Abstract
Vasopeptidase inhibition (VPI), a therapeutic strategy by dual inhibition of both ACE and neutral endopeptidase 24.11, has not shown a prognostic benefit over ACE inhibition in chronic severe heart failure (CHF). Nevertheless, the effects of early treatment by VPI on cardiac remodelling have not been well assessed. We analysed the effects of early chronic VPI (50 mg/kg/day Omapatrilat) on cardiac remodelling and neurohumoral function during the progression of rapid ventricular pacing-induced heart failure in rabbits (early left ventricular dysfunction [ELVD]: 10 days at 330 bpm, CHF: further 10 days at 360 bpm). VPI-treated animals (ELVD-VPI n = 6; CHF-VPI n = 8) and placebo treated animals (ELVD n = 6; CHF n = 7) were compared with control rabbits (CTRL n = 5). LV fractional shortening (FS) and enddiastolic diameter (LVEDD) were assessed by echocardiography (12 MHz probe). LV BNP- and LV IL-6 gene expression was analysed quantitatively by real time PCR. Neurohumoral function was assessed by ANP, cGMP, plasma renin activity (PRA) and Aldosterone. In ELVD, LVEDD and atrial mass were significantly increased (both p < 0.05). This increase was markedly attenuated by VPI (both p < 0.05 vs. placebo). CHF was associated with a further increase in atrial mass and an increase in LV mass (both p < 0.05), which was again attenuated by VPI (atrial mass, p < 0.05 vs. untreated). LV BNP mRNA was significantly increased in CHF (p < 0.05 vs. control), and chronic VPI completely abolished this increase in ELVD and significantly attenuated it in CHF (p < 0.05 vs. CHF-placebo). Beyond that, the increase of cGMP was augmented by chronic VPI (p < 0.05 vs. placebo in CHF) in heart failure and that of Aldosterone was attenuated (p < 0.05 vs. placebo in ELVD), whereas PRA was temporarily increased (p < 0.05 vs. placebo in ELVD). Combined inhibition of ACE and NEP by VPI significantly inhibits early cardiac remodelling and LV BNP gene expression. If initiated early enough, it may slow down cardiac remodelling and represents a promising therapeutic strategy in progressive heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congestive heart failure is a chronic and progressive disease with a further increasing incidence and prevalence. Despite recent improvements in therapy, morbidity and mortality still remain high (Roger et al. 2012). Further improvements in pharmacotherapy therefore are urgently needed. Current established pharmacotherapy aims at antagonism of the renin–angiotensin system (RAS, through inhibition of ACE, blockade of the angiotensin 2- and aldosterone receptor) as well as antagonism of the sympathetic nervous system (SNS, through blockade of the beta-adrenergic receptor). Antagonism of these systems has been shown to ameliorate cardiac remodelling and improve cardiac function, symptoms, morbidity and outcome (Dickstein et al. 2008; Hunt 2005).
In addition to blockade of the RAS and SNS, augmentation of the vasodilatory, antimitogenic and natriuretic peptide system (NPS) through inhibition of NEP 24.11, the proteolytic enzyme, which partially degrades the biologically active natriuretic peptide, has been put forward (Abassi et al. 2005; Daull et al. 2006; Emoto et al. 2005; Linz et al. 2006; Martin et al. 2005). NEP inhibition has been shown to enhance cyclic GMP (Bani et al. 2000; Johnson et al. 2006; Lainchbury et al. 1999; Murohara and Johnston 1992; Stasch et al. 1996), the second messenger of the NPS, and improve vasodilatation and natriuresis (Quaschning 2005; Quaschning et al. 2002; Regamey et al. 2002; Roques 1998). With respect to chronic experimental heart failure, NEP inhibition has recently shown to delay the onset of sodium retention during the transition from early to severe heart failure and improve the natriuretic response to volume expansion while enhancing the natriuretic peptides and depressing aldosterone activation in tachycardia-induced heart failure (Martin et al. 2005).
Progressive, tachycardia-induced heart failure is a model of great relevance for the human disease and closely resembles progressive cardiomyopathy (Spinale 1994; Schneider et al. 2009; Birner et al. 2007; Elsner and Riegger 1995a, b; Luchner et al. 1996, 1998, 2000, 2001; Moe and Armstrong 1999). The model was initially used as acute heart failure model in the dog (Elsner and Riegger 1995a) and has since then been developed to a chronic progressive model which evolves from early left ventricular dysfunction (ELVD) through a transitional phase towards heart failure over several weeks. This model has also been adapted to rabbits and is characterized by a progressive decrease in blood pressure, progressive left ventricular dysfunction and dilatation, impaired repolarisation reserve and progressive left ventricular activation of BNP and IL6-gene expression (Schneider et al. 2009; Luchner et al. 2001; Birner et al. 2012a, b).
In the current study, it was our objective to assess the effects of combined inhibition of the RAS and NEP upon cardiac remodelling and neurohumoral function. Given the recently demonstrated effects of chronic inhibition of NEP as demonstrated in the canine model (Martin et al. 2005), we hypothesized that dual inhibition of ACE and NEP might as well attenuate cardiac remodelling in the progressive rabbit model.
In order to address our hypothesis, we assessed the effects of chronic RAS and NEP inhibition by the vasopeptidase inhibitor Omapatrilat in rabbits with chronic progressive tachycardia-induced heart failure.
Methods
Animal model
Thirty-two male rabbits (chinchilla bastard) were used for the study (see Fig. 1 for study protocol). Twenty-seven rabbits underwent implantation of a programmable cardiac pacemaker (Medtronic Minix 8340, Minneapolis, MN). Under anesthesia (Ketamine 60 mg/kg and Xylazine 5 mg/kg i.m.), the right internal jugular vein was dissected and cannulated with a single-lumen central venous catheter (Braun, Germany). The catheter was then advanced into the right ventricle under fluoroscopic guidance. A two-French transvenous screw-in pacemaker lead (Medtronic, Minneapolis, MN) was advanced through the catheter into the right ventricular apex and implanted endocardially. The pacemaker was implanted subcutaneously into the right abdominal wall and the pacemaker lead was connected subcutaneously with the pacemaker. All rabbits were allowed to recover for at least 10 days after surgery before the pacemaker was started for the induction of heart failure. Proper pacemaker function was checked intraoperatively at the time of programming, and subsequently, all 10 days. All studies were approved by the governmental animal care committee.
Fifteen rabbits (CHF group) underwent pacing with a stepwise increase of stimulation frequencies over 30 days. During the first 10 days, animals were paced at 330 beats per minute (bpm). This protocol results in ELVD, as defined by significant LV systolic dysfunction with cardiac enlargement and decreased perfusion pressure but no clinical signs of heart failure. The pacing rate was then increased to 360 bpm for 10 days, and ELVD evolved to CHF with further cardiac enlargement and further decreased perfusion pressure together with clinical signs of fluid retention (ascites, pleural and pericardial effusion). Since invasive measurement of arterial blood pressure demonstrated a hypotensive effect of VPI already in ELVD and a more sustained effect in advanced CHF, the pacing rate was not increased above 360 bpm in order to avoid symptomatic hypotension. Usually, the progressive model in the rabbit allows pacing rates up to and above 380 bpm without symptomatic hypotension
At baseline (control), after being paced at 330 bpm for 10 days (ELVD) and at the end of the protocol (CHF), conscious arterial pressure was measured invasively via the medial ear artery and a 2D-guided M-mode echocardiogram was obtained. At the end of the pacing protocol, rabbits were euthanized and tissue was rapidly harvested. Hearts were trimmed on ice, snap frozen in liquid nitrogen and stored at −80 °C until further processing.
A second group of 12 rabbits was paced at 330 bpm for 10 days only and served as tissue donors for the ELVD group, and a third group of five untreated rabbits served as tissue donors for the control group. Again, invasive hemodynamic measurements and an echocardiogram were obtained to assess cardiac function before animals were euthanized and tissue was rapidly harvested and deep-frozen.
Drinking water of the laboratory animals was either substituted with Omapatrilat to reach a daily VPI dose of 50 mg/kg BW (VPI group), or remained untreated (placebo group). Pharmacological intervention was started after initiation of cardiac pacing and was sustained during the whole pacing period.
Figure 2 exemplarily displays structural cardiac remodelling as it is induced by the animal model.
Analytical methods
For analysis of LV BNP- and IL-6 expression, mRNA was extracted from LV samples utilizing a commercially available kit (Fasttrack, Invitrogen). Briefly, tissue was homogenized (Polytron PT 1200) in a detergent-based buffer containing RNAse/Protein Degrader and incubated in a slow-shaking waterbath. DNA was precipitated and sheared and Oligo (dT) cellulose was added for adsorption of polyadenylated mRNA. DNA, proteins, cell debris and non-polyadenylated RNA were washed off and mRNA eluted off the Oligo (dT) cellulose. The yield of mRNA was determined in a spectrophotometer by absorption of 260 nm UV light. Afterwards, 1 μg of mRNA was reverse transcribed with Omniscript RT Kit (Qiagen) following the manufacturer’s instructions using Oligo (dT) primers. Real-time PCR was performed using QuantiTect SYBR Green PCR Kit (Qiagen) in a 20 μl volume, and genes of interest were normalized to GAPDH gene expression. Primers were: GAPDH forward primer: 5′- ATCACTGCCACCCAGAAGAC-3′, reverse primer: 5′-GTGAGTTTCCCGTTCAGCTC-3′, BNP forward primer: 5′-CTCCTCTTCTTGCACCTGTC-3′, reverse primer: 5′-GTGTTTCCTGAGCACATTGC-3′, IL-6 forward primer: 5′-CTTCAGGCCAAGTTCAGGAG-3′, and reverse primer: 5′-GGGTGGCTTCTTCATTCAAA-3′.
Plasma renin activity, plasma aldosterone- (DiaSorin, Germany, respectively) and plasma ANP- (Phoenix Pharmaceuticals, USA) concentrations were determined using commercially available RIA kits, and plasma cGMP concentration was quantified by a commercial ELISA (GE Healthcare, Fairfield, USA) according to the manufacturer’s instructions, respectively.
Echocardiography
A long and short-axis echocardiogram (HP Sonos 5500, 12 MHz probe) was performed under light sedation (5 mg midazolam i.m.) in a supine position from the left parasternal window. LV enddiastolic (LVEDd) and endsystolic (LVESd) dimensions and diastolic and systolic thickness of the left ventricular anterior wall (AEDth and AESth) and posterior wall (PEDth and PESth) as well as left atrial diameter (LAd) were determined from three repeated 2D-guided M-mode tracings using the ASE convention. From those measurements, fractional shortening (FS) was calculated as: FS = (LVEDd − LVESd)/LVEDd
Hemodynamic measurements
Conscious arterial pressure was determined invasively via the medial ear artery under light sedation after pausing pacemaker stimulation.
Statistical analysis
Results of the quantitative studies were expressed as mean ± standard error of the mean. Comparison between the control, ELVD and CHF groups were performed by analysis of variance (ANOVA) followed by Fisher’s least significant difference test. Comparison between the atrial and LV tissues as well as between IL-6, BNP, cGMP, PRA and aldosterone were performed by paired Student’s t test. Statistical significance was defined as p < 0.05.
Results
Haemodynamics and echocardiography
The results concerning haemodynamics and echocardiography are depicted in Table 1. In comparison to untreated animals, VPI reduced blood pressure in both ELVD (p < 0.05 for systolic BP) and CHF (p < 0.05 for systolic and diastolic BP, respectively).
Regarding echocardiography, animals with ELVD were characterized by significant systolic and diastolic left ventricular dilatation (both p < 0.05), left ventricular wall thinning and left ventricular systolic dysfunction (p < 0.05). In heart failure, left ventricular systolic dilatation as well as left ventricular dysfunction and left ventricular wall thinning progressed further. In treated animals, left ventricular dilatation was attenuated, and in ELVD, the treated animals exhibited significantly smaller left ventricular enddiastolic diameters as compared to placebo (p < 0.05). Of note, no significant differences were observed between treatment groups regarding left ventricular systolic function.
During the progression of heart failure, left atrial diameter increased progressively in ELVD and in heart failure (p < 0.05). This increase was completely blunted in treated ELVD animals (p < 0.05 vs. placebo) and markedly attenuated in heart failure.
Effects on cardiac remodelling
Progressive heart failure was characterized by progressive increases in total heart weight, right ventricular mass and atrial mass index in ELVD and heart failure (Table 2). These increases were markedly blunted in the treated group, and atrial mass index in the treated group was significantly smaller as compared to placebo both in ELVD (p < 0.05) and in heart failure (p < 0.05). Total cardiac mass index was likewise significantly smaller in the treated animals with heart failure as compared to placebo (p < 0.05).
Neurohumoral function
Animals with progressive heart failure were characterized by a progressive increase in aldosterone both in ELVD and heart failure (Table 3). Plasma renin activity was unchanged in animals with ELVD as compared to control but was increased in heart failure. In treated animals, aldosterone was suppressed as compared to placebo both in ELVD (p < 0.05) and in heart failure. Regarding plasma renin activity, a differential pattern was observed with a temporal increase in ELVD (p < 0.05) but without significant changes in heart failure. Likewise, plasma ANP progressively increased in ELVD (p < 0.05) and CHF, and was temporarily blunted in treated animals in ELVD (p < 0.05). Plasma cGMP was significantly increased in CHF (p < 0.05 vs. control) and markedly higher in treated animals, both in ELVD and in CHF.
BNP and IL-6 gene expression
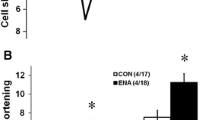
Progressive heart failure was characterized by progressive increases in left ventricular BNP expression in ELVD (p < 0.05) and heart failure (p < 0.05). In VPI-treated animals, these increases were completely blunted in ELVD as compared to control and were significantly attenuated in heart failure (p < 0.05, Fig. 3).
A very similar pattern was observed regarding left ventricular IL-6 expression, which was increased in ELVD (p < 0.05) and in heart failure (Fig. 4). Again, these increases were blunted in treated animals with ELVD and markedly attenuated in heart failure.
Discussion
The current study was undertaken to assess the effects of combined inhibition of the RAS and NEP on cardiac remodelling in progressive, tachycardia-induced heart failure. It demonstrates that combined RAS and NEP inhibition, in conjunction with a potent blood pressure lowering effect, has beneficial effects upon neurohumoral function and cardiac remodelling. These effects are already present during early left ventricular dysfunction and sustained in more advanced heart failure. The current study underscores the potential of early combined antagonism of the RAS and NEP on cardiac remodelling in heart failure.
Effects of combined RAS and NEP inhibition on haemodynamics and cardiac remodelling
Early combined RAS and NEP inhibition had sustained effects on progressive experimental heart failure. Specifically, we observed that increases in left atrial diameter which are normally seen with progressive heart failure were blunted in the treated group and this effect was sustained even in more advanced heart failure. Regarding left ventricular diameters and function, we observed an attenuated increase in left ventricular enddiastolic diameter in the treated group during ELVD. Interestingly, left ventricular systolic function did not differ between the treated and untreated group, most likely due to the effects of tachypacing. The observation of a decreased left atrial size in the treated group was confirmed upon autopsy with a significantly attenuated left atrial hypertrophy in the treated group both in ELVD and in advanced heart failure. Of note, the extent of left atrial hypertrophy in advanced heart failure was blunted in the treated group to even a lower degree of atrial hypertrophy than is usually observed in untreated animals with ELVD. Since VPI treatment also resulted in a significant decline in both systolic and diastolic blood pressure, alleviated atrial and ventricular remodelling could mechanistically be assigned to hemodynamic and cardioprotective VPI effects with current evidence more favoring the latter one (Lapointe et al. 2002).
The current data confirm previous studies which have addressed the concept of combined RAS and NEP inhibition (Lapointe et al. 2002; Maki et al. 2003a, b; Mishima et al. 2002; Xu et al. 2004) and extend these studies to the model of progressive, pacing-induced heart failure. Importantly, our data indicate an early effect on cardiac remodelling during progressive heart failure which is already evident during the phase of early left ventricular dysfunction.
Effects on neurohumoral function and left ventricular gene expression
In the current study, combined RAS and NEP inhibition had neurohumoral effects which are consistent with the expected mechanism of action. Specifically, cyclic GMP, the second messenger of the natriuretic peptides, was increased in the treated group both in ELVD and in advanced heart failure. In contrast, aldosterone was attenuated in the treated group throughout the observation period. These neurohumoral observations again confirm previous experimental studies (Martin et al. 2005; Bani et al. 2000; Johnson et al. 2006; Lainchbury et al. 1999; Murohara and Johnston 1992; Stasch et al. 1996) and extend these observations to the model of progressive tachycardia-induced heart failure and to the disease states of ELVD as well as advanced heart failure.
Regarding the molecular effects of combined RAS and NEP inhibition, we have assessed the left ventricular gene expression of BNP and IL-6. BNP is a well-established molecular and circulatory marker of heart failure severity and hypertrophic left ventricular remodelling (Latini et al. 2002; Munagala et al. 2004). IL-6 is a cytokine with pro-inflammatory, pro-apoptotic and pro-hypertrophic effects and increased left ventricular IL-6 has been associated with adverse left ventricular function and prognosis in heart failure (Birner et al. 2007; Staudt et al. 2002). In the current study, we observed strong and sustained effects of combined RAS and NEP inhibition upon left ventricular gene expression. Specifically, left ventricular BNP was suppressed in advanced heart failure to a level as untreated ELVD despite longer duration of pacing, higher pacing rate and stronger depression of left ventricular ejection fraction. In the treated ELVD group, left ventricular BNP expression was unchanged as compared to control despite several days of tachypacing and significant left ventricular dysfunction and dilatation. A similar pattern of activation was observed regarding the expression of IL-6 with a blunted increase of IL-6 in treated animals with advanced heart failure which was attenuated to a level as observed in untreated animals with ELVD and a completely blunted activation of IL-6 in treated animals with ELVD. The current data contribute to the potential mechanisms which may underlie the beneficial effects of combined RAS and NEP inhibition and include haemodynamic unloading, inhibition of the local and circulating RAS, activation of the local and circulating natriuretic peptide system and depression of pro-inflammatory cytokines.
Relevance of combined RAS and NEP inhibition
In the current study, combined RAS and NEP inhibition was achieved through the vasopeptidase inhibitor Omapatrilat. This substance has proven effective in experimental studies (Abassi et al. 2005; Cha et al. 2006; Nishikimi et al. 2006), shown potent blood pressure lowering effects in the human (Kostis et al. 2004) and beneficial effects which were equal or even superior to Enalapril in subjects with chronic heart failure, higher blood pressure and less than severe left ventricular dysfunction (Kostis et al. 2004). Nevertheless, this compound has not been developed further in the human due to an increased incidence of Quincke’s oedema, presumably due to the accumulation of bradykinine. However, for experimental purposes, the molecule provides the desired effects of combined RAS and NEP inhibition and no adverse effects such as angio-oedema were observed in our current animal study. More importantly, the current data again underscore the fundamental importance of the concept of combined RAS and NEP inhibition which has recently also been extended from cardio-protection to vaso-protection (Quaschning et al. 2003) and reno-protection (Davis et al. 2003). Further, this concept has been advanced by studies which have evaluated the combination of angiotensin receptor blockade together with NEP inhibition (Hegde et al. 2011) by novel compounds which achieve multiple inhibitions without interference with bradykinine breakdown. These molecules are now named angiotensin receptor nephrilysin inhibitors (ARNIs), and one compound—LCZ696—has already shown excellent antihypertensive properties (Ruilope et al. 2010) and is currently tested in chronic heart failure (ongoing PARADIGM-HF study). Insofar, these compounds show great promise for being a new treatment option in arterial hypertension and chronic heart failure (Cuculi and Erne 2011). Nevertheless, in light of the somehow disappointing results from studies evaluating a combined inhibition of the renin–angiotensin–aldosterone system (including the OVERTURE trial comparing Omapatrilat with enalapril (Packer et al. 2002)), results from ongoing clinical trials need to be awaited in order to finally judge the clinicial effectiveness of this concept.
Conclusion
The current study demonstrates multiple beneficial effects of combined RAS and NEP inhibition in and upon the progression of tachycardia-induced heart failure in the rabbit. These effects include beneficial neurohumoral and haemodynamic changes as well as attenuated cardiac remodelling. These findings warrant that the pharmacological concept of combined RAS and NEP inhibition continues to be developed further.
References
Abassi ZA, Yahia A, Zeid S, Karram T, Golomb E, Winaver J et al (2005) Cardiac and renal effects of omapatrilat, a vasopeptidase inhibitor, in rats with experimental congestive heart failure. Am J Physiol Heart Circ Physiol 288(2):H722–H728
Bani M, Colantoni A, Guillaume M, Macchi F, Moroni G, Persiani S (2000) A double-blind, placebo-controlled study to assess tolerability, pharmacokinetics and preliminary pharmacodynamics of single escalating doses of Z13752A, a novel dual inhibitor of the metalloproteases ACE and NEP, in healthy volunteers. Br J Clin Pharmacol 50(4):338–349
Birner CM, Ulucan C, Fredersdorf S, Rihm M, Löwel H, Stritzke J et al (2007) Head-to-head comparison of BNP and IL-6 as markers of clinical and experimental heart failure: superiority of BNP. Cytokine 40(2):89–97
Birner C, Husser O, Jeron A, Rihm M, Fredersdorf S, Resch M et al (2012a) Differential expression of potassium channels and abnormal conduction in experimental tachycardia-induced heart failure. Naunyn Schmiedebergs Arch Pharmacol 385(5):473–480
Birner C, Dietl A, Deutzmann R, Schmid P, Jungbauer C, Resch M et al (2012) Inter- and intraventricular proteomic profiling implies mitochondrial dysfunction in tachycardia-induced heart failure. J Card Fail 18:660–673
Cha Y, Dzeja PP, Redfield MM, Shen WK, Terzic A (2006) Bioenergetic protection of failing atrial and ventricular myocardium by vasopeptidase inhibitor omapatrilat. Am J Physiol Heart Circ Physiol 290(4):H1686–H1692
Cuculi F, Erne P (2011) Combined neutral endopeptidase inhibitors. Expert Opin Investig Drugs 20(4):457–463
Daull P, Blouin A, Beaudoin M, Gadbois S, Belleville K, Cayer J et al (2006) The hemodynamic and metabolic profiles of Zucker diabetic fatty rats treated with a single molecule triple vasopeptidase inhibitor, CGS 35601. Exp Biol Med (Maywood) 231(6):824–829
Davis BJ, Johnston CI, Burrell LM, Burns WC, Kubota E, Cao Z et al (2003) Renoprotective effects of vasopeptidase inhibition in an experimental model of diabetic nephropathy. Diabetologia 46(7):961–971
Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJV, Ponikowski P, Poole-Wilson PA et al (2008) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 10(10):933–989
Elsner D, Riegger GA (1995a) Experimental heart failure produced by rapid ventricular pacing in the dog. J Card Fail 1(3):229–247
Elsner D, Riegger GA (1995b) Characteristics and clinical relevance of animal models of heart failure. Curr Opin Cardiol 10(3):253–259
Emoto N, Raharjo SB, Isaka D, Masuda S, Adiarto S, Jeng AY et al (2005) Dual ECE/NEP inhibition on cardiac and neurohumoral function during the transition from hypertrophy to heart failure in rats. Hypertension 45(6):1145–1152
Hegde LG, Yu C, Renner T, Thibodeaux H, Armstrong SR, Park T et al (2011) Concomitant angiotensin AT1 receptor antagonism and neprilysin inhibition produces omapatrilat-like antihypertensive effects without promoting tracheal plasma extravasation in the rat. J Cardiovasc Pharmacol 57(4):495–504
Hunt SA (2005) ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 46(6):e1–e82
Johnson AG, Pearce GL, Danoff TM (2006) A randomized, double-blind, placebo-controlled, parallel-group study to assess the efficacy and safety of dual ACE/NEP inhibitor GW660511X in mild-to-moderate hypertensive patients. J Hum Hypertens 20(7):496–503
Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E (2004) Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 17(2):103–111
Lainchbury JG, Richards AM, Nicholls MG, Espiner EA, Yandle TG (1999) Brain natriuretic peptide and neutral endopeptidase inhibition in left ventricular impairment. J Clin Endocrinol Metab 84(2):723–729
Lapointe N, Blais C, Adam A, Parker T, Sirois MG, Gosselin H et al (2002) Comparison of the effects of an angiotensin-converting enzyme inhibitor and a vasopeptidase inhibitor after myocardial infarction in the rat. J Am Coll Cardiol 39(10):1692–1698
Latini R, Masson S, de Angelis N, Anand I (2002) Role of brain natriuretic peptide in the diagnosis and management of heart failure: current concepts. J Card Fail 8(5):288–299
Linz W, Schäfer S, Afkham F, Gerl M, Schmidts H, Rütten H (2006) Vasopeptidase inhibition prevents target organ damage and improves survival in spontaneously hypertensive rats. J Renin Angiotensin Aldosterone Syst 7(3):155–161
Luchner A, Stevens TL, Borgeson DD, Redfield MM, Bailey JE, Sandberg SM et al (1996) Angiotensin II in the evolution of experimental heart failure. Hypertension 28(3):472–477
Luchner A, Stevens TL, Borgeson DD, Redfield M, Wei CM, Porter JG et al (1998) Differential atrial and ventricular expression of myocardial BNP during evolution of heart failure. Am J Physiol 274(5 Pt 2):H1684–H1689
Luchner A, Jougasaki M, Friedrich E, Borgeson DD, Stevens TL, Redfield MM et al (2000) Activation of cardiorenal and pulmonary tissue endothelin-1 in experimental heart failure. Am J Physiol Regul Integr Comp Physiol 279(3):R974–R979
Luchner A, Muders F, Dietl O, Friedrich E, Blumberg F, Protter AA et al (2001) Differential expression of cardiac ANP and BNP in a rabbit model of progressive left ventricular dysfunction. Cardiovasc Res 51(3):601–607
Maki T, Nasa Y, Tanonaka K, Takahashi M, Takeo S (2003a) Direct inhibition of neutral endopeptidase in vasopeptidase inhibitor-mediated amelioration of cardiac remodeling in rats with chronic heart failure. Mol Cell Biochem 254(1–2):265–273
Maki T, Nasa Y, Tanonaka K, Takahashi M, Takeo S (2003b) Beneficial effects of sampatrilat, a novel vasopeptidase inhibitor, on cardiac remodeling and function of rats with chronic heart failure following left coronary artery ligation. J Pharmacol Exp Ther 305(1):97–105
Martin FL, Stevens TL, Cataliotti A, Schirger JA, Borgeson DD, Redfield MM et al (2005) Natriuretic and antialdosterone actions of chronic oral NEP inhibition during progressive congestive heart failure. Kidney Int 67(5):1723–1730
Mishima T, Tanimura M, Suzuki G, Todor A, Sharov VG, Tanhehco EJ et al (2002) Effects of chronic neutral endopeptidase inhibition on the progression of left ventricular dysfunction and remodeling in dogs with moderate heart failure. Cardiovasc Drugs Ther 16(3):209–214
Moe GW, Armstrong P (1999) Pacing-induced heart failure: a model to study the mechanism of disease progression and novel therapy in heart failure. Cardiovasc Res 42(3):591–599
Munagala VK, Burnett JC, Redfield MM (2004) The natriuretic peptides in cardiovascular medicine. Curr Probl Cardiol 29(12):707–769
Murohara Y, Johnston CI (1992) Effect of neutral endopeptidase inhibitor in rats with congestive heart failure. Clin Exp Pharmacol Physiol 19(5):380–383
Nishikimi T, Mori Y, Ishimura K, Ishikawa Y, Koshikawa S, Akimoto K et al (2006) Chronic effect of combined treatment with omapatrilat and adrenomedullin on the progression of heart failure in rats. Am J Hypertens 19(10):1039–1048
Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau J et al (2002) Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 106(8):920–926
Quaschning T (2005) Vasopeptidase inhibition for blood pressure control: emerging experience. Curr Pharm Des 11(25):3293–3299
Quaschning T, Ruschitzka F, Lüscher TF (2002) Vasopeptidase inhibition: effective blood pressure control for vascular protection. Curr Hypertens Rep 4(1):78–84
Quaschning T, Galle J, Wanner C (2003) Vasopeptidase inhibition: a new treatment approach for endothelial dysfunction. Kidney Int Suppl 84:S54–S57
Regamey F, Maillard M, Nussberger J, Brunner HR, Burnier M (2002) Renal hemodynamic and natriuretic effects of concomitant angiotensin-converting enzyme and neutral endopeptidase inhibition in men. Hypertension 40(3):266–272
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB et al (2012) Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 125(1):e2–e220
Roques BP (1998) Cell surface metallopeptidases involved in blood pressure regulation: structure, inhibition and clinical perspectives. Pathol Biol 46(3):191–200
Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP (2010) Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet 375(9722):1255–1266
Schneider H, Husser O, Rihm M, Fredersdorf S, Birner C, Dhein S et al (2009) Safety of the novel atrial-selective K+ -channel blocker AVE0118 in experimental heart failure. Naunyn Schmiedebergs Arch Pharmacol 379(3):225–232
Spinale FG, Eble DM, Mukherjee R, Johnson WS, Walker JD (1994) Left ventricular and myocyte structure and function following chronic ventricular tachycardia in rabbits. Basic Res Cardiol 89(5):456–467
Stasch JP, Hirth-Dietrich C, Ganten D, Wegner M (1996) Renal and antihypertensive effects of neutral endopeptidase inhibition in transgenic rats with an extra renin gene. Am J Hypertens 9(8):795–802
Staudt A, Landsberger M, Staudt Y, Felix SB (2002) Die Rolle der Zytokine bei der Herzinsuffizienz. Herz 27(7):691–698
Xu J, Carretero OA, Liu Y, Yang F, Shesely EG, Oja-Tebbe N et al (2004) Dual inhibition of ACE and NEP provides greater cardioprotection in mice with heart failure. J Card Fail 10(1):83–89
Acknowledgements
Supported by the Deutsche Forschungsgemeinschaft (Lu 562/3-1,2), an institutional grant of the Universität Regensburg (ReForM-A and -B), the Bayerische Forschungsförderung (Az 446/01) and by an educational grant by Bristol Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Birner, C., Ulucan, C., Bratfisch, M. et al. Antihypertrophic effects of combined inhibition of the renin–angiotensin system (RAS) and neutral endopeptidase (NEP) in progressive, tachycardia-induced experimental heart failure. Naunyn-Schmiedeberg's Arch Pharmacol 385, 1117–1125 (2012). https://doi.org/10.1007/s00210-012-0791-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-012-0791-6