Abstract
The aim of this study was to evaluate whether the sub-chronic systemic ethanol exposure has direct effect on cavernosal smooth muscle contractions induced by KCl (depolarizing) and phenylephrine (α1-receptor agonist), and the possible involvement of RhoA/Rho-kinase pathway. Sub-chronic systemic ethanol was applied to mice with inhalation route for 14 days. The blood levels in ethanol-treated mice averaged 121.2 ± 9.1 mg/dl. KCl (80 mM) and phenylephrine (10 nM–100 μM) induced sustained contractions in corpus corporal strips from sham-treated mice. Sub-chronic ethanol treatment reduced the contractions to KCl. However, phenylephrine-induced contractions were not affected by ethanol treatment. Rho-kinase inhibitor fasudil (50 μM) and Y-27632 (50 μM) inhibited contractions to KCl and phenylephrine in sham-treated mice. Ethanol treatment increased the inhibitory effect of Rho-kinase inhibitors on contractions to phenylephrine. The relaxations induced by fasudil (100 μM) and Y-27632 (500 μM) did not change in ethanol treatment group. In ethanol-treated group, the expression of RhoA decreased compared to sham-treated group. Also, ROCK1 expression was reduced by ethanol but not statically significant to sham-treated group; however, the expression of ROCK2 increased in ethanol group. From these findings, it seems that phenylephrine and KCl-induced contractions depends on RhoA/Rho-kinase-mediated Ca2+ sensitization. Also, these results suggest that the ethanol treatment decreased the expression of RhoA, and the inhibitory effect of ethanol on KCl-induced contractions may be due to, at least in part, the inhibition of a RhoA/Rho-kinase in mouse corpus cavernosum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Penile erection is a complex neurovascular process of the corpus cavernosum (CC) smooth muscle (Burnett 2006), and relaxation of corporal smooth muscle is essential for normal erectile function (Andersson 2001). With regard to the mechanisms of penile erection, the cavernous trabecular smooth muscle is considered an extension of the systemic vasculature. Therefore, risk factors known to affect the vascular system may also impair of the cavernous erectile tissue. Chronic ethanol consumption is considered as a risk factor for erectile dysfunction (ED). ED is one of the common complications of chronic ethanol consumption (Peugh and Belenko 2001). Van Thiel and Lester (1979) have indicated that 61 % of patients with alcohol dependence reported sexual dysfunction, the most common being ED followed by ejaculation incompetence and reduced sexual desire. Findings from experimental research show that modest ethanol doses can both increase sexual drive and decrease erectile capacity in men (Caspari et al. 1999). Since ethanol has vasodilator effect and suppression of anxiety, small amounts of ethanol was reported to improve erection and to enhance libido; however, excessive intake of ethanol can cause erectile problems, impairing penile tumescence and reducing sexual performance (Miller and Gold 1988). There is little evidence about the background of impairment effect of ethanol on contractions of corpus cavernosal tissue. In rabbit corporal cavernosal tissue, chronic ethanol administration increased contraction to phenylephrine and KCl (Saito et al. 1994). The reduced relaxation to acetylcholine might suggest that ethanol induces endothelial dysfunction in mouse and rat CC (Aydinoglu et al. 2008; Lizarte et al. 2009). Supporting these data, the impaired carbachol- and electrical field stimulation-induced relaxant responses were observed in high chronic ethanol-fed groups of rabbit CC (Yazir et al. 2012). Furthermore, heavy ethanol consumption increased plasma endothelin-1(ET-1) levels and the contractions induced by the peptide in rat CC, suggesting ET-1 could play a role in the pathogenesis of ED associated with chronic ethanol consumption (Leite et al. 2013). On the other hand, Kim et al. (2013) suggested that long-term ethanol consumption may preserve the erectile function in type 2 diabetes mellitus through the downregulation of the RhoA/Rho-kinase pathway.
Recently, Muniz et al. (2015) revealed that chronic ethanol consumption induced ED and increased the contraction to ET-1 by a mechanism involving the increased activation of the RhoA/Rho-kinase (ROK; ROCK) pathway on rat CC. The small GTPase RhoA and one of its downstream effectors, Rho-kinase, have been shown to play important roles in Ca2+-sensitization that can modulate smooth muscle contractility independently of intracellular Ca2+ concentration [Ca2+]i (Kitazawa et al. 1989; Somlyo and Somlyo 2000; Fukata et al. 2001). The potential role of RhoA/Rho-kinase pathway on modulating the contraction of penile vascular and nonvascular smooth muscle has been addressed by various groups using different experimental approaches (Jin and Burnett 2006; Chitaley et al. 2003). Mills et al. (2001) reported that the RhoA/Rho-kinase pathway plays an important role in the maintenance of the vasoconstrictive state of the cavernosal vasculature, and the vasoconstrictor effects of α-adrenergic stimulation is mediated by the Rho-kinase pathway in rats. Recently, Rho-kinase is expressed in human CC (Waldkirch et al. 2012), and RhoA/Rho-kinase signaling pathway is involved in the noradrenergic contractile response in human cavernosal smooth muscle (Gur et al. 2012). There is inadequate and contradictory evidence about the influence of systemic ethanol consumption on corpus cavernosal contractility and RhoA/Rho-kinase pathway. In this study, we investigated whether the sub-chronic systemic ethanol exposure has effect on mouse cavernosal smooth muscle contractions induced by KCl (depolarizing) and phenylephrine (α1-receptor agonist), and the possible involvement of RhoA/Rho-kinase pathway in mouse CC strips.
Materials and methods
Experimental animals
Male Swiss albino mice weighing 20–25 g were obtained from Çukurova University Experimental Research and Application Center of Medical Science (DETAUM). Mice were located in Plexiglas cages and kept under environmental conditions (12-h light/darkness cycles) and allowed free access to food and water. Protocols were approved by local the Ethic Committee of the University of Çukurova. This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23 revised 1996). Two groups of animals were used as ethanol-treated mice and sham-treated mice.
Ethanol treatment
For exposure via the inhalation route, we used a method described by “Goldstein (Goldstein and Pal 1971)”. Mice were put in an inhalation chamber and continuously exposed to ethanol vapor by inhalation for 14 days. The inhalation chamber consisted of Plexiglas box (7.5 L) through which ethanol vapor continuously passed. The 95 % ethanol was delivered to filter paper at a rate of 0.0035 ml/min by an infusion pump. The sham-treated group was not exposed to ethanol vapor but only placed in air vapor chambers under conditions similar to those of the ethanol. At the end of the 2 weeks, sham-treated and ethanol-treated mice were removed from the chamber. In our previous study, increased blood ethanol level was detected by this model (Aydinoglu et al. 2008; Aydinoglu et al. 2015). Blood ethanol levels were monitored throughout the experiments and showed elevated levels of alcohol in the ethanol groups of mice. As previously reported, no significant weight loss or nutritional disturbances were observed in mice treated with ethanol.
Tissue preparations
Male mice were killed by cervical dislocation. The penises from both sham-treated and ethanol-treated mice were removed and placed in a Petri dish containing (composition in mM) NaCl 119, KCl 4.6, CaCl2 1.5, MgCl2 1.2, NaHCO3 15, NaPO4 1.2, and glucose 11. The glans penis and urethra were excised and fibrous septum between two corpus cavernosum strips were cut and each corpus cavernosum (0.3 × 0.3 × 4 mm) was carefully dissected from the adherent tissues, keeping the tunica albuginea intact. Cavernosal strips were mounted under 0.2 g tension in organ bath (10 ml) containing Krebs solution. The bath medium was maintained at 37 °C and gassed with 5 % CO2 and 95 % O2. The tissue strips were allowed to equilibrate for a period of 60 min during equilibration; the bath solution was replaced every 15 min. The responses were recorded with isotonic transducer (Ugo Basile, 7006) on a recorder (Ugo Basile Gemini, 7070).
Functional studies
To study the effects of ethanol, depolarizing (KCl)- and agonist (phenylephrine, α1-receptor agonist)- induced contractions were obtained in isolated cavernosal strips from sham-treated and ethanol-treated mice. 80 mM KCl-induced contractions were produced in CC isolated from both groups. In this set of experiments, following to equilibration, period tissues were pre-contracted with phenylephrine (5 μM) to calculate to the percentage of contractile response to KCl. The tissue was then washed out with Krebs solution and strips were left to re-equilibration for 30 min. After this period, KCl-induced contractions were obtained.
In another series of experiments, phenylephrine-induced contractions were investigated. Following to equilibration period of 60 min, cavernosal strips were exposed to 80 mM KCl in order to calculate the percentage of contractile response to phenylephrine. The tissues were then washed out with Krebs solution and the strips were left to re-equilibration for 30 min. After this period, cumulative phenylephrine (10 nM–100 μM) concentration-response curve was obtained. A plateau response was obtained after the addition of each test, the phenylephrine concentration before the addition of a subsequent dose. Only one phenylephrine concentration-response curve or 80 mM KCl was studied one each tissue. KCl/phenylephrine response data have been normalized separately for control and ethanol groups in the absence of ROCK inhibitors.
To investigate the involvement of Rho-kinase in contractions induced by KCl and phenylephrine in isolated cavernosal strips from both of sham-treated and ethanol-treated mice, contractile responses to KCl (80 mM) and phenylephrine (10 nM–100 μM) were constructed in the presence of two different Rho-kinase inhibitors fasudil and (R)-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide (Y-27632). In this set of experiments, tissues were incubated with fasudil (50 μM) or Y-27632 (50 μM) for 30 min and then responses to phenylephrine or KCl were obtained. The experiments in the absence or presence of Rho-kinase inhibitors were done sequentially within a single muscle strip. The addition of Rho-kinase inhibitors did not affect the basal tonus of the cavernosal strips.
In addition, the relaxant responses to Rho-kinase inhibitors were studied on the isolated cavernosal strips from both of sham-treated and ethanol-treated mice. In this series, contractile responses to KCl (80 mM) or phenylephrine (10 nM–100 μM) were obtained, and then fasudil (100 μM) or Y-27632 (500 μM) was added to organ baths.
Expression of RhoA, ROCK1, and ROCK2
RhoA, ROCK1, and ROCK2 protein expression were determined by western blot analysis of cavernosal tissues isolated from both of sham-treated and ethanol-treated mice. The frozen tissues were homogenized in ice cold RIPA buffer system (sc-24948) and total protein content determinated by Bradford method. Proteins from cavernosal tissues were obtained and boiled the presence of 1× Laemmli gel loading buffer containing SDS and β-mercaptoethanol as reducing agent at pH 6.8 and kept at −20 °C until use. Proteins were separated in a 12 % SDS-PAGE gel containing 4 % of stacking gel, under denaturing conditions at 100 V for 1 h and 45 min at room temperature. Proteins in the gel were then transferred to a PVDF membrane (Bio-Rad) which was previously rehydrated in methanol and equilibrated with transfer buffer. Then, a sandwich cassette was prepared according to the manufacturer’s instructions (Bio-Rad), and proteins were electro-blotted onto the PVDF membrane for 1 h and 45 min at 4 °C. After transfer, the membrane was briefly washed with phosphate-buffered saline (PBS) or tris-buffered saline (TBS) containing 1 % Tween-20. Bovine serum albumin (BSA) or skim milk at concentrations varying from 5 to 10 % was used in the wash buffer as blocking agents. Membranes were blocked for 1 h at room temperature with gentle and constant agitation and incubated with the following primary antibodies from Cell Signaling Technology; RhoA (dilution 1:1000, CST-2117), β-actin (dilution 1:1000, CST-4970), Rho-kinase 1 (1:1000, CST-4035), and Rho-kinase 2 (1:1000, CST-8236) in blocking buffer in dilution 1:1000, overnight at 4 °C. The membranes were washed three times for 10 min each with washing buffer and incubated with an appropriate HRP-conjugated secondary antibody in dilution of 1:2000 at room temperature for 1 h with constant agitation. After briefly drying, the membrane was incubated with 3 ml of HRP ECL substrate mixture (1.5 ml hydrogen peroxide and 1.5 ml enhancer) (Pierce) and incubated for 1 min at room temperature. The membranes were wrapped with stretch film and placed in the X-ray cassette and exposed to an X-ray film (KODAK) for 1 to 10 min in a dark room. The films were scanned and quantified using the ImageJ program.
Drugs and solutions
The following drugs were used: fasudil hydrochloride, Y-27632 dihydrochloride ([(R)-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide supplied from Tocris), phenylephrine hydrochloride (Sigma Chemical Co., St. Louis, MO, USA), potassium chloride (Merck, Darmstadt, Germany), and ethanol (SISMED Medical Limited Co.). All drugs were dissolved in distilled water.
Data analysis
Data are expressed as the mean ± SD from n experiments. Contractions were recorded as changes in the displacement (mm) from baseline and expressed as a percentage of peak contractions induced by KCl (80 mM) and phenylephrine (5 μM) as measured prior to addition of inhibitor in sham-treated and ethanol-treated mice. The absolute value of the response to 80 mM KCl and phenylephrine was 25.5 ± 10 and 34.2 ± 5 for control and 16.8 ± 6.9 and 34.5 ± 5 for ethanol groups, respectively. Relaxations were expressed as percentage change from the KCl and phenylephrine-contracted level in sham- and ethanol-treated mice. Agonist concentration-response curves were fitted using a non-linear interactive fitting program (Graphpad Prism®, version 5). The sensitivity to the agonist was expressed as pD2 (negative logarithm of the agonist concentration required for half-maximum response) and Emax (maximum effect elicited by the agonist). Statistically significant differences between sham-treated and ethanol-treated strips were calculated by analysis of variance (ANOVA) and t test corrected for multiple comparisons (Bonferroni corrections). P values less than 0.05 were considered as significant.
Results
Body weight and blood ethanol levels
The body weights of the mice prior to treatment averaged 29.75 ± 2.6 g in the sham-treated group (control) and 29.50 ± 1.9 g in the ethanol group. After 2 weeks, no differences were not found in body weights between control and ethanol groups (control 37.25 ± 5.7 g and ethanol 35.76 ± 2.6 g). The blood levels in ethanol-treated mice averaged 121.2 ± 9.1 mg/dl (∼26 mM, n = 6). No ethanol was detected in the blood of control mice.
Effect of sub-chronic ethanol treatment on KCl- and phenylephrine-induced contractions in mouse CC
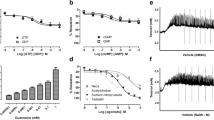
The depolarizing (KCl)- and agonist (phenylephrine, α1-receptor agonist)-induced contractions were obtained in isolated CC strips from control. KCl (80 mM) produced sustained contraction in corpus cavernous smooth muscle from control group (Fig. 1a). The response to KCl (80 mM) was 76.41 ± 10.5 % in control group. Ethanol treatment significantly reduced the response to high KCl when compared to control group (48.73 ± 9 %; Fig. 1g). There were significant differences in the KCl-induced contractile responses between the control and ethanol-treated groups (P < 0.001; Fig. 1g). Phenylephrine (10 nM–100 μM) produced sustained contractions in a concentration-dependent manner in the corpus cavernous strips of control groups (Fig. 2a). In contrast to KCl, the contractions induced by phenylephrine were not affected by ethanol treatment (Fig. 2d). There were no significant differences in the sensitivity and maximal response of phenylephrine between the control and the ethanol-treated groups (Table 1).
Effect of sub-chronic ethanol treatment on KCl-induced contractions and contribution of Rho-kinase. Representative traces showing the contractile response to KCl (80 mM) in the CC strips isolated from control (a) and sub-chronic ethanol-treated mice (d), and the effect of fasudil (50 μM) or Y-27632 (50 μM) on the contractile responses to KCl in control (b, c) and ethanol-treated mice (e, f). The scatter graph showing the contractile response to KCl (80 mM) and the effects of fasudil (50 μM) or Y-27632 (50 μM) on these contractile responses in CC strips isolated from control and ethanol-treated mice (g). All values are mean ± SD (n = 6). *P < 0.05 significantly different from control; + P < 0.05 significantly different from ethanol; # P < 0.05 significantly different from fasudil; ∞ P < 0.05 significantly different from Y-27632; one-way ANOVA and unpaired t test followed by Bonferroni’s comparison test
Effect of sub-chronic ethanol treatment on phenylephrine-induced contractions and contribution of Rho-kinase. Representative traces showing the cumulative concentration-response curve to phenylephrine (Phe;10 nM–100 μM) in CC strips isolated from control (a) and sub-chronic ethanol-treated mice (d), and the effects of fasudil (50 μM) and Y-27632 (50 μM) on the contractile responses to phenylephrine in control (b, c) and ethanol-treated mice (e, f). The effects of fasudil (50 μM) and Y-27632 (50 μM) on the concentration-response curve to Phe in control and ethanol-treated mice (e). All values are mean ± SD (n = 6).*P < 0.05 significantly different from control; + P < 0.05 significantly different from ethanol; # P < 0.05 significantly different from fasudil or Y-27632; one-way ANOVA and unpaired t test followed by Bonferroni’s comparison test
The effect of Rho-kinase inhibitors on KCl-induced contractions in control and sub-chronic ethanol-treated mice
Pre-treatment with fasudil (50 μM) and Y-27632 (50 μM) significantly reduced the maximal contractions of KCl in control (Fig. 1b and c) and ethanol-treated groups (Fig. 1e and f). The maximum contractions to KCl were significantly reduced by pre-treatment with fasudil and Y-27632 from 76.41 ± 10.5 to 19.61 ± 7.1 % (P < 0.0001) and 11.45 ± 4 % (P < 0.0001), respectively, in control group. In ethanol-treated group, fasudil and Y-27632 significantly attenuated KCl-evoked contractions (Fig. 1g). Pre-treatment with fasudil and Y-27632 significantly decreased the contractile response from 48.73 ± 9 to 9.77 ± 2.9 % (P < 0.0001) and 6.71 ± 3 % (P < 0.0001), respectively, in ethanol-treated group. There were significant differences of KCl-evoked contractions (P < 0.05) between control and ethanol-treated group in the presence of Rho-kinase inhibitors (Fig. 1g). However, the reduction by Rho-kinase inhibitors as percentage was similar between control and ethanol-treated group.
The effect of Rho-kinase inhibitors on phenylephrine-induced contractions in control and sub-chronic ethanol-treated mice
Rho-kinase inhibitors fasudil (50 μM) and Y-27632 (50 μM) significantly reduced phenylephrine (10 nM–100 μM)-induced contraction in control group (Fig. 2b and c). Maximal contractile responses to phenylephrine were also significantly reduced by fasudil and Y-27632 (P < 0.0001; Fig. 2g and h; Table 1) in control group. Fasudil but not Y-27632 markedly decreased pD2 values (P < 0.0001; Table 1). In ethanol-treated group, fasudil and Y-27632 significantly reduced phenylephrine-induced contraction compared to control group (Fig. 2e and f). Maximum contractile responses to phenylephrine and pD2 value were decreased by fasudil and Y-27632 (P < 0.0001; Fig. 2g and h; Table 1) in ethanol-treated group.
The relaxant effect of Rho-kinase inhibitors on KCl- and phenylephrine-induced contractions in control and sub-chronic ethanol-treated mice
We studied the relaxant responses to Rho-kinase inhibitors on elevated tone with KCl and phenylephrine in isolated cavernosal strips from both of control and ethanol-treated mice. In control and ethanol-treated mice, fasudil (100 μM) and Y-27632 (500 μM) produced relaxation on contraction to KCl (80 mM) and phenylephrine (10 nM–100 μM). There were no significant differences in the maximum relaxant response to both of Rho-kinase inhibitors on contractions induced by KCl and phenylephrine in ethanol-treated group compared to control (Fig. 3a and b).
The relaxant effects of Rho-kinase inhibitors on phenylephrine- and KCl-evoked contractions in sham-treated and sub-chronic ethanol-treated mice CC. The scatter graphs showing the relaxant effects of fasudil (100 μM) or Y-27632 (500 μM) in CC strips isolated from control and ethanol-treated group which pre-contracted by cumulative phenylephrine (10 nM–100 μM; a) and KCl (80 mM; b). All values are mean ± SD (n = 6)
Effect of sub-chronic ethanol treatment on RhoA, ROCK1, and ROCK2 expressions in the mouse CC
In order to determine RhoA/Rho-kinase pathway in cavernosal strips, RhoA, ROCK1, and ROCK2 protein expression were studied with the western blot method. RhoA, ROCK1, and ROCK2 expressions were detected in the control group. The relative expression versus β-actin of the RhoA was significantly decreased in the ethanol-treated group compared to the control (Fig. 4a). Also, ROCK1 expression was lower but not statically significant in ethanol-treated group versus control, whereas expression of ROCK2 significantly increased in ethanol-treated group (Fig. 4b).
The effect of sub-chronic ethanol treatment on RhoA, ROCK1, and ROCK2 expressions in the mouse CC. Representative image of western blot analysis for RhoA (a), ROCK-1/ROCK-2 (b), and β-actin in isolated CC tissues from control and ethanol-treated mice and relative protein expression levels of RhoA, ROCK1, ROCK2 versus β-actin in isolated CC strips from control and ethanol-treated mice. Values were normalized by the intensity of each band relative to the intensity of the loading control: values of β-actin (1.2, 2.2, 1.3, 1.6, 2.1). All values are mean ± SD (n = 3). *P < 0.05 significantly different from control (unpaired t test)
Discussion
Our findings suggest that the inhibitory effect of sub-chronic systemic ethanol treatment on KCl-induced contractions may be due to inhibition of a RhoA/Rho-kinase-mediated Ca2+-sensitizing mechanism, and the RhoA/Rho-kinase signaling pathway plays an important role in phenylephrine and KCl contractions in mouse corpus cavernosum.
In the present study, the sub-chronic systemic ethanol treatment inhibited contractions induced by KCl compared to control. However, phenylephrine-induced contractions did not change by ethanol treatment, confirming findings in mouse (Aydinoglu et al. 2008) and rat CC (Lizarte et al. 2009). In contrast to our findings, both phasic and tonic contractions induced by phenylephrine and KCl was significantly augmented by chronic ethanol administration in rabbit corpus cavernosal tissue (Saito et al. 1994). Also, chronic ethanol consumption increased the contractile responses induced by ET-1 in the isolated rat CC, suggesting that chronic ethanol consumption induces erectile dysfunction (Leite et al. 2013; Muniz et al. 2015). These contradictory results of ethanol on contractions may be dependent on blood ethanol levels. The blood ethanol concentration is an important variable to the development of ED associated with ethanol consumption (Arackal and Benegal 2007). In the present study, the blood ethanol concentration is low according to human and rat plasma ethanol levels (Urso et al. 1981; Muniz et al. 2015), suggesting ethanol at low concentrations may improve erectile function due to the inhibition of contractions. Furthermore, these differences may be attributable to different doses and/or durations of ethanol treatment, different experimental conditions, or type of contractile drug.
The accumulated evidence in the literature suggests the importance of Rho-kinase activity in the maintenance of corporal vasoconstriction and penile detumescence (Rees et al. 2001; Chitaley et al. 2001; Teixeira et al. 2005; Waldkirch et al. 2012). Early studies reported that ethanol has shown to exert directly inhibition on gall bladder contraction induced by agonist and KCl via decreasing the calcium sensitivity of the contractile apparatus of the smooth muscle (Masui et al. 1993). Our recent study has also indicated the role of the RhoA/Rho-kinase pathway in the ethanol-induced inhibition of contractions produced by agonist and high KCl in mouse lung parenchymal tissue (Aydinoglu et al. 2015). Recently, Muniz et al. (2015) suggested a role for the RhoA/Rho-kinase pathway in chronic ethanol-induced hyper-reactivity of the isolated rat CC to ET-1. Consistent with these concepts, we found that a decrease in the protein expression levels of RhoA and ROCK1 in the corpus cavernosal tissue of ethanol-treated mice suggests that ethanol inhibits the RhoA/Rho-kinase expression in this tissue. Furthermore, Rho-kinase inhibitors fasudil and Y-27632 caused the decrease of KCl contractions in ethanol group, and this inhibition significantly was greater compared to control, supporting that the ethanol-induced attenuation of contractions produced by high KCl may be due to inhibition of a RhoA/Rho-kinase-mediated Ca2+-sensitizing mechanism. However, the inhibitory effect of ethanol on KCl-induced contractions in control groups cannot be ignored, and greater inhibition of ROCK in ethanol groups may be attributed to the inhibitory effects of ethanol on KCl-contractions.
In our study, although ethanol treatment did not influence phenylephrine-induced contraction, Rho-kinase inhibitors, fasudil and Y27632, produced much more inhibition on phenylephrine-induced contractions in ethanol-treated tissues compare to control, suggesting that ethanol may affect the Rho-kinase signaling pathway. The inhibitory effect of ethanol on KCl-induced contraction but not those to phenylephrine implied the greater importance of RhoA/Rho-kinase-dependent Ca2+ sensitization in response to contraction to voltage-operated Ca2+ channels than GPCR activation in ethanol-treated mouse CC. Kim et al. (2013) investigated the influence of ethanol intake on the RhoA/Rho-kinase signaling pathway in CC, and they suggested that ethanol intake may preserve the erectile function in type 2 diabetes mellitus through the downregulation of the RhoA/Rho-kinase pathway. Consistent with Kim et al. (2013), our present findings suggest that ethanol at low blood concentrations maybe can improve erectile function due to the inhibition of contractions through the RhoA/Rho-kinase inhibition. Furthermore, sub-chronic ethanol treatment did not cause additional augmentation in the relaxant responses of Rho-kinase inhibitors on KCl- and phenylephrine-induced contractions, suggesting that RhoA-kinase inhibitors may produce approximate maximum inhibition on the RhoA/Rho-kinase pathway in mouse CC (Fig. 3a). There are contradictory results about the effect of chronic ethanol treatment on the RhoA/Rho-kinase pathway in corpus cavernosal smooth muscle, and there is less knowledge about the effects of ethanol on the RhoA/Rho-kinase pathway what mechanism involves to the alterations. It is needed to further studies to reveal underlying mechanisms by which ethanol cause inhibition on KCl- and phenylephedrine-induced contraction via inhibition of the RhoA/Rho-kinase signaling pathway.
Also, there are investigations supporting that RhoA/Rho-kinase-mediated cell signaling way is involved in contraction to both G protein-coupled receptor (GPCR) and non-G-protein-coupled mechanism (voltage-operated Ca2+ channels) activations in various smooth muscles (Somlyo and Somlyo 2000; Gosens et al. 2004; Ratz et al. 2005; Schaafsma et al. 2006). Consistent with these reports, we observed that Rho-kinase inhibitors fasudil and Y-27632 reduced contractions induced by phenylephrine (a selective α1- receptor agonist) and KCl (membrane depolarizer via voltage-operated Ca2+ channels activation), suggesting the RhoA/Rho-kinase signaling pathway plays an important role in phenylephrine- and KCl-induced Ca2+ sensitization in mouse CC smooth muscle. In agreement with the present results, recent studies reported that the phenylephrine- and KCl-induced contraction involves RhoA/Rho-kinase-mediated Ca2+ sensitization in retractor penis muscles (Teixeira et al. 2005), rabbit aortic vascular smooth muscle (Sakurada et al. 2003), and guinea-pig gallbladder (Quinn et al. 2006). Our findings is the first report of inhibition of KCl-induced contraction by Rho-kinase inhibitors in CC, supporting a notion that K-induced contraction involves the activation of Rho-kinase with resultant inhibition of myosin light chain phosphatase (MLCP) in rat tail artery (Mita et al. 2002). Recent studies reported that the G-protein-mediated Ca2+ sensitization is mainly through the PKCα/PKCδ-CPI-17Thr38 and RhoA/Rho-kinase signaling pathway in vascular smooth muscle (Kitazawa et al. 2003; Kizub et al. 2010), whereas KCl also causes Ca2+ sensitization solely by the activation of Rho-kinase not by activation of both Rho-kinase and PKC like many GPCR stimuli (Eto et al. 2001; Mita et al. 2002; Sakamoto et al. 2003; Ratz et al. 2005). Also, Cernecka et al. (2015) recently implied that Rho-kinase involvement in β-receptor downstream signaling seems to depend on the contractile agent, and receptor-dependent and receptor-independent agents use different signaling pathways to cause bladder contraction. Our finding that a greater phenylephrine-induced contraction than that of KCl may support the hypothesis that the mechanism for activating Rho-kinase may not be exactly the same between α1- receptor agonist and KCl. We also observed that Rho-kinase inhibitors produced relatively a greater reduction of contraction to KCl than to phenylephrine, in agreement with those of bovine airway smooth muscle (Gosens et al. 2004), suggesting that phenylephrine and KCl may not likely to act equally on the RhoA/Rho-kinase to exert their constrictive effect. It is possible that the greater inhibition induced by Rho-kinase inhibitors in KCl-induced contraction reflects a greater contribution of Rho-kinase to the constrictor response to KCl. On the other hand, it may be likely that phenylephrine causes excessive activation of Rho-kinase, and, thus, the Rho-kinase inhibitors induce a smaller inhibition on phenylephrine-induced contraction. However, it remains unknown whether adrenergic agonist utilize this signaling pathway more than does KCl. We cannot exclude the possibility that fasudil and Y-27632, at higher concentrations, can inhibit other kinases, (e.g., PKC); however, we used fasudil and Y-27632 at the concentration that should inhibit RhoA/Rho-kinase but no other kinases (Janssen et al. 2004; Levent and Büyükafşar 2004; Quinn et al. 2006; Aydinoglu et al. 2015). In the view of these studies, the increased RhoA/Rho-kinase activity may lead to the abnormal contractility of the CC and contribute to the pathogenesis of ED (Andersson 2003).
In conclusion, these results indicate the sub-chronic ethanol treatment decreased the expression of RhoA in mouse CC. Also, the inhibitory effect of ethanol on KCl-induced contractions may be due to, at least in part, the inhibition of a RhoA/Rho-kinase in mouse CC. Furthermore, the RhoA/Rho-kinase signaling pathway plays an important role in phenylephrine and KCl contractions in mouse corpus cavernosum.
References
Andersson KE (2001) Pharmacology of penile erection. Pharmacol Rev 53(3):417–450
Andersson KE (2003) Erectile physiological and pathophysiological pathways involved in erectile dysfunction. J Urol 170(2 Pt 2):S6–S13
Arackal BS, Benegal V (2007) Prevalance of sexual dysfunction in male subjects with alcohol dependence. Indian J Psychiatry 49(2):109–112
Aydinoglu F, Yilmaz SN, Coskun B, Daglioglu N, Ogulener N (2008) Effects of ethanol treatment on the neurogenic- and endothelium-dependent relaxation of corpus cavernosum smooth muscle in the mouse. Pharmacol Rep 60:725–734
Aydinoglu F, Ergurhan Kiroglu O, Astarci E, Balli E, Ogulener N (2015) Effects of ethanol on RhoA/Rho-kinase-mediated calcium sensitization in mouse lung parenchymal tissue. Eur J Pharmacol 764:318–327. doi:10.1016/j.ejphar.2015.07.021
Burnett AL (2006) The role of nitric oxide in erectile dysfunction: implications for medical therapy. J Clin Hypertens (Greenwich) 8(12):53–62
Caspari D, Huebgen EM, Derouet H (1999) Interdisciplinary assessment and follow-up of patients with erectile dysfunction-psychiatric aspects. Int J Impot Res 11:213–217. doi:10.1038/sj.ijir.3900420
Cernecka H, Kersten K, Maarsingh H, Elzinga CR, de Jong IJ, Korstanje C, Michel MC, Schmidt M (2015) β3-Adrenoceptor-mediated relaxation of rat and human urinary bladder: roles of BKCa channels and Rho kinase. Naunyn Schmiedebergs Arch Pharmacol 388(7):749–759. doi:10.1007/s00210-015-1128-z
Chitaley K, Wingard CJ, Clinton Webb R, Branam H, Stopper VS, Lewis RW, Mills TM (2001) Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med 7(1):119–122. doi:10.1038/83258
Chitaley K, Webb R, Mills TM (2003) The ups and downs of Rho-kinase and penile erection: upstream regulators and downstream substrates of rho-kinase and their potential role in the erectile response. Int J Impot Res 15(2):105–109
Eto M, Kitazawa T, Yazawa M, Mukai H, Ono Y, Brautigan DL (2001) Histamine-induced vasoconstriction involves phosphorylation of a specific inhibitor protein for myosin phosphatase by protein kinase C alpha and delta isoforms. J Biol Chem 276:29072–29078. doi:10.1074/jbc.M103206200
Fukata Y, Amano M, Kaibuchi K (2001) Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci 22:32–39. doi:10.1016/S0165-6147(00)01596-0
Goldstein DB, Pal N (1971) Alcohol dependence produced in mice by inhalation of ethanol: grading withdrawal reaction. Science 172:288–290
Gosens R, Schaafsma D, Meurs H, Zaagsma J, Nelemans SA (2004) Role of Rho-kinase in maintaining airway smooth muscle contractile phenotype. Eur J Pharmacol 483:71–78. doi:10.1016/j.ejphar.2003.10.027
Gur S, Kadowitz PJ, Sikka SC, Bivalacqua TJ, Hellstrom WJG (2012) Inhibition of sympathetic neuroeffector transmission in human corpus cavernosum. B JUI 110:856–862. doi:10.1111/j.1464-410X.2011.10822.x
Janssen LK, Tazzeo T, Zuo J, Pertens E, Keshavjee S (2004) KCl evokes contraction of airway smooth muscle via activation of RhoA and Rho-kinase. Am J Physiol Lung Cell Mol Physiol 287:L852–L858. doi:10.1152/ajplung.00130.2004
Jin L, Burnett AL (2006) RhoA/Rho-kinases in erectile tissue: mechanisms of disease and therapeutic insights. Clin Sci (Lond) 110(2):153–165
Kim SJ, Bae WJ, Han JH, Hong SH, Kim SW, Hwang TK, Kim DJ, Lee JY (2013) The influence of ethanol intake on RhoA/Rho kinase signaling pathway in corpus cavernosum of OLETF rats. Int Urol Nephrol 45(2):429–438. doi:10.1007/s11255-012-0342-6
Kitazawa T, Kobayashi S, Horiuti K, Somlyo AV, Somlyo AP (1989) Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem 264(10):5339–5342
Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M (2003) Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol 546(3):879–889. doi:10.1113/jphysiol.2002.029306
Kizub IV, Pavlova OO, Johnson CD, Soloviev AI, Zholos AV (2010) Rho kinase and protein kinase C involvement in vascular smooth muscle myofilament calcium sensitization in arteries from diabetic rats. Br J Pharmacol 159:1724–1731. doi:10.1111/j.1476-5381.2010.00666.x
Leite LN, Lacchini R, Carnio EC, Queiroz RH, Tanus-Santos JE, de Oliveira AM, Tirapelli CR (2013) Ethanol consumption increases endothelin-1 expression and reactivity in the rat cavernosal smooth muscle. Alcohol Alcohol 48(6):657–666. doi:10.1093/alcalc/agt057
Levent A, Büyükafşar K (2004) Expression of Rho-kinase (ROCK-1 and ROCK-2) and its substantial role in the contractile activity of the sheep ureter. Br J Pharmacol 143:431–437. doi:10.1038/sj.bjp.0705961
Lizarte FS, Claudino MA, Tirapelli CR, Morgueti M, Tirapelli DP, Batalhão ME, Carnio EC, Queiroz RH, Evora PR, Tucci S Jr, Cologna A, Antunes E, Martins AC, Tirapelli LF (2009) Chronic ethanol consumption induces cavernosal smooth muscle dysfunction in rats. Urology 74(6):1250–1256. doi:10.1016/j.urology.2009.04.043
Masui H, Wakabayashi I, Hatake K, Yoshimoto S, Sakamoto K (1993) Effects of ethanol on contractile response of gall bladder isolated from guinea pig. Eur J Pharmacol 248:103–110
Miller NS, Gold MS (1988) The human sexual response and alcohol and drugs. J Subst Abuse Treat 5(3):171–177. doi:10.1016/0740-5472(88)90006-2
Mills TM, Pollock DM, Lewis RW, Branam HS, Wingard CJ (2001) Endothelin-1-induced vasoconstriction is inhibited during erection in rats. Am J Physiol Regul Integr Comp Physiol 281(2):R476–R483
Mita M, Yanagihara H, Hishinuma S, Saito M, Walsh MP (2002) Membrane depolarization-induced contraction of rat caudal arterial smooth muscle involves Rho-associated kinase. Biochem J 364:431–440. doi:10.1042/BJ20020191
Muniz JJ, Leite LN, De Martinis BS, Carneiro FS, Tirapelli CR (2015) Chronic ethanol consumption induces erectile dysfunction: role of oxidative stress. Life Sci 15:44–53. doi:10.1016/j.lfs.2015.09.017
Peugh J, Belenko S (2001) Alcohol, drugs and sexual function: a review. J Psychoactive Drugs 33(3):223–232
Quinn T, Feighery R, Baird AW (2006) Role of Rho-kinase in guinea pig gallbladder smooth muscle contraction. Eur J Pharmacol 534:210–217. doi:10.1016/j.ejphar.2006.01.016
Ratz PH, Berg KM, Urban NH, Miner AS (2005) Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol 288:C769–C783. doi:10.1152/ajpcell.00529.2004
Rees RW, Ralph DJ, Royle M, Moncada S, Cellek S (2001) Y-27632, an inhibitor of Rho-kinase, antagonizes noradrenergic contractions in the rabbit and human penile corpus cavernosum. Br J Pharmacol 133:455–458. doi:10.1038/sj.bjp.0704124
Saito M, Broderick GA, Wein AJ, Levin RM (1994) Effect of chronic ethanol consumption on the pharmacological response of the rabbit corpus cavernosum. Pharmacology 49(6):386–391. doi:10.1159/000139257
Sakamoto K, Hori M, Izumi M, Oka T, Kohama K, Ozaki H, Karaki H (2003) Inhibition of high K+-induced contraction by the ROCKs inhibitor Y-27632 in vascular smooth muscle: possible involvement of ROCKs in a signal transduction pathway. J Pharmacol Sci 92:56–69
Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y (2003) Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res 93:548–556. doi:10.1161/01.RES.0000090998.08629.60
Schaafsma D, Boterman M, de Jong AM, Hovens I, Penninks JM, Nelemans SA, Meurs H, Zaagsma J (2006) Differential Rho-kinase dependency of full and partial muscarinic receptor agonists in airway smooth muscle contraction. Br J Pharmacol 147:737–743. doi:10.1038/sj.bjp.0706665
Somlyo AP, Somlyo AV (2000) Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522:177–185. doi:10.1111/j.1469-7793.2000.t01-2-00177.x
Teixeira CE, Jin L, Ying Z, Palmer T, Webb RC (2005) Ca2+ sensitization and the regulation of contractility in rat anococcygeus and retractor penis muscle. Biochem Pharmacol 69:1483–1492. doi:10.1016/j.bcp.2005.02.018
Urso T, Gavaler JS, Van Thiel DH (1981) Blood ethanol levels in sober alcohol users seen in an emergency room. Life Sci 28(9):1053–1056
Van Thiel DH, Lester R (1979) The effect of chronic alcohol abuse on sexual function. Clin Endocrinol Metab 8:499–510
Waldkirch ES, Ückert S, Sohn M, Kuczyk MA, Hedlund P (2012) Rho kinase (ROK)-related proteins in human cavernous arteries: an immunohistochemical and functional approach. J Sex Med 9(5):1337–1343. doi:10.1111/j.1743-6109.2012.02662.x
Yazir Y, Gocmez SS, Utkan T, Komsuoglu-Celikyurt I, Gacar N, Sarioglu Y (2012) Effects of chronic low- and high-dose ethanol intake on the nitrergic relaxations of corpus cavernosum and penile nitric oxide synthase in the rabbit. Int J Impot Res 24(5):185–190. doi:10.1038/ijir.2012.14
Acknowledgments
This work was supported by Cukurova University Research Foundation (TF2007BAP8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Protocols were approved by local the Ethic Committee of the University of Cukurova. This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23 revised 1996).
Rights and permissions
About this article
Cite this article
Kumcu, E.K., Aydinoglu, F., Astarci, E. et al. The effect of sub-chronic systemic ethanol treatment on corpus cavernosal smooth muscle contraction: the contribution of RhoA/Rho-kinase. Naunyn-Schmiedeberg's Arch Pharmacol 389, 249–258 (2016). https://doi.org/10.1007/s00210-015-1204-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-015-1204-4