Abstract
Proliferation and apoptosis of endothelial cells are crucial angiogenic processes that contribute to carcinogenesis and tumor progression. Emerging evidence implicates the regulation of proliferation and apoptosis by reactive oxygen species (ROS) such as superoxide and hydrogen peroxide (H2O2). In the present study, we investigated the roles of the ROS-generating Nox4- and Nox2-containing reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidases in proliferation of human endothelial cells by examining the impact of these enzyme systems on (1) specific proliferative and tumorigenic kinases, extracellular regulated kinase1/2 (ERK1/2) and Akt, (2) cytoskeletal organization, and (3) the mechanisms that influence cellular apoptosis. ROS production and the expression of NADPH oxidase subunit Nox4, but not Nox2, were markedly higher in proliferating than in quiescent endothelial cells. Addition of the H2O2 scavenger catalase or downregulation of Nox4 protein with specific siRNA reduced ROS levels, cell proliferation, and ERK1/2 phosphorylation but had no effect on either cell morphology or caspase 3/7 activity. Although downregulation of Nox2 protein with siRNA also reduced ROS production and cell proliferation, it caused an increase in caspase 3/7 activity, reduced Akt phosphorylation, and caused cytoskeletal disorganization. Therefore, in endothelial cells, Nox4-derived H2O2 activates ERK1/2 to promote proliferation, whereas Nox2-containing NADPH oxidase maintains the cytoskeleton and prevents apoptosis to support cell survival. Our study provides a new understanding of the molecular mechanisms that underpin endothelial cell survival and a rationale for the combined suppression of Nox4- and Nox2-containing NADPH oxidases for unwanted angiogenesis in cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mechanisms of proliferation and apoptosis of vascular endothelial cells are finely regulated yet contrasting processes, that when deregulated underpin pathophysiological effects such as angiogenesis during cancer (Hanahan and Weinberg 2000; Fesik 2005). Yet, while endothelial cell proliferation and apoptosis are fundamentally distinct processes, recent evidence indicates that both are largely regulated by what might appear on the surface to be similar changes in cellular redox state (Irani 2000; Stone and Collins 2002). In fact, a number of studies have implicated a role for reactive oxygen species (ROS), such as superoxide and hydrogen peroxide (H2O2), in both cell proliferation and apoptosis (Rao and Berk 1992; Brown et al. 1999; Zanetti et al. 2001, 2002; Deshpande et al. 2002; Kim et al. 2006) that are necessary for tumor growth and maintenance. For example, it is well established that ROS are crucial cell signaling molecules that transduce effects of angiogenic factors and cytokines such as vascular endothelial growth factor (VEGF), angiopoietin-1, and TNF-α (Chen et al. 2004; Chen et al. 2006; Harfouche et al. 2005; Ushio-Fukai et al. 2002; Ushio-Fukai 2007). Moreover, ROS, such as H2O2, promote cell proliferation by influencing the tumorigenic mitogen-activating protein kinase pathways, such as by phosphorylating extracellular regulated kinase1/2 (ERK1/2). While it is intuitive that the lowering of ROS levels will arrest cell proliferation, what may be surprising is that this could be attributed to the promotion of cell apoptosis. In fact, ROS are also acknowledged to influence important cell survival factors such as Akt and to be crucial mediators in the re-organization of the cytoskeleton during proliferation (Moldovan et al. 2000). The observations that similar ROS can influence opposing cell functions such as proliferation and apoptosis by regulating the activity of tumor-promoting kinases such as ERK1/2 and Akt are intriguing and highlight important new roles of ROS in tumor biology. Furthermore, by limiting the formation of ROS from the outset, i.e., through the inhibition of enzymatic production of ROS, it could be possible to suppress tumor progression by inhibiting proliferation and, at the same time, by inducing apoptosis of endothelial cells in the vasculature supplying the tumor.
The enzyme family most likely to influence the production of ROS, and thus endothelial cell proliferation (Abid et al. 2000; Ushio-Fukai et al. 2002) and apoptosis, is the reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase family. NADPH oxidase enzyme complexes consist of a flavocytochrome b558 reductase composed of a “Nox” catalytic subunit, a smaller p22phox subunit, regulatory subunits including the organizer proteins, p47phox or NoxO1, activator proteins p67phox or NoxA1, and the small molecular weight G protein Rac1 (Opitz et al. 2007; Selemidis 2008; Selemidis et al. 2008). At least five isoforms of the Nox subunit have been identified (namely Nox1 to Nox5); however, it appears that Nox2, Nox4, and Nox5 are expressed in endothelial cells (Li and Shah 2002; Bedard and Krause 2007; Peshavariya et al. 2007; Selemidis et al. 2007, 2008; Selemidis 2008) that are likely to produce significant amounts of superoxide and H2O2 for pathophysiological processes necessary for tumor progression (Xia et al. 2007).
Evidence supporting a role for ROS produced by Nox2- and/or Nox4-containing NADPH oxidase in the proliferation of endothelial cells already exists. For example, expression of p22phox, which modulates both Nox2 and Nox4 but not Nox5 enzyme activity (Kawahara et al. 2005), is substantially higher in proliferating endothelial cells than quiescent cells (Bayraktutan 2005). Furthermore, transfection of cells with anti-sense for p22phox dramatically reduces both ROS production and cell proliferation (Bayraktutan 2005). Furthermore, overexpression of Nox2 or Nox4 in endothelial cells increases ROS production and stimulates cell proliferation, whereas siRNA silencing of either Nox isoform decreases both ROS production and cell proliferation (Petry et al. 2006; Datla et al. 2007).
Although endothelial cells utilize both Nox2 and Nox4 in their proliferation, the differential subcellular localization of these Nox proteins is compatible with the new concept that they influence cell proliferation and survival by distinct mechanisms. For example, the specific localization of Nox4 to the nuclear membrane and endoplasmic reticulum (Van Buul et al. 2005; Chen et al. 2008) puts it in an ideal position to generate ROS within these sites to then activate kinases such as ERK1/2 and promote cell proliferation. By contrast, the association of Nox2 and its regulatory subunits p47phox and p67phox to the cytoskeleton (Li and Shah 2002; Ikeda et al. 2005) would place this Nox isoform in a position to influence the re-arrangement of the cytoskeleton during cell proliferation. In light of the subcellular localization patterns of Nox4 and Nox2, in the present study, we tested the hypothesis that Nox4 produces ROS to directly cause endothelial cell proliferation, whereas Nox2 supports proliferation by being anti-apoptotic via its effects on the cytoskeleton. Our study aims to provide new information as to how ROS emanating from Nox-containing oxidases contribute to the endothelial cell survival and proliferation that is pivotal for tumor progression.
Materials and methods
Cell culture
Human microvascular endothelial cells (HMECs; dermal in origin; passages 10–14) were a kind gift from Prof Philip Hogg from the University of New South Wales, Sydney, Australia. Both HMECs and human umbilical vein endothelial cells (HUVEC; Cambrex Corporation) were cultured in Microvascular endothelial growth medium (EGM-MV; Bullekit, Clonetics) containing 10% fetal bovine serum (FBS) in endothelial basal medium using standard cell culture techniques.
Cell proliferation assay: effect of ROS scavengers, enzyme inhibitors, and siRNA for Nox4 and Nox2
HMECs were seeded (105 cells/well, day 0) in six-well tissue culture plates at a level of confluency (~25%) for proliferation to take place. After 24 h, cells were replenished with fresh growth medium containing 10% FBS in the absence or presence of either (1) catalase (1,000 U/mL to scavenge endogenous H2O2), (2) diphenyleneiodonium (DPI; 1 μM to inhibit flavin adenine dinucleotide (FAD)-containing enzymes), (3) NG-nitro-l-arginine-methyl ester (l-NAME; 100 μM and 1 mM; to inhibit nitric oxide synthase (NOS)), (4) allopurinol (100 μM; to inhibit xanthine oxidase), (5) indomethacin (3 μM; to inhibit cyclooxygenase), (6) 17-octadecanoic acid (17-ODYA; 100 μM; to inhibit cytochrome P450), (7) U0126 (10 μM; to inhibit mitogen-activated protein kinase1/2 (MEK1/2)), or (8) LY294002 (to inhibit PI3-kinase; 10 μM) for 48 h. Some HMECs were starved of FBS for 48 h to remove the stimulus for cell growth (i.e., serum starvation group).
For siRNA experiments, cells were starved of serum and antibiotics for 24 h and then treated with control siRNA, Nox4 siRNA, or Nox2 siRNA (final concentration 100 nM) in the presence of lipofectamine 2000 for 5 h. Cells were then washed with phosphate-buffered saline (PBS) and incubated with complete growth medium containing 10% FBS for 72 h. Following either 48 h (for pharmacological inhibitor and serum removal studies) or 72 h (for siRNA transfection studies), cells were collected by trypsinisation, resuspended in complete growth medium, and then mixed with an equal volume of trypan blue (0.4%) solution. Cell numbers were then determined using a hemocytometer.
MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) cell viability assay
A tetrazolium-based assay was also used to determine cell number and, thus, the degree of cell proliferation. Following either 48 h (for pharmacological inhibitor and serum removal studies) or 72 h (for siRNA transfection studies), cells were washed with PBS and incubated with 100 μl of CellTiter 96TM AQ solution and 400 μl of phenol-free Dulbecco’s modified essential medium (DMEM) for 1 h at 37°C in a 5% CO2 incubator. Absorbance at 490 nm was then determined using a Polarstar plate reader (BMG, Germany) after incubation.
Expression of Nox proteins and ERK1/2 and Akt in proliferating versus quiescent cells
Expression levels of Nox4, Nox2, ERK1/2, phosphorylated ERK1/2, Akt, and phosphorylated Akt were determined in endothelial cells during different proliferative states, as described previously (Kondrikov et al. 2006). HMECs or HUVEC were grown in 100 mm tissue culture plates and allowed to reach confluence, after which time cells were seeded using a ratio of 1:8 (37.5 × 104 cells per plate) or 1:2 (150 × 104 cells per plate) in separate 100 mm tissue culture plates. Cells were allowed to grow for 48 h, at which time they had doubled in number and reached 25% (i.e., proliferating) and 100% (quiescent) confluence, respectively. Cells were then harvested and used for Western blot analysis.
Superoxide and ROS detection
HMECs and HUVEC were seeded to achieve proliferating and quiescent states in the manner mentioned above either in white (for chemiluminescence assays) or black (for fluorescence assays) 96-well Optiplates (Packard, USA) and allowed to grow for 48 h. Superoxide and total ROS levels were also assessed in HMECs (1) in the presence of pharmacological inhibitors (45 min treatment period) including the superoxide scavenger, tiron, the FAD inhibitor and nonselective inhibitor of NADPH oxidase, DPI, and (2) following control and specific siRNA for Nox4 and Nox2. Superoxide production was quantified using lucigenin (5 μM)-enhanced chemiluminescence, whereas total ROS production was quantified by dichlorodihydrofluorescein diacetate (H2DCF-DA; 10 μM) fluorescence as we have described previously (Peshavariya et al. 2007).
Western blot analysis
The effect of Nox2 and Nox4 siRNA on Nox2 and Nox4 protein expression and on phosphorylation of ERK1/2 and Akt was determined after 48 h of transfection using Western blotting, as described previously (Selemidis et al. 2007). Expression of Nox2 and Nox4 protein was also determined in Triton-X soluble and insoluble HMEC fractions as described previously (Li and Shah 2002). The Gene Genius Bio imaging system was used to capture the images and for densitometry analysis.
Fluorescence microscopy for actin organization
HMECs were grown in eight-well chamber slides. Cells were transfected either with control, Nox2, or Nox4 siRNA as described above. After 24 h of transfection, cells were incubated with serum-free media overnight. Cells were then treated with 10% serum-containing medium for 1 h. In some cases, cells were treated with DPI (1 μM) or latrunculin B (0.5 μM) for 1 h before serum treatment. Then, cells were washed three times with PBS and fixed with 4% paraformaldehyde for 10 min. Cells were washed three times and permeabilized with 0.1% triton-X for 5 min. Nonspecific binding sites were blocked for 1 h in 3% bovine serum albumin prepared in PBS. Cells were treated with 200 μl of phalloidin-Alexa488 (1:40 dilution) overnight at 4°C as per manufacturer’s instruction. Unbound phalloidin was washed with PBS. Cell nuclei were stained with propidium iodide (0.1 μg/ml) for 1 min, and then, fluorescence due to F-actin was observed using a fluorescent microscope (Zeiss axiovision).
Apoptosis assay
Caspase 3/7 activity was determined to quantify the degree of apoptosis induced following addition to the cells of catalase, DPI, Nox-isoform specific siRNA, or the cytoskeletal disrupting agent, latrunculin B (0.5 μM). Caspase 3/7 activities were measured with Caspase-Glo® 3/7 luminescence assay as per the manufacturer’s (Promega) instructions. Briefly, cells were seeded in six-well plates to a level of ~25% confluency as described above. After 48 h (for catalase and DPI studies) and 72 h (for siRNA studies), the media were removed, and 100 μl of Caspase-Glo® 3/7 solution was added to each well. The entire volume was then transferred to a 96-well white Optiplate and incubated for 48 h. Luminescence was measured with the Polarstar plate reader.
Statistical analysis
All results are expressed as mean ± standard error of the mean (SEM). Statistical comparisons were made using one-way analysis of variance (ANOVA) with Tukey–Kramer post hoc tests. In some cases, paired t tests were used to compare data within the same group. P < 0.05 was considered significant.
Materials and their suppliers
EGM-MV culture media and fetal bovine serum were purchased from Clonetics (CA, USA). Nox2 siRNA, control siRNA and anti-Nox4 goat polyclonal antibodies (product ID (N-15): sc-21860) were purchased from Santa Cruz Biotechnology (USA). Specific Nox4 (product ID 118807) and control siRNA were from Ambion (USA). Anti gp91phox (Nox2) rabbit polyclonal antibodies were purchased from Upstate Biotechnology (USA). Anti-β-actin mouse monoclonal antibodies were purchased from Abcam (USA). Anti phospho-ERK and total ERK, phospho-Akt, and total Akt rabbit polyclonal antibodies were purchased from Cell signaling (MA, USA). Cell-Titer 96TM AQ proliferation and Caspase-Glo® 3/7 assay kits were obtained from Promega (Madison, WI, USA). All other chemicals were obtained from Sigma Chemical (MO, USA).
Results
Superoxide and ROS
HMECs generated superoxide as detected by lucigenin-enhanced chemiluminescence, which was virtually abolished by the superoxide scavenger tiron (11 ± 3.7% of control, P < 0.001) or the nonselective FAD inhibitor DPI (8.3 ± 3.8% of control, P < 0.001). Intracellular ROS generation detected by H2DCF-DA fluorescence was also significantly inhibited by tiron (68 ± 2% of control, P < 0.001) and by DPI (65 ± 11% of control P < 0.001) but was unaffected by l-NAME (1 mM; 113 ± 5% of control) treatment.
ROS scavengers and NADPH oxidase inhibition suppress proliferation of HMECs
Treatment for 48 h with catalase (1,000 U/ml) or DPI (1 μM) inhibited HMEC proliferation, as evidenced by a significant reduction in cell number (P < 0.001; Fig. 1a, c) and MTS absorbance (P < 0.001, Fig. 1b, d) compared to control. In contrast, allopurinol, indomethacin, and 17-ODYA had no effect on proliferation (Fig. 1c, d). In addition, l-NAME (100 μM or 1 mM) had no significant effect on cell proliferation (Fig. 1c, d; and for 1 mM; control 31.6 × 104 ± 1.7 cells versus l-NAME 33.2 × 104 ± 1.7 cells, n = 4). Serum starvation for 48 h markedly inhibited cell proliferation (Fig. 1a, b).
Effects of H2O2 scavenging and inhibitors of potential enzymatic sources of ROS on HMECs proliferation. HMECs were treated with either catalase (1,000 U/mL), DPI (1 μM), l-NAME (100 μM), allopurinol (100 μM), indomethacin (3 μM), or 17-ODYA (100 μM) or, in some cases, were serum-starved for 48 h. Cell proliferation was assessed by trypan blue cell counting (a, c) or the MTS cell viability assay (b, d). Values (mean ± SEM from five experiments) are expressed as (a, c) cell counts or (b, d) absorbance at 490 nm expressed as a percentage of the control. *P < 0.05 value following a one-way ANOVA with Tukey–Kramer post hoc analysis
Nox expression and ROS production in confluent versus proliferating endothelial cells
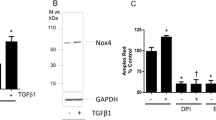
Western blot analysis revealed a significant, ~4-fold higher expression of Nox4 in proliferating HMECs compared to quiescent cells (Fig. 2a, b). By contrast, the expression level of Nox2 was similar in proliferating and quiescent endothelial cells (Fig. 2a, b). Superoxide and ROS production, as assessed by lucigenin chemiluminescence and H2DCF-DA fluorescence, respectively, were significantly higher in proliferating versus quiescent HMECs (P < 0.05, Fig. 2c, d). Similar studies performed in HUVEC revealed that Nox4 expression, but not Nox2 expression, was higher in proliferating cells than in confluent cells (Fig. 2e, f).
ROS production and expression of Nox4 and Nox2 in proliferating and confluent HMECs and HUVEC. Representative Western blots of Nox4 and Nox2 expression, as well as β-actin expression in confluent and proliferating a HMECs and e HUVEC. b, f Densitometry analysis of Nox4 and Nox2 protein expression where values (mean ± SEM from four experiments) are represented as optical density/mm2 (OD/mm2), normalized to the respective β-actin level and then as fold-induction above confluent cells. Superoxide and total ROS levels were determined by c lucigenin-enhanced chemiluminescence and d H2DCF-DA fluorescence in HMECs. Values (mean ± SEM from five experiments) are expressed as counts per second (CPS; c) and relative fluorescence units (RFU; d) were normalized per 103 cells and expressed as a percentage of the values obtained in confluent cells. *P < 0.05 value following a Students’ paired t test
Effect of siRNA for Nox4 and Nox2 on ROS levels and cell proliferation
Transfection of HMECs with Nox2 siRNA significantly reduced expression of Nox2 but not Nox4 protein (Fig. 3a). Nox2 siRNA also decreased superoxide and total ROS levels in HMECs (Fig. 3b). Nox4 siRNA reduced Nox4 protein expression but had no effect on Nox2 expression (Fig. 3a). While Nox4 siRNA only had a relatively small, albeit significant, inhibitory effect on superoxide production (i.e., by 25%), its effects on total ROS appeared to be substantially greater (i.e., ~40% inhibition; Fig. 3b).
Effects of siRNA silencing of Nox4 and Nox2 protein on a Nox4 and Nox2 expression, b superoxide and ROS levels, and c cell proliferation in HMECs. HMECs were either untreated (control) or treated with control siRNA, Nox2, or Nox4 specific siRNA molecules for 48 h. a Representative immunoblots of Nox2 and Nox4 expression from five individual experiments. b Superoxide and total ROS were measured by either lucigenin-enhanced chemiluminescence or H2DCF-DA fluorescence from a total of five experiments. Cell proliferation was assessed by c trypan blue cell counting or d the MTS cell viability assay. Values (mean ± SEM from four experiments) are expressed as cell counts (c) and absorbance at 490 nm expressed as a percentage of the control (d).*P < 0.05 value following a one-way ANOVA with Tukey–Kramer post hoc analysis
Also, Nox2 and Nox4 siRNA significantly inhibited cell proliferation, as assessed by either cell counting (Fig. 3c) or MTS absorbance (Fig. 3d).
Nox isoform-derived ROS regulate ERK1/2 and Akt in endothelial cell proliferation
The MEK inhibitor U0126 (10 μM) markedly suppressed the phosphorylation of ERK1/2 without affecting the total expression of the protein (Fig. 4a). This attenuation of phosphorylated ERK1/2 by U0126 significantly suppressed endothelial cell proliferation (Fig. 4c). The PI-3 K inhibitor, LY294002 (10 μM) almost abolished the phosphorylation of Akt without affecting overall expression levels of the protein (Fig. 4b). LY294002 also caused a significant reduction in cell proliferation (Fig. 4c).
Effect of MEK1/2 and PI3-kinase inhibition and siRNA for either Nox4 or Nox2, on phosphorylated ERK1/2 and phosphorylated Akt, and cell proliferation. a, b Representative Western blots of phosphorylation and total levels of ERK1/2 and Akt in the absence or presence of the MEK1/2 inhibitor UO126 (1 μM) or the PI3-kinase inhibitor, LY294002 (1 μM) in HMECs. c Cell proliferation as assessed by trypan blue cell counting where the values are mean ± SEM from five experiments. d, e Representative Western blots of phosphorylated ERK1/2 and Akt following siRNA silencing of either Nox4 or Nox2. f, g Densitometry analysis of the ratio of phosphorylated ERK/total ERK and phosphorylated Akt/total Akt where values (mean ± SEM from five experiments) are represented as optical density per square millimeter (OD/mm2), normalized to the respective β-actin level and then as a percentage of the confluent cells
We also assessed the degree of ERK1/2 and Akt phosphorylation in quiescent versus proliferating endothelial cells. Proliferating endothelial cells had significantly higher levels of phosphorylated ERK1/2 and Akt compared to quiescent cells (Fig. 4d, f). Despite the increase in phosphorylation, the overall protein expression of both ERK1/2 and Akt were not different between quiescent and proliferating endothelial cells (Fig. 4d, f). Selective siRNA gene silencing of Nox2 had no significant effect on phosphorylated ERK1/2 but significantly reduced phosphorylation of Akt (Fig. 4e, g). By contrast, Nox4 siRNA significantly reduced both ERK1/2 and Akt phosphorylation (Fig. 4e, g). Neither Nox2 nor Nox4 siRNA had any effect on total ERK1/2 and Akt expression levels (Fig. 4e).
Nox2-derived ROS but not Nox4 modulates endothelial cell apoptosis
Incubation of HMECs with DPI (1 μM), or Nox2 siRNA silencing of Nox2, increased caspase 3/7 activity (P < 0.05, Fig. 5a, b). Also, the actin polymerisation inhibitor, latrunculin B (0.5 μM) caused a significant increase in caspase 3/7 activity (Fig. 5a). In contrast, caspase 3/7 activity was unaffected by siRNA silencing of Nox4 or catalase (1,000 U/ml; Fig. 5a, b).
Caspase 3/7 activity in HMECs. Cells were treated with either a catalase (1,000 U/mL), DPI (1 μM) the NADPH oxidase inhibitor for 48 h, or latrunculin B (0.5 μM) or b Nox2 and Nox4 siRNA for 72 h. Values (mean ± SEM from four experiments) are CPS and then expressed as a percentage of the control. *P < 0.05 value following a one-way ANOVA with Tukey–Kramer post hoc analysis
Nox2 but not Nox4 influences cytoskeletal organization
The differential effects of Nox2 and Nox4 isoforms of NADPH oxidase on kinases ERK1/2 and Akt, and apoptosis, may be due to their subcellular localization. Indeed, we found that Nox2 protein was present in triton-X insoluble fractions suggesting that this protein is associated with the cytoskeleton, as shown in Fig. 6. By contrast, Nox4 protein was mainly found in the triton-X soluble fractions, similar to glyceraldehyde 3-phosphate dehydrogenase (Fig. 6).
Using phase contrast microscopy, it was evident that transfection of HMECs with Nox2 siRNA but not Nox4 siRNA dramatically influenced the morphology of the cells (Fig. 7a). A similar effect on cell morphology occurred with DPI (1 μM) and latrunculin B (0.5 μM, Fig. 7a). Nox2 siRNA, DPI, and latrunculin B also reduced phalloidin fluorescence in HMECs suggesting a disorganization of filamentous actin (Fig. 7b). By contrast, downregulation of Nox4 with siRNA had no visible effect on phalloidin fluorescence (Fig. 7b).
Discussion
ROS play a fundamental role in all aspects of cancer biology, in particular by not only influencing the survival of endothelial cells of new blood vessels that supply but also the tumor cells themselves. In the present study, we have shown that ROS, most likely H2O2 generated by Nox4-containing NADPH oxidases, cause proliferation of endothelial cells via the activation of ERK1/2 and Akt. Furthermore, the data reveal that Nox2 oxidase-dependent superoxide production is anti-apoptotic by regulating the morphology of the cytoskeleton. These findings may be taken to indicate that Nox2 and Nox4 act in concert and, most probably via their unique subcellular localization, to govern whether endothelial cells will be in a state of apoptosis, quiescence or proliferation. Thus, dual inhibition of Nox4- and Nox2-containing NADPH oxidases may be a novel means for simultaneously causing suppression of proliferation and induction of apoptosis, respectively, two processes that are fundamental for halting the angiogenesis that supports tumor development.
We provide several lines of evidence to support a fundamental role for ROS in the proliferation and survival of endothelial cells. First, we have shown using two methods of ROS detection that proliferating endothelial cells produce markedly higher levels of superoxide and H2O2 than quiescent cells. Second, the addition of a powerful stimulus for proliferation, i.e. serum, to endothelial cells substantially raised ROS production and cell proliferation. Third, the scavenging of endogenous H2O2 with catalase markedly suppressed the ability of endothelial cells to proliferate. The most likely source of this ROS for cell proliferation is NADPH oxidase. To establish this, we treated cells with DPI, a FAD inhibitor and reputed NADPH oxidase inhibitor. DPI reduced superoxide and total ROS levels by about 90% and 50%, respectively. This effect of DPI was probably due to an action on NADPH oxidase activity since selective inhibition of other known FAD-containing enzymes and potential sources of superoxide in human endothelial cells, including eNOS (with l-NAME), xanthine oxidase (with allopurinol), cytochrome P450 (with 17-ODYA), and cyclooxygenase (with indomethacin), all had no effect on superoxide levels (Peshavariya et al. 2007) and cell proliferation.
Although HMECs and HUVEC express both Nox4 and Nox2, we found that Nox4 expression is markedly higher in proliferating cells than in quiescent cells, whereas Nox2 expression remained largely constant between the two states of proliferation. A specific role for Nox4 in endothelial cell proliferation can be postulated from these data; however, they do not discriminate between cause and effect. Thus, to address this, we employed an siRNA approach to selectively downregulate Nox4 expression. Endothelial cells transfected with Nox4 siRNA prior to being stimulated by serum to proliferate had lower levels of Nox4 protein, reduced levels of both superoxide and ROS, and failed to proliferate. Overall, these findings suggest that, in human endothelial cells, a Nox4-containing NADPH oxidase provides ROS that are crucial for cell proliferation.
Several previous studies have demonstrated that Nox2-containing NADPH oxidase is important for endothelial cell proliferation (Abid et al. 2000; Ushio-Fukai et al. 2002; Petry et al. 2006). For example, Ushio-Fukai et al. showed that VEGF-dependent superoxide production and proliferation of HUVEC were suppressed by a reduction in Nox2 protein expression using Nox2 anti-sense oligonucleotides (Ushio-Fukai et al. 2002). Similarly, Petry et al. provided evidence that siRNA gene silencing of Nox2 inhibited endothelial cell proliferation (Petry et al. 2006). The findings of the present study are consistent with both these studies in that they indicate that Nox2 activity is crucial for endothelial cell proliferation to occur. Here we have provided evidence that Nox2-derived superoxide/ROS is unlikely to be directly responsible for causing cell proliferation, as the expression level of Nox2 was not higher in proliferating cells than confluent cells and was not altered by the stimulus for cell proliferation (i.e., serum). Despite these possibilities, any expressional changes of Nox2 are likely to influence cell proliferation, as shown by our siRNA data. Indeed, gene silencing of Nox2 protein expression with siRNA in the present study caused a marked increase in caspase activity indicating that Nox2 is anti-apoptotic. Thus, Nox2 expression and the levels of ROS it generates are necessary for promoting the survival of cells, which supports the processes of cell proliferation.
Our findings that siRNA silencing of Nox2 but not Nox4 causes apoptosis suggest that these proteins, probably through their unique subcellular localization and generation of ROS, activate distinct downstream signaling targets. In endothelial cells, Nox4 is expressed within the perinuclear regions of the cell (Petry et al. 2006) and most likely on the membranes of the nucleus and endoplasmic reticulum (Chen et al. 2008; Li and Shah 2002; Van Buul et al. 2005). Our data showing that Nox4 is predominantly expressed in the triton-X soluble fraction also supports the idea that Nox4 is localized predominantly in the nucleus and endoplasmic reticulum (ER). This localization of Nox4 is likely to position the protein in key sites within the cell to regulate cell proliferation through generation of H2O2. By contrast, Nox2 is expressed in the plasma membrane of the cell, and it is also associated with actin (Ikeda et al. 2005; Van Buul et al. 2005). We found Nox2 to precipitate within the triton-X insoluble fraction that contains the cytoskeleton components of the cell. Thus Nox2 might regulate the morphology of the cytoskeleton during cell proliferation and migration. Indeed, the processes of actin polymerization are redox-sensitive and dependent on superoxide production (Moldovan et al. 2000). In the present study, downregulation of Nox2 with siRNA caused a marked alteration in the filamentous actin organization, as assessed by phalloidin fluorescence. This effect on cell morphology by Nox2 downregulation was similar to that of the actin polymerization inhibitor, latrunculin B. Furthermore, both the downregulation of Nox2 with siRNA and latrunculin B treatment caused cell apoptosis. In stark contrast, downregulation of Nox4 with siRNA had no effect on the cytoskeleton and did not result in apoptosis. It is plausible that a unique property of Nox2-containing NADPH oxidases that associate with actin, at least in endothelial cells, is to provide superoxide to regulate the redox-sensitive processes of actin polymerisation, which ultimately influences cytoskeletal morphology during proliferation. However, we are by no means suggesting that this is the only way that Nox2 can influence cell proliferation. In fact, it is likely that membrane-bound Nox2, the activity of which is dependent on Rac-1, redox regulates tyrosine kinase receptors such as the VEGF receptor during cell proliferation as demonstrated by Ushio-Fukai et al. (2002).
A recent study by Pendyala et al. 2008 shows that, under hyperoxia conditions, Nox4 and Nox2 may compensate for one another (Natarajan et al. 2008). Although we did not see such an effect in HMECs in our study, the difference may be due to either the cell type employed or to the conditions of the assay (hyperoxia vs normoxia in our study). Despite these differences, it is important in future studies that possible compensatory mechanisms are taken into consideration with both siRNA transfection studies and use of Nox knockout mice.
In the present study, the scavenging of H2O2 with catalase, like downregulation of Nox4 expression with siRNA, suppressed endothelial cell proliferation without causing apoptosis, confirming a number of previous studies that this ROS has proliferative activity. H2O2 can stimulate a variety of kinases including ERK1/2 and Akt, which activate transcription factors such as HIF-1α, AP-1, NF-κB, and Ets-1 that are involved in cell proliferation and survival (Griendling et al. 2000; Irani 2000). We also investigated the relationship between the state of cell growth and expression and phosphorylation of ERK1/2 and Akt, in proliferating versus confluent cells. Our findings verify that both ERK1/2 and Akt are involved in endothelial cell proliferation, as they were both in a higher state of phosphorylation in proliferating cells than in confluent cells. Furthermore, inhibition of ERK1/2 phosphorylation with U0126, which is an inhibitor of MEK1/2, a kinase that acts upstream of ERK, and inhibition of Akt phosphorylation with the PI-3 kinase inhibitor LY294002 both suppressed endothelial cell proliferation. Interestingly, ERK1/2 and Akt phosphorylation are regulated by ROS produced by Nox4-containing NADPH oxidase, as phosphorylation of both kinases was prevented by siRNA silencing of Nox4. Thus, the findings of our study support the concept that nuclear- and ER-bound Nox4 generates H2O2 that phosphorylates ERK1/2 localized within these regions to cause cell proliferation. The recent study by Chen et al. 2008 is consistent with our observations and further indicates that the phosphorylation of ERK1/2 is dependent on the oxidation/inactivation of PTP1B that is also a Nox4-derived H2O2-dependent process (Chen et al. 2008). By contrast, Nox2 silencing with siRNA had no effect on ERK1/2 phosphorylation but reduced the phosphorylation of Akt. This differential regulation of kinase activation likely represents just one example of many potential redox-signaling pathways activated by Nox4 and Nox2 within their specific subcellular locations (Alom-Ruiz et al. 2008).
In summary, the present study has demonstrated that Nox4-containing NADPH oxidases are critical enzymes for endothelial cell proliferation via generation of ROS and activation of ERK1/2 and Akt. In contrast to Nox4, endothelial Nox2 is vital for cytoskeletal organization and cell survival. Our work demonstrates for the first time that the regulation of proliferation and apoptosis occurs via distinct Nox isoforms and highlights the potential for isoform-specific Nox inhibition for suppression of proliferation and the promotion of apoptosis in cancer therapy.
Abbreviations
- DPI:
-
Diphenylene iodonium
- FAD:
-
Flavin adenine dinucleotide
- FBS:
-
Fetal bovine serum
- H2-DCFDA:
-
Dichlorodihydrofluorescein-diacetate
- HMECs:
-
Human microvascular endothelial cells
- HUVEC:
-
Human umbilical vein endothelial cells
- H2O2 :
-
Hydrogen peroxide
- ERK1/2:
-
Extracellular regulated kinase1/2
- l-NAME:
-
NG-Nitro-l-arginine methyl ester
- 170-ODYA:
-
17-Octadecynoic acid
- ROS:
-
Reactive oxygen species
- PI-3 K:
-
Phosphatidyl inositol 3-kinase
- TNF-α:
-
Tumor necrosis factor-alpha
References
Abid MR, Kachra Z et al (2000) NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett 486(3):252–256
Alom-Ruiz SP, Anilkumar N et al (2008) Reactive oxygen species and endothelial activation. Antioxid Redox Signal 10(6):1089–1100
Bayraktutan U (2005) Coronary microvascular endothelial cell growth regulates expression of the gene encoding p22-phox. Free Radic Biol Med 39(10):1342–1352
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313
Brown MR, Miller FJ Jr et al (1999) Overexpression of human catalase inhibits proliferation and promotes apoptosis in vascular smooth muscle cells. Circ Res 85(6):524–533
Chen JX, Chen Y et al (2004) Dual functional roles of Tie-2/angiopoietin in TNF-alpha-mediated angiogenesis. Am J Physiol Heart Circ Physiol 287(1):H187–H195
Chen JX, Zeng H et al (2006) Angiopoietin-1-induced angiogenesis is modulated by endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol 291(4):H1563–H1572
Chen K, Kirber MT et al (2008) Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181(7):1129–1139
Datla SR, Peshavariya H et al (2007) Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol 27(11):2319–2324
Deshpande NN, Sorescu D et al (2002) Mechanism of hydrogen peroxide-induced cell cycle arrest in vascular smooth muscle. Antioxid Redox Signal 4(5):845–854
Fesik SW (2005) Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer 5(11):876–885
Griendling KK, Sorescu D et al (2000) Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 20(10):2175–2183
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Harfouche R, Malak NA et al (2005) Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. Faseb J 19(12):1728–1730
Ikeda S, Yamaoka-Tojo M et al (2005) IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol 25(11):2295–2300
Irani K (2000) Oxidant signaling in vascular cell growth, death, and survival : a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res 87(3):179–183
Kawahara T, Ritsick D et al (2005) Point Mutations in the Proline-rich Region of p22phox Are Dominant Inhibitors of Nox1- and Nox2-dependent Reactive Oxygen Generation. J Biol Chem 280(36):31859–31869
Kim YM, Kim KE et al (2006) Hydrogen peroxide produced by angiopoietin-1 mediates angiogenesis. Cancer Res 66(12):6167–6174
Kondrikov D, Han HR et al (2006) Growth and density-dependent regulation of NO synthase by the actin cytoskeleton in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 290(1):L41–L50
Li JM, Shah AM (2002) Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem 277(22):19952–19960
Moldovan L, Moldovan NI et al (2000) Redox changes of cultured endothelial cells and actin dynamics. Circ Res 86(5):549–557
Natarajan V, Pendyala S et al (2008) Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal . doi:10.1089/ARS.2008.2203
Opitz N, Drummond GR et al (2007) The ‘A’s and ‘O’s of NADPH oxidase regulation: a commentary on “Subcellular localization and function of alternatively spliced Noxo1 isoforms”. Free Radic Biol Med 42(2):175–179
Pendyala S, Usatyuk P, Gorshkova IA, Garcia JG, Natarajan V (2008) Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal 11(4):841–860. doi:10.1089/ars.2008.2231
Peshavariya HM, Dusting GJ et al (2007) Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic Res 41(6):699–712
Petry A, Djordjevic T et al (2006) NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 8(9–10):1473–1484
Rao GN, Berk BC (1992) Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res 70(3):593–599
Selemidis S (2008) Suppressing NADPH oxidase-dependent oxidative stress in the vasculature with nitric oxide donors. Clin Exp Pharmacol Physiol 35(11):1395–1401
Selemidis S, Dusting GJ et al (2007) Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc Res 75(2):349–358
Selemidis S, Sobey CG et al (2008) NADPH oxidases in the vasculature: Molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther 120(3):254–291
Stone JR, Collins T (2002) The role of hydrogen peroxide in endothelial proliferative responses. Endothelium 9(4):231–238
Ushio-Fukai M (2007) VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 9(6):731–739
Ushio-Fukai M, Tang Y et al (2002) Novel role of gp91(phox)-containing NAD(P) H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res 91(12):1160–1167
Van Buul JD, Fernandez-Borja M et al (2005) Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 7(3–4):308–317
Xia C, Meng Q et al (2007) Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res 67(22):10823–10830
Zanetti M, Zwacka R et al (2001) Superoxide anions and endothelial cell proliferation in normoglycemia and hyperglycemia. Arterioscler Thromb Vasc Biol 21(2):195–200
Zanetti M, Katusic ZS et al (2002) Adenoviral-mediated overexpression of catalase inhibits endothelial cell proliferation. Am J Physiol Heart Circ Physiol 283(6):H2620–H2626
Acknowledgments
Supported by the National Health and Medical Research Council (NHMRC) of Australia. Fellowships from the NHMRC supported HP (327401), GJD (400303), GRD (465109), CGS (350327) and SS (232324).
Author disclosure statement
There are no competing financial interests for all authors involved.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peshavariya, H., Dusting, G.J., Jiang, F. et al. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn-Schmied Arch Pharmacol 380, 193–204 (2009). https://doi.org/10.1007/s00210-009-0413-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-009-0413-0