT-185 (20 mg/kg), and the daily food consumption was measured. There were five to six mice in each experimental group. VUF5681 was dissolved or diluted with saline containing 0.5% DMSO.
Effects of an H3 receptor inverse agonist on cisplatin-induced anorexia
Mice were s.c. injected with an H3 receptor selective inverse agonist, ciproxifan (0.5, 1, or 5 mg/kg; Gbahou et al. 2003), 5 min before cisplatin (7.5 mg/kg) injection. Ciproxifan was then administered every 24 h throughout the observation period, and the daily food consumption was measured. Five to eight mice were used in each experimental group. As ciproxifan was dissolved in saline containing 0.5% DMSO, control animals received saline containing 0.5% DMSO.
Effects of orexin receptor antagonists on H4 receptor antagonist-induced improvement of anorexia
Mice were s.c. injected with both an OX2 receptor selective antagonist, JNJ10397049 (1 or 5 mg/kg, s.c.; Shoblock et al. 2011) and JNJ7777120 (10 mg/kg) 5 min before cisplatin (7.5 mg/kg) injection. JNJ10397049 and JNJ7777120 were then administered every 24 h throughout the observation period, and daily food consumption was measured. There were six to eight mice in each experimental group. JNJ10397049 was also dissolved or diluted in saline containing 0.5% DMSO. To investigate the involvement of OX1 receptors in the development of cisplatin-induced anorexia, an OX1 receptor selective antagonist, SB408124 (30 mg/kg, s.c.; Shoblock et al. 2011) was administered instead of JNJ10397049. There were five mice in each experimental group.
Effect of the combination of an H3 receptor inverse agonist and H4 receptor antagonist on cisplatin-induced anorexia
The experimental method was almost identical to that described in the “Effects of orexin receptor antagonists on H4 receptor antagonist-induced improvement of anorexia” section, except that ciproxifan (0.5 or 1 mg/kg) was used instead of JNJ10397049. There were five to eight mice in each experimental group.
Drugs
Cisplatin (Sigma-Aldrich, St. Louis, MO, USA), sodium pentobarbital (Somnopentyl®, Kyoritsu Seiyaku Corporation, Tokyo, Japan), JNJ7777120 and ciproxifan (AdooQ Bioscience LLC, Irvine, CA, USA), DMSO (Sigma-Aldrich Japan, Tokyo, Japan), YNT-185 dihydrochloride and 1 M HCl (FUJIFILM Wako Pure Chemical, Osaka, Japan), SB408124 and JNJ10397049 (Cayman Chemical, Ann Arbor, MI, USA), and VUF5681 dihydrobromide (R&D Systems, Minneapolis, MN, USA) were purchased from Katayama Chemical Industries (Osaka, Japan). Doses are expressed as the freebase.
Statistical analysis
The data on food intake are expressed as the mean value ± standard deviation (S.D.). Differences in the results were analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison tests. A P value of less than 0.05 was considered significant. All statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA).
Results
Effects of an H4 receptor antagonist on cisplatin-induced expression of PPO mRNA
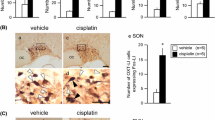
Two-way factorial ANOVA revealed significant effects of drug treatment (F(2, 36) = 7.673, P < 0.001). The administration of cisplatin significantly decreased PPO mRNA expressions in the hypothalamus at 26 h after drug administration (P < 0.05; Fig. 1). Decreased expression was maintained at 50 h after cisplatin administration (P < 0.05; Fig. 1). The administration of JNJ7777120 significantly attenuated the cisplatin-induced reduction of PPO mRNA expression in the hypothalamus (P < 0.05; Fig. 1).
The effects of cisplatin (7.5 mg/kg) on the expression of prepro-orexin (PPO) mRNA in the hypothalamus of mice. Total RNA was extracted at 2, 26, and 50 h after the administration of cisplatin. There were five mice used in each group. The number of animals in each group is indicated in parentheses. Columns and bars represent the mean ± S.D., respectively, expressed as a ratio of PPO to glyceraldehyde-3-phosphate dehydrogenase. Differences in the results were analyzed using two-way repeated measure ANOVA, followed by post-hoc Bonferroni’s test. *P < 0.05 vs. saline + vehicle. †P < 0.05, ‡P < 0.01 vs. cisplatin + vehicle
Food intake in mice during the habituation period or in control mice
During the habituation period, an individual mouse ate between 2.5 and 3.5 g of food pellets. Mice treated with an i.p. injection of saline (control mice) exhibited no change in food intake during the experimental period.
Effects of an H3 receptor silent antagonist and OX2 receptor agonist on cisplatin-induced anorexia
Two-way factorial ANOVA revealed significant effects of drug treatment (F(5, 90) = 35.78, P < 0.0001), day (F(2, 90) = 87.84, P < 0.0001), and drug treatment × day (F(10, 40) = 4.084, P < 0.0001). Hydrochloric acid acidified saline alone did not affect the food intake in mice (pre-administration: 2.77 ± 0.41 g, post-administration: 2.67 ± 0.40 g). As shown in Fig. 2, the daily administration of YNT-185 also significantly suppressed the development of anorexia on the second and third days after cisplatin injection. The inhibitory effects of YNT-185 at a dose of 20 mg/kg were superior to those of YNT-185 at a dose of 40 mg/kg. VUF5681 at a dose of 5 mg/kg alone did not affect food intake in mice (pre-administration: 3.12 ± 0.41 g, post-administration: 3.07 ± 0.44 g). We observed that the combination of VUF-5681 and YNT-185 significantly inhibited the therapeutic effects of YNT-185 on anorexia, and food intake in mice treated with VUF-5681 at 5 mg/kg was reduced to 50% of that of control mice (Fig. 2).
The effects of an OX2 receptor agonist (YNT-185) and an H3 receptor silent antagonist (VUF5681) on cisplatin-induced anorexia in mice. YNT-185 (20 or 40 mg/kg), VUF5681 (1 or 5 mg/kg), and HCl-acidified saline (pH 2.4) were subcutaneously administered 5 min before, and 24 and 48 h after the intraperitoneal administration of cisplatin (7.5 mg/kg). The mean amount of food consumed for the 3 days before cisplatin administration for each mouse was defined as the baseline intake, and subsequent daily consumption is expressed as a percent of the baseline intake. There were five to eight mice used in each group. The number of animals in each group is indicated in parentheses. Columns and bars represent the mean ± S.D. of the baseline intake. Differences in the results were analyzed using the two-way repeated measure ANOVA, followed by Bonferroni’s multiple comparison tests. *P < 0.05, ***P < 0.001 vs. saline + vehicle. †P < 0.05, ‡P < 0.01 vs. cisplatin + acidified saline. §P < 0.05, §§P < 0.01 vs. cisplatin + YNT-185 20 mg/kg
Effects of a histamine H3 receptor inverse agonist on cisplatin-induced anorexia
Two-way factorial ANOVA revealed significant effects of drug treatment (F(4, 99) = 51.36, P < 0.0001), day (F(2, 99) = 34.18, P < 0.0001), and drug treatment × day (F(8, 99) = 3.098, P < 0.01). Ciproxifan at a dose of 1 mg/kg alone did not affect the food intake in mice, but ciproxifan at a dose of 5 mg/kg alone reduced food intake by approximately 85% compared with control mice (pre-administration: 3.23 ± 0.26 g, post-administration: 2.73 ± 0.25 g). As shown in Fig. 3, the daily administration of ciproxifan at a dose of 0.5 mg/kg did not affect cisplatin-induced anorexia. On the other hand, ciproxifan at a dose of 1 mg/kg, similar to YNT-185, significantly inhibited the development of anorexia on the second and third days after cisplatin injection. We observed that their food intake returned to 70% of that of the control mice. However, sufficient therapeutic effects on anorexia were not achieved with ciproxifan at a dose of 5 mg/kg.
The effects of an H3 receptor inverse agonist (ciproxifan) on cisplatin-induced anorexia in mice. Ciproxifan (0.5, 1, and 5 mg/kg) and saline containing 0.5% dimethyl sulfoxide were subcutaneously administered 5 min before, and 24 and 48 h after the intraperitoneal administration of cisplatin (7.5 mg/kg). The mean amount of food consumed for the 3 days before cisplatin administration for each mouse was defined as the baseline intake, and subsequent daily consumption is expressed as the percent of the baseline intake. There were five to eight mice used in each group. Columns and bars represent the mean ± S.D. of the baseline intake. The number of animals in each group is indicated in parentheses. The data were analyzed for significant differences using two-way repeated measures ANOVA followed by Bonferroni’s multiple comparison tests. **P < 0.01, ***P < 0.001 vs. saline + vehicle. †P < 0.05, ‡P < 0.01 vs. cisplatin + vehicle
Effects of orexin receptor antagonist on H4 receptor antagonist-induced improvement of anorexia
Two-way factorial ANOVA revealed significant effects of drug treatment (F(3, 78) = 46.76, P < 0.0001), day (F(2, 78) = 16.65, P < 0.0001), and drug treatment × day (F(6, 78) = 2.649, P < 0.05). JNJ10397049 at a dose of 5 mg/kg alone slightly decreased food intake in mice, but there was no significant difference among the control mice (pre-administration: 3.05 ± 0.59 g, post-administration: 2.81 ± 0.53 g). The combination of JNJ10397049 and JNJ7777120 reduced the therapeutic effects of JNJ7777120 on cisplatin-induced anorexia on the second and third days after cisplatin injection (Fig. 4). SB408124 at a dose of 30 mg/kg did not affect food intake in mice (pre-administration: 3.36 ± 0.39 g, post-administration: 3.25 ± 0.30 g). The combination of SB408124 at a dose of 30 mg/kg and JNJ7777120 slightly reduced the therapeutic effects of JNJ7777120 on cisplatin-induced anorexia, but there was no significant difference among the control mice (Fig. 4).
The effects of an OX1 receptor antagonist (SB408124) or OX2 receptor antagonist (JNJ10397049) on H4 receptor antagonist-induced improvement of anorexia in mice. SB408124 (30 mg/kg) or JNJ10397049 (1 and 5 mg/kg) and JNJ7777120 (10 mg/kg) were subcutaneously administered 5 min before, and 24 and 48 h after the intraperitoneal administration of cisplatin (7.5 mg/kg). The mean amount of food consumed for the 3 days before cisplatin administration for each mouse was defined as the baseline intake, and subsequent daily consumption is expressed as the percent of the baseline intake. There were six to eight mice used in each group. Columns and bars represent the mean ± S.D. of the baseline intake. The number of animals in each group is indicated in parentheses. Differences in the results were analyzed using two-way repeated measure ANOVA, followed by Bonferroni’s multiple comparison tests. *P < 0.05, **P < 0.01, ***P < 0.001 vs. saline + vehicle. †P < 0.05, ‡P < 0.01 vs. cisplatin + vehicle. §P < 0.05 vs. cisplatin + JNJ7777120 10 mg/kg
Effects of H3 receptor ligands on H4 receptor antagonist-induced improvement of anorexia
Two-way factorial ANOVA revealed significant effects of drug treatment (F(5, 111) = 47.31, P < 0.0001), day (F(2, 111) = 18.39, P < 0.0001), and drug treatment × day (F(10, 111) = 1.821, P < 0.05). Cisplatin-induced anorexia was significantly suppressed by the combination of JNJ7777120 plus ciproxifan at a dose of 0.5 mg/kg. Its inhibitory effect was superior to that of only JNJ7777120, and the amount of food intake returned to the control level during the experimental periods; however, the combination of JNJ7777120 plus ciproxifan at a dose of 1 mg/kg significantly reduced the therapeutic effects on cisplatin-induced anorexia (Fig. 5).
The effects of an H3 receptor inverse agonist (ciproxifan) on H4 receptor antagonist-induced improvement of anorexia in mice. Ciproxifan (0.5 or 1 mg/kg) and JNJ7777120 (10 mg/kg) were subcutaneously administered 5 min before, and 24 and 48 h after the intraperitoneal administration of cisplatin (7.5 mg/kg). The mean amount of food consumed for the 3 days before cisplatin administration for each mouse was defined as the baseline intake, and subsequent daily consumption is expressed as the percent of the baseline intake. There were five to eight mice used in each group. Columns and bars represent the mean ± S.D. of the baseline intake. The number of animals in each group is indicated in parentheses. Differences in the results were analyzed using two-way repeated measure ANOVA, followed by Bonferroni’s multiple comparison tests. *P < 0.05, **P < 0.01, ***P < 0.001 vs. saline + vehicle. †P < 0.05, ‡P < 0.01 vs. cisplatin + vehicle. §P < 0.05, §§P < 0.01 vs. cisplatin + JNJ7777120 10 mg/kg
Discussion
Orexin, which is synthesized by neurons located mainly in the perifornical area of the posterolateral hypothalamus, is a neuropeptide that regulates arousal, wakefulness, and appetite (Tsujino and Sakurai 2013; Sakurai 2014). Weymann et al. (2014) reported that chemotherapeutic agents induce the suppression of hypothalamic orexin neuron activity, and the intracerebroventricular administration of orexin inhibits chemotherapeutic agent-induced fatigue-like behavior in rats. Furthermore, Guo et al. (2018) recently reported that cisplatin inhibited mRNA expression of PPO and that orexin-A, one of the endogenous agonists of orexin receptors, improves cisplatin-induced anorexia and gastric motility in rats. In the present study, similar to Guo’s results, we confirmed that cisplatin induced the decrease of PPO mRNA expression in the hypothalamus of mice. In addition, we found that treatment with JNJ7777120 significantly inhibited the decreased cisplatin-induced PPO mRNA expression in the hypothalamus. The decrease in the period of PPO mRNA expression was comparable to the onset period of anorexia; thus, we considered that deterioration of the orexinergic system is also a mediator of symptom development.
Previous studies reported the characterization of two types of orexin receptors: the OX1 receptor and OX2 receptor (Tsujino and Sakurai 2013). It has remained unclear which orexin receptor is involved in the onset of cisplatin-induced anorexia based on Guo’s results, because orexin-A is known to bind to both OX1 and OX2 receptors with a high affinity (Tsujino and Sakurai 2013). Histaminergic neurons are localized to the tuberomammillary nucleus (TMN) of the posterior hypothalamus, and send extensive projections throughout the central nervous system (Wada et al. 1991). Although OX1 and OX2 receptors are also widely distributed throughout the central nervous system, the TMN predominantly expresses OX2 receptors rather than OX1 receptors (Eriksson et al. 2011). Mieda et al. (2013) reported that orexins excite TMN neurons mainly via the OX2 receptor and enhance histamine release from the TMN. In the present study, we found that an OX2 receptor selective agonist, YNT-185, significantly inhibited cisplatin-induced anorexia in mice (Fig. 2), and the therapeutic effects of YNT-185 were abolished by the H3 receptor silent antagonist VUF5681 (Fig. 2). It was previously reported that YNT-185, which is peripherally administered, penetrates the blood brain barrier and significantly induces wakefulness in mice, and that YNT-185 affects the firing rate of histaminergic neurons (Irukayama-Tomobe et al. 2017; Takenoshita et al. 2018). Nagahara et al. (2015) reported that this agent shows higher potency (EC50 for OX2 receptor is 28 nM) and selectivity for the OX2 receptor than OX1 receptor (binding affinity to OX2 receptor is 100-fold higher than that to OX1 receptor). Pretreatment with an OX2 receptor antagonist rather than OX1 receptor antagonist partially inhibited the therapeutic effects of the H4 receptor antagonist against cisplatin-induced anorexia in mice (Fig. 4); therefore, from these observations, we next hypothesized that enhancement of the histaminergic system by H3 receptor inverse agonists has therapeutic effects on cisplatin-induced anorexia. As shown in Fig. 3, we actually observed that the single administration of ciproxifan at a dose of 1 mg/kg significantly inhibited cisplatin-induced anorexia. The therapeutic effects on cisplatin-induced anorexia were not observed even with the administration of the other H3 receptor ligands immethridine (an agonist) and VUF5681 (a silent antagonist) (please see Appendix data). Furthermore, we observed that the daily administration of JNJ7777120 plus ciproxifan completely inhibited cisplatin-induced anorexia throughout the experimental period. We recently reported that cisplatin significantly increased TNF-α mRNA expression in the hypothalamus, the period of expression increase paralleled the onset period of cisplatin-induced anorexia, and pretreatment with JNJ7777120 completely inhibited the increased expression (Yamamoto et al. 2018). Zhan et al. (2011) reported that TNF-α inhibits PPO mRNA and OX2 receptor mRNA expression. From these facts, we consider that the pathway for both H4 receptor-mediated production of TNF-α and inhibition of the orexinergic system in the brain induces inhibition of the histaminergic system via the OX2 receptor, which initiates the development of cisplatin-induced anorexia in mice (Fig. 6a), and that activation of not only the orexinergic pathway but also the histaminergic pathway is involved in the therapeutic effect of a histamine H4 receptor antagonist against cisplatin-induced anorexia (Fig. 6b–d). It is known that a number of ligands of the H4 receptor have a high affinity for the H3 receptor because this receptor exhibits the highest sequence homology with the H3 receptor (Liu et al. 2001). Recent studies have revealed that H3 and H4 receptors are involved in anti-cancer activity, such as the inhibition of angiogenesis and tumor invasion, and induction of apoptosis (Tanaka et al. 2016; Chen and Hu 2018; Sterle et al. 2018). Thus, we considered that ligands which exhibit H4 receptor antagonistic activity together with H3 receptor inverse agonistic activity serve as more powerful therapeutic agents for the treatment of not only chemotherapy-induced toxicity but also the cancer itself.
A hypothetical diagram of possible mechanisms of the therapeutic effect of the H4 receptor antagonist against cisplatin-induced anorexia. a Inhibition of the orexinergic and histaminergic systems by TNF-α production via H4 receptors may induce cisplatin-induced anorexia; therefore, b H4 receptor antagonists, c OX2 receptor antagonists, and d H3 receptor inverse agonists may be potentially useful treatments. On the contrary, since a higher dose of e the OX2 receptor antagonist and f the H3 receptor inverse agonist may excessively activate the histaminergic system, they may suppress feeding behavior via activating H1 receptors in the central nervous system. For these reasons, we may need to be more careful regarding the activities of orexinergic and histaminergic systems
In our study, although there was no significant change, we found that treatment with immethridine or VUF5681 alone slightly inhibited cisplatin-induced anorexia (Appendix data). The therapeutic applications of H3 receptor agonists for inflammation have been considered (Kitbunnadaj et al. 2003), and Shi et al. (2017) demonstrated that immethridine actually inhibited the expression profiles of TNF-α in dendritic cells. Baker (2008) reported that VUF5681 acts as a partial agonist of the H3 receptor. Thus, we need to consider the therapeutic potential of H3 receptor agonists that act as anti-inflammatory drugs to treat cisplatin-induced anorexia.
However, we did not observe a dose-response relationship with the therapeutic effects of ciproxifan or YNT-185 in this study. We also confirmed that the administration of JNJ7777120 plus a higher dose of ciproxifan did not affect cisplatin-induced anorexia. Histamine and histamine H1 receptors in the brain are known as essential for regulating the circadian rhythm of feeding behavior. Although activating H1 receptors in the central nervous system of rodents suppressed food intake, the administration of H1 receptor antagonists or decrease in the amount of central histamine by α-fluoromethylhistidine, an irreversible histidine decarboxylase inhibitor, increased food consumption and body weight (Ookuma et al. 1993; Morimoto et al. 2001). Gbahou et al. (2003) reported that ciproxifan increased the release of endogenous histamine in the central nervous system of mice. Based on these observations, excessive activation of the histaminergic system in the central nervous system by OX2 receptor agonists or H3 receptor inverse agonists may reduce feeding behavior via H1 receptors (Fig. 6e, f). Further investigations are needed to identify the most suitable dose, injection route, and injection times of H3 receptor ligands in order to inhibit cisplatin-induced anorexia.
Alhadeff et al. (2017) reported that activating the central nucleus of amygdala glutamate receptor signaling via parabrachial neurons (PBN) mediates cisplatin-induced anorexia and body weight loss. Previous reports demonstrated that immunoreactive fibers of histaminergic neurons are present in the lateral PBN, and that lateral glutamatergic PBN neurons regulate orexin-containing neurons in the hypothalamus (Panula et al. 1989; Niu et al. 2010); thus, activation of the lateral PBN is also involved in the maintenance of anorexia. Further investigations are needed to elucidate mechanisms of histaminergic and orexinergic systems in the central nervous system underlying cisplatin-induced anorexia in mice.
Cancer and its treatments are known to adversely affect patients’ sleep-wake cycle and change their typical sleep patterns. Liu and Ancoli-Israel (2008) reported that sleep disturbances were present in 30 to 75% of newly diagnosed or recently treated cancer patients. Gbahou et al. (2003) and Irukayama-Tomobe et al. (2017) reported that ciproxifan and YNT-185 induced a state of arousal in mice by altering the sleep-wake cycle. Additional studies are required for the elucidation of associations between sleep disorder and the development cisplatin-induced anorexia using this animal model.
In summary, this is the first report that activation of the orexinergic and histaminergic pathway is involved in the therapeutic effect of an H4 receptor antagonist against cisplatin-induced anorexia. We considered that ligands with H4 receptor antagonistic activity together with H3 receptor inverse agonistic activity can serve as more useful treatments for cisplatin-induced anorexia.
Abbreviations
- ANOVA:
-
Analysis of variance
- DMSO:
-
Dimethyl sulfoxide
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- HCl:
-
Hydrochloric acid
- i.p.:
-
Intraperitoneally
- PBN:
-
Parabrachial neurons
- PPO:
-
Prepro-orexin
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- s.c.:
-
Subcutaneously
- S.D.:
-
Standard deviation
- TMN:
-
Tuberomammillary nucleus
- TNF-α:
-
Tumor necrosis factor-alpha
References
Alhadeff AL, Holland RA, Zheng H, Rinaman L, Grill HJ, De Jonghe BC (2017) Excitatory hindbrain-forebrain communication is required for cisplatin-induced anorexia and weight loss. J Neurosci 37:362–370
Baker JG (2008) Antagonist affinity measurements at the Gi-coupled human histamine H3 receptor expressed in CHO cells. BMC Pharmacol 8:9
Chen J, Hu XY (2018) Inhibition of histamine receptor H3R suppresses prostate cancer growth, invasion and increases apoptosis via the AR pathway. Oncol Lett 16:4921–4928
Eriksson KS, Sergeeva O, Brown RE, Haas HL (2011) Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci 21:9273–9279
Gbahou F, Rouleau A, Morisset S, Parmentier R, Crochet S, Lin JS, Ligneau X, Tardivel-Lacombe J, Stark H, Schunack W, Ganellin CR, Schwartz JC, Arrang JM (2003) Protean agonism at histamine H3 receptors in vitro and in vivo. Proc Natl Acad Sci U S A 100:11086–11091
Guo F, Xu L, Gao S, Sun X, Zhang N, Gong Y (2018) Effect of orexin-A in the arcuate nucleus on cisplatin-induced gastric side effects in rats. Neurosci Res S0168-0102(18):30128–30127
Irukayama-Tomobe Y, Ogawa Y, Tominaga H, Ishikawa Y, Hosokawa N, Ambai S, Kawabe Y, Uchida S, Nakajima R, Saitoh T, Kanda T, Vogt K, Sakurai T, Nagase H, Yanagisawa M (2017) Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models. Proc Natl Acad Sci U S A 114:5731–5736
Kitbunnadaj R, Zuiderveld OP, De Esch IJ, Vollinga RC, Bakker R, Lutz M, Spek AL, Cavoy E, Deltent MF, Menge WM, Timmerman H, Leurs R (2003) Synthesis and structure-activity relationships of conformationally constrained histamine H3 receptor agonists. J Med Chem 46:5445–5457
Liu L, Ancoli-Israel S (2008) Sleep disturbances in cancer. Psychiatr Ann 38:627–634
Liu C, Ma XJ, Jiang X, Wilson SJ, Hofstra CL, Blevitt J, Pyati J, Li X, Chai W, Carruthers N, Lovenberg TW (2001) Cloning and pharmacological characterization of a fourth histamine receptor (H4) expressed in bone marrow. Mol Pharmacol 59:420–426
Mieda M, Tsujino N, Sakurai T (2013) Differential roles of orexin receptors in the regulation of sleep/wakefulness. Front Endocrinol (Lausanne) 4:57
Moreno-Delgado D, Torrent A, Gómez-Ramírez J, de Esch I, Blanco I, Ortiz J (2006) Constitutive activity of H3 autoreceptors modulates histamine synthesis in rat brain through the cAMP/PKA pathway. Neuropharmacology 51:517–523
Morimoto T, Yamamoto Y, Yamatodani A (2001) Brain histamine and feeding behavior. Behav Brain Res 124:145–150
Morisset S, Rouleau A, Ligneau X, Gbahou F, Tardivel-Lacombe J, Stark H, Schunack W, Ganellin CR, Schwartz JC, Arrang JM (2000) High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature 408:860–864
Nagahara T, Saitoh T, Kutsumura N, Irukayama-Tomobe Y, Ogawa Y, Kuroda D, Gouda H, Kumagai H, Fujii H, Yanagisawa M, Nagase H (2015) Design and synthesis of non-peptide, selective orexin receptor 2 agonists. J Med Chem 58:7931–7937
Niu JG, Yokota S, Tsumori T, Qin Y, Yasui Y (2010) Glutamatergic lateral parabrachial neurons innervate orexin-containing hypothalamic neurons in the rat. Brain Res 1358:110–122
Ookuma K, Sakata T, Fukagawa K, Yoshimatsu H, Kurokawa M, Machidori H, Fujimoto K (1993) Neuronal histamine in the hypothalamus suppresses food intake in rats. Brain Res 628:235–242
Panula P, Pirvola U, Auvinen S, Airaksinen MS (1989) Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience 28:585–610
Plata-Salamán CR (1991) Dexamethasone inhibits food intake suppression induced by low doses of interleukin-1 beta administered intracerebroventricularly. Brain Res Bull 27:737–738
Sakurai T (2014) Roles of orexins in the regulation of body weight homeostasis. Obes Res Clin Pract 8:e414–e420
Shi Y, Li Z, Chen R, Zhang J, Hu X, He C, Su Q, Ma H, Ren H, Qian M, Cui S, Jiang W (2017) Immethridine, histamine H3-receptor (H3R) agonist, alleviated experimental autoimmune encephalomyelitis via inhibiting the function of dendritic cells. Oncotarget 8:75038–75049
Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, Palmer J, Bonaventure P, Carruthers NI, Lovenberg TW, Boggs J, Galici R (2011) Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology 215:191–203
Smith LB, Leo MC, Anderson C, Wright TJ, Weymann KB, Wood LJ (2014) The role of IL-1β and TNF-α signaling in the genesis of cancer treatment related symptoms (CTRS): a study using cytokine receptor-deficient mice. Brain Behav Immun 38:66–76
Sterle HA, Nicoud MB, Massari NA, Táquez Delgado MA, Herrero Ducloux MV, Cremaschi GA, Medina VA (2018) Immunomodulatory role of histamine H4 receptor in breast cancer. Br J Cancer 120:128–138. https://doi.org/10.1038/s41416-018-0173-z. [Epub ahead of print]
Taguchi K, Iihara H, Ishihara M, Komori Y, Tanizawa K, Matsuura K, Itoh Y (2009) Comparison of antiemetic efficacy between single and repeated treatments with a 5-HT3 receptor antagonist in breast cancer patients with high-risk emetogenic chemotherapy. Anticancer Res 29:1721–1725
Takenoshita S, Sakai N, Chiba Y, Matsumura M, Yamaguchi M, Nishino S (2018) An overview of hypocretin based therapy in narcolepsy. Expert Opin Investig Drugs 27:389–406
Tanaka S, Sakaguchi M, Yoneyama H, Usami Y, Harusawa S (2016) Histamine H3 receptor antagonist OUP-186 attenuates the proliferation of cultured human breast cancer cell lines. Biochem Biophys Res Commun 480:479–485
Tsujino N, Sakurai T (2013) Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci 7:28
van der Werf JF, Timmerman H (1989) The histamine H3 receptor: a general presynaptic histaminergic regulatory system? Trends Pharmacol Sci 10:159–162
Wada H, Inagaki N, Yamatodani A, Watanabe T (1991) Is the histaminergic neuron system a regulatory center for whole-brain activity? Trends Neurosci 14:415–418
Weymann KB, Wood LJ, Zhu X, Marks DL (2014) A role for orexin in cytotoxic chemotherapy-induced fatigue. Brain Behav Immun 37:84–94
Wood LJ, Weymann K (2013) Inflammation and neural signaling: etiologic mechanisms of the cancer treatment-related symptom cluster. Curr Opin Support Palliat Care 7:54–59
Yamamoto K, Yamatodani A (2018) Strain differences in the development of cisplatin-induced pica behavior in mice. J Pharmacol Toxicol Methods 91:66–71
Yamamoto K, Okui R, Yamatodani A (2018) Effects of a histamine H4 receptor antagonist on cisplatin-induced anorexia in mice. Neurosci Lett 676:103–107
Zhan S, Cai GQ, Zheng A, Wang Y, Jia J, Fang H, Yang Y, Hu M, Ding Q (2011) Tumor necrosis factor-alpha regulates the hypocretin system via mRNA degradation and ubiquitination. Biochim Biophys Acta 1812:565–571
Zhou T, Yang K, Thapa S, Liu H, Wang B, Yu S (2017) Differences in symptom burden among cancer patients with different stages of cachexia. J Pain Symptom Manag 53:919–926
Funding
This study was partially supported by JSPS KAKENHI Grant Number JP17K08951 and the Drug Discovery Science Division, Open and Transdisciplinary Research Initiatives, Osaka University.
Author information
Authors and Affiliations
Contributions
KY and AY conceived and designed the research. KY and RO conducted the experiments and analyzed the data. KY wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix data: effects of histamine H3 receptor agonist or silent antagonist on cisplatin-induced anorexia
Appendix data: effects of histamine H3 receptor agonist or silent antagonist on cisplatin-induced anorexia
The experimental method was almost identical to that described in the “Materials and methods” section (“Effects of an H3 receptor inverse agonist on cisplatin-induced anorexia” section). The DBA/2 mice were housed in an automatic kaolin and food intake-monitoring system (FDM300SW). On the day of the experiment, mice were subcutaneously (s.c.) injected with an H3 receptor selective agonist, immethridine (5 mg/kg), or H3 receptor selective silent antagonist, VUF5681 (5 mg/kg), 5 min before cisplatin (7.5 mg/kg) injection. Immethridine or VUF5681 was then administered every 24 h throughout the observation period, and the daily food consumption was measured. Five to eight mice were used in each experimental group. As both H3 receptor ligands were dissolved in saline containing 0.5% DMSO, control animals received saline containing 0.5% DMSO. Immethridine dihydrobromide and VUF5681 dihydrobromide (R&D Systems, Minneapolis, MN, USA) were purchased from Katayama Chemical Industries (Osaka, Japan). Doses are expressed as the freebase. Columns and bars represent the mean ± S.D. of the baseline intake. The number of animals in each group is indicated in parentheses. Differences in the results were analyzed using the two-way repeated measures ANOVA, followed by Bonferroni’s multiple comparison tests. *P < 0.05, ***P < 0.001 vs. saline + vehicle. As shown in the Appendix data, the administration of immethridine or VUF5681 did not change cisplatin-induced anorexia in mice during the observation period.

Rights and permissions
About this article
Cite this article
Yamamoto, K., Okui, R. & Yamatodani, A. Activation of orexinergic and histaminergic pathway involved in therapeutic effect of histamine H4 receptor antagonist against cisplatin-induced anorexia in mice. Naunyn-Schmiedeberg's Arch Pharmacol 392, 925–936 (2019). https://doi.org/10.1007/s00210-019-01646-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01646-x