Abstract
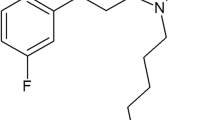
The objective of this study was to characterize the anticonvulsant and acute adverse-effect potentials of topiramate (TPM) and gabapentin (GBP)—two second-generation antiepileptic drugs administered alone and in combination in the maximal electroshock (MES)-induced seizures and chimney test in mice. The anticonvulsant and acute adverse effects of the combination of TPM with GBP at the fixed ratio of 1:1 were determined using the type I isobolographic analysis for nonparallel dose–response relationship curves (DRRCs). To ascertain any pharmacokinetic contribution to the observed interaction between TPM and GBP, total brain concentrations of both drugs were determined. The isobolographic analysis of interaction for TPM and GBP, whose DRRCs were not parallel in both MES and chimney tests, was accompanied with a presentation of all required calculations allowing the determination of lower and upper lines of additivity. The isobolographic analysis revealed that TPM combined with GBP at the fixed-ratio combination of 1:1 interacted supraadditively (synergistically) in terms of suppression of MES-induced seizures, and simultaneously, the combination produced additive interaction with respect to motor coordination impairment (adverse effects) in the chimney test. The evaluation of pharmacokinetic characteristics of interaction for the combination of TPM with GBP revealed that neither TPM nor GBP affected their total brain concentrations in experimental animals, and thus, the observed interaction in the MES test was pharmacodynamic in nature. In conclusion, the combination of TPM with GBP, because of supraadditivity in the MES test and additivity in terms of motor coordination impairment in the chimney test as well as lack of pharmacokinetic interactions between drugs, fulfilled the criterion of a favorable combination, worthy of recommendation in further clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Relatively recently, there has appeared a trend to characterize interactions among antiepileptic drugs (AEDs) in preclinical studies using isobolographic analysis (Löscher and Wauquier 1996; Deckers et al. 2000; Leach 2000). Indeed, the isobolographic analysis is a valuable method allowing the precise classification of exact types of interactions among drugs, comprehensively evaluating their nature as: supraadditive (synergistic), subadditive (relatively antagonistic), infraadditive (absolutely antagonistic), indifferent or additive (Berenbaum 1989; Greco et al. 1995; Tallarida 2000; Luszczki and Czuczwar 2003, Luszczki et al. 2003a,b, 2006b). There are two types of isobolographic analysis: type I—used if all examined drugs are fully active, and type II—if one of the drugs produces no effect and is considered as virtually ineffective in an experimental model (Berenbaum 1989; Porreca et al. 1990; Tallarida 2000). Moreover, the isobolographic analysis is based on some specific presumptions allowing the exact classification of observed interactions in vivo. One of these presumptions is the parallelism of dose–response relationship curves (DRRCs) for drugs administered alone. The test for parallelism provides certainty that the analyzed drug dose ratios are constant for every investigated effect (Tallarida 2000). Sometimes, the DRRCs for drugs are not parallel, and there appears a problem related to the misinterpretation of obtained results and false classification of interactions. In such cases, one should perform the modified isobolographic analysis for different regression line slopes according to Grabovsky and Tallarida (2004) and Tallarida (2006, 2007).
In experimental studies, topiramate (TPM), a newer second generation AED, has been found to be fully effective in suppressing maximal electroshock (MES)-induced seizures in mice (Shank et al. 1994). In contrast, gabapentin (GBP), another second generation AED, has been classified as virtually ineffective in the mouse MES test (Bartoszyk et al. 1986; Dalby and Nielsen 1997), although GBP at doses of 75 and 100 mg/kg significantly elevated the threshold for electroconvulsions in mice (Luszczki et al. 2003a). On the other hand, one experimental report has documented that GBP produced a clear-cut anticonvulsant effect against MES-induced seizures in mice, and its median effective dose (ED50) was 78.2 (46.6–127) mg/kg (White et al. 2002). These controversial results, concerning the antiseizure effects of GBP in the MES test in mice, prompted me to determine thoroughly the anticonvulsant and acute adverse-effect (neurotoxic) profiles for GBP administered alone.
Accumulating experimental evidence indicates that TPM potentiated the anticonvulsant activity of numerous coadministered AEDs. Previously, it has been shown isobolographically that TPM interacted synergistically with phenobarbital, carbamazepine (Shank et al. 1994), lamotrigine (Luszczki et al. 2003c), levetiracetam (Sills et al. 2004; Luszczki et al. 2006a), felbamate, oxcarbazepine (Luszczki and Czuczwar 2004a), and loreclezole (Luszczki et al. 2005b) in the MES test in mice. Similarly, GBP combined with conventional and some newer AEDs (i.e., phenytoin, carbamazepine, phenobarbital, valproate, lamotrigine, talampanel, and oxcarbazepine) exerted synergistic interactions in isobolography, at various fixed-ratios in the MES test in mice (Borowicz et al. 2002; Luszczki et al. 2005a). Considering the above-mentioned combinations and their synergistic interactions in the MES test, it was expected that the combination of TPM with GBP would also be synergistic in this experimental model of epilepsy. It is widely accepted that the MES test is considered as an experimental model of tonic-clonic seizures and, to a certain extent, of partial convulsions with or without secondary generalization in humans (Löscher et al. 1991).
To characterize the type of interactions between TPM and GBP, the two AEDs whose DRRCs were not parallel in the MES-induced seizures in mice, the type I isobolographic analysis for different regression line slopes was used. Additionally, the adverse-effect profiles for the combinations of AEDs were investigated in relation to motor coordination impairment in the chimney test also by using the type I isobolographic analysis for different (nonparallel) regression line slopes. To ascertain whether the observed anticonvulsant effects for the combination of GBP with TPM were consequent to a pharmacodynamic and/or a pharmacokinetic interaction, total brain TPM and GBP concentrations were evaluated.
Materials and methods
Animals and experimental conditions
All experiments were performed on adult male albino Swiss mice weighing 22–26 g. The mice were kept in colony cages with free access to food and tap water, under standardized housing conditions (natural light–dark cycle, ambient temperature of 22 ± 1°C, relative humidity of 55 ± 5%). After 7 days of adaptation to laboratory conditions, the animals were randomly assigned to experimental groups consisting of eight mice. Each mouse was used only once. All tests were performed between 0900 and 1400 hours. Procedures involving animals and their care were conducted in accordance with current European Community and Polish law on the experimentation and protection of animals. Additionally, all efforts were made to minimize animal suffering and to use only the number of animals necessary to produce reliable scientific data. The experimental protocols and procedures listed were approved by the Local Ethics Committee at the Medical University of Lublin (License no.: 368/2002/349/2002) and complied with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Drugs
The following AEDs were used in this study: GBP (Neurontin; Parke-Davis, Freiburg, Germany) and TPM (Topamax; Cilag AG, Schaffhausen, Switzerland). Both drugs were suspended in a 1% solution of Tween 80 (Sigma) in saline and were administered intraperitoneally (i.p.), as two separate injections, in a volume of 5 ml/kg body weight. The control animals received adequate amounts of vehicle (1% solution of Tween 80 in saline). Fresh drug solutions were prepared on each day of experimentation and administered 60 min before MES, chimney test, and brain sampling for the measurement of AED concentrations. This pretreatment time before testing of the AEDs was based on information about their biological activity from the literature and our previous experiments (Luszczki and Czuczwar 2004a,b). The time of peak maximum anticonvulsant effects for TPM and GBP (60 min) was used as reference time in all experimental tests and pharmacokinetic estimation of brain AED concentrations.
MES test
Electroconvulsions were produced by means of an alternating current (0.2 s stimulus duration, 50 Hz, maximum stimulation voltage of 500 V) delivered via ear-clip electrodes by a generator (Rodent Shocker, Type 221, Hugo Sachs Elektronik, Freiburg, Germany). The electrical system of the stimulator was self-adjustable so that changes in impedance did not result in alterations of current intensity (i.e., the system provides constant current stimulation). The criterion for the occurrence of seizure activity was the tonic hind limb extension (i.e., the hind limbs of animals outstretched 180° to the plane of the body axis). The protective activities of TPM and GBP administered alone were evaluated and expressed as their median effective doses (ED50 values in mg/kg) against MES-induced seizures. The animals were administered different drug doses so as to obtain a variable percentage of protection against MES, allowing the construction of a dose–response relationship curve (DRRC) for every AED administered separately. Moreover, to ascertain the effects of TPM and GBP in combination, the animals were administered with the mixture of different drug doses (at a fixed-ratio combination of 1:1) so as to obtain a variable percentage of protection against MES, allowing the construction of a DRRC for the AED combination. The anticonvulsant activity of the fixed-ratio combination of 1:1 was evaluated and expressed as the ED50 mix value, corresponding to the total dose of the mixture, necessary to protect 50% of mice against tonic hind limb extension in the MES test. Subsequently, the ED50 and ED50 mix values with their 95% confidence limits were calculated according to log-probit method (Litchfield and Wilcoxon 1949). The experimental procedure has been described in more detail elsewhere (Luszczki and Czuczwar 2003, 2004b; Luszczki et al. 2003b,c).
Chimney test
The effects of TPM and GBP administered alone as well as in combination on motor coordination impairment were quantified by the chimney test of Boissier et al. (1960). In this test, animals had to climb backwards up a transparent plastic tube (3 cm inner diameter, 25 cm length), and motor coordination impairment was indicated by the inability of the animals to climb backward up the tube within 60 s. The acute adverse effects of TPM and GBP, administered alone and in combination, were expressed as their median toxic doses (TD50 and TD50 mix values in mg/kg), representing the doses of AEDs, which impaired motor coordination in 50% of the animals tested (Löscher and Nolting 1991). To evaluate each TD50 value, at least four groups of animals (each group consisted of eight mice) injected with various doses of GBP, TPM, or their combination at the fixed ratio of 1:1 were challenged with the chimney test. The DRRC for each AED was fitted on the basis of the percentage of mice showing motor deficits by means of the log-probit method according to Litchfield and Wilcoxon (1949). This experimental procedure has been described in detail elsewhere (Luszczki et al. 2005a, 2006b). Moreover, the protective index for each AED administered alone was calculated by dividing a given TD50 value, evaluated in the chimney test, by the respective ED50 value determined in the MES test. The protective index is considered an index of the margin of safety and tolerability between anticonvulsant doses and doses of AEDs exerting acute adverse effects (e.g., sedation, ataxia, impairment of motor coordination, or other neurotoxic manifestations) in preclinical studies (Löscher et al. 1991).
Measurement of total brain AED concentrations
The animals were given TPM+ vehicle, GBP+ vehicle, or a combination of TPM and GBP. The fixed-ratio combination for estimating brain concentrations of TPM and GBP was chosen as 1:1 (TPM/GBP) from the MES test. Mice were killed by decapitation at times chosen to coincide with that scheduled for the MES test. The whole brains of the mice were removed from the skulls, weighed, and homogenized using Abbott buffer (2:1 vol/weight) in an Ultra-Turrax T8 homogenizer (IKA-Werke, Staufen, Germany). The homogenates were centrifuged at 10,000 × g for 10 min. The supernatant samples (75 μl) containing TPM were analyzed by fluorescence polarization immunoassay using a TDx analyzer and reagents exactly as described by the manufacturer (Seradyn, Indianapolis, IN, USA). Simultaneously, the brain supernatant samples (100 μl) containing GBP were transferred into the high-pressure liquid chromatography (HPLC) technique of drug detection. Details concerning the technique of estimation of GBP concentrations with HPLC have been presented elsewhere (Luszczki et al. 2003a; Luszczki and Czuczwar 2004b). The brain AED concentrations were expressed in micrograms per milliliter of brain supernatants as means ± SD of at least eight separate brain preparations.
Isobolographic analysis of interactions
Isobolographic analysis of interactions between GBP and TPM was performed according to the methodology previously detailed (Luszczki et al. 2003a,b, 2006b) and modified according to Grabovsky and Tallarida (2004) and Tallarida (2006, 2007) for drugs with nonparallel DRRCs. The evaluation of ED50 and TD50 values of AEDs injected alone was performed using log-probit method according to Litchfield and Wilcoxon (1949). Subsequently, based upon these ED50 and TD50 values, median additive doses of the mixture of GBP with TPM, i.e., doses of the mixture that theoretically should protect 50% of the animals tested against MES-induced seizures (ED50 add) and doses of the mixture that theoretically should impair motor coordination in 50% of the animals tested in the chimney test (TD50 add), were calculated from two equations of additivity presented by Tallarida (2006, 2007). For the lower line of additivity, the equation at a 50% effect is: y = ED50_TPM − [ED50_TPM / (ED50_GBP / x)q/p] for the ED50 add value, and y = TD50_TPM − [TD50_TPM / (TD50_GBP / x)q/p] for the TD50 add value, where y is a dose of TPM, x is a dose of GBP, and q and p are curve-fitting parameters (Hill coefficients) for TPM and GBP, respectively. In contrast, for the upper line of additivity, the equation at a 50% effect is: y = ED50_TPM [(ED50_GBP − x) / ED50_GBP]q/p for the ED50 add value, and y = TD50_TPM [(TD50_GBP − x) / TD50_GBP]q/p for the TD50 add value. To calculate the curve-fitting parameters (q and p), probits of response for TPM and GBP administered alone were transformed to percent of effect. It is important to note that when two drugs produce maximal effect but are “heterodynamic” (i.e., have nonparallel DRRCs), the additivity is represented as an area bounded by two defined curves (lower and upper isoboles of additivity). For supraadditivity (synergy), the experimentally derived ED50 mix or TD50 mix points are placed below this region bounded by the lower and upper isoboles of additivity, and for subadditivity (antagonism), above the area of additivity (Tallarida 2006, 2007). In isobolography, it is accepted that half the ED50_1 of one drug plus half the ED50_2 of the second drug should be as effective therapeutically as a full dose of either drug administered separately. This concept of adding fractions of the ED50s of drugs is a basic principle of isobolography (Loewe 1953; Berenbaum 1989). Subsequently, proportions of GBP and TPM in the mixture were calculated for the fixed-ratio combination of 1:1, and the mixture of GBP with TPM was administered to animals. The evaluation of the experimentally derived ED50 mix at the fixed ratio of 1:1 was based upon the dose of mixture protecting 50% of animals tested against MES-induced seizures in mice. Similarly, in the chimney test, the TD50 mix value was isobolographically determined.
To visualize types of the interactions between TPM and GBP, the isobolograms were drawn by plotting the points reflecting the respective doses of GBP on the x-axis and doses of TPM on the y-axis. The lower and upper isoboles of additivity are presented as curves concaved downward connecting the ED50 or TD50 values for GBP and TPM administered alone. The dotted line starting from the point (0,0) corresponds to the fixed ratio of 1:1 for the combination of GBP with TPM. The points A′ and A″ depict the theoretically calculated ED50 add or TD50 add values for both lower and upper isoboles of additivity. The point A represents the experimentally derived ED50 mix (or TD50 mix) value for total dose of the mixture expressed as proportions of GBP and TPM that produced 50% anticonvulsant effects (or 50% impairment of motor coordination) in mice.
Statistics
The ED50 and TD50 values for TPM and GBP administered alone (with their 95% confidence limits) were calculated by computer log-probit analysis according to Litchfield and Wilcoxon (1949). The respective 95% confidence limits were transformed into standard errors (SE) as described previously (Luszczki et al. 2003b, 2006b). Simultaneously, the test for parallelism of DRRCs of TPM and GBP was performed as an indispensable condition for testing AED interactions with isobolography, according to the method presented by Litchfield and Wilcoxon (1949). For more details, see Appendix in Luszczki and Czuczwar (2006). The experimentally derived ED50 mix and TD50 mix values were statistically compared to their respective ED50 add and TD50 add values using unpaired Student’s t test (Porreca et al. 1990). The SE for additive ED50 add and TD50 add values were calculated in approximation according to Tallarida (2006), whereas SE for experimentally derived ED50 mix and TD50 mix values were denoted directly from the log-probit analysis. Total brain AED concentrations were statistically analyzed using the unpaired Student’s t test.
Software used
Microsoft’s Excel spreadsheet was used to perform the required calculations and to graph the results in the form of DRRCs and isobolograms. This spreadsheet was programmed to compute all calculations automatically and determine the lower and upper isoboles of additivity. The ED50 add and TD50 add values with their SE (for the combination of TPM with GBP at the fixed ratio of 1:1) were also denoted in this program.
Results
Effects of GBP and TPM administered alone on the MES-induced seizures
The anticonvulsant effects of GBP, expressed as the percentage of protection against MES-induced tonic hind limb extension in mice, were ranged between 12.5% for GBP at 200 mg/kg and 75% for GBP at 1,400 mg/kg (Table 1). The log-probit analysis of DRRC for GBP allowed the calculation of its ED50 value, which was 638.5 (450.0–906.1) mg/kg (Table 1; Fig. 1a). In contrast, TPM exerted a clear-cut anticonvulsant effect in the MES test, and its ED50 value, denoted from the log-probit method, was 49.55 (39.6–62.0) mg/kg (Table 1; Fig. 1a). In this case, TPM at 30 mg/kg produced a 12.5% protection against MES-induced seizures, whereas the drug at 80 mg/kg offered an 87.5% anticonvulsant effect in the MES test (Table 1).
Log-probit analysis and dose–response relationship curves for GBP and TPM administered alone in the MES-induced seizures (a) and chimney test (b) in mice. Doses of TPM and GBP administered alone were transformed into logarithms, whereas the protective effects offered by the AEDs against MES-induced seizures or motor coordination deficits in animals produced by the AEDs in the chimney test were transformed into probits (Litchfield and Wilcoxon 1949). Linear regression equations of DRRCs are presented on the graph, where y is the probit of response and x is the logarithm (to the base 10) of drug dose. The dotted lines represent on the graph median effective doses (ED 50 ) or median toxic doses (TD 50 ) of TPM and GBP. The test for parallelism of DRRCs (for TPM and GBP) revealed that lines are not parallel (Litchfield and Wilcoxon 1949)
Effects of GBP and TPM administered alone on motor coordination impairment in the chimney test
GBP produced clear-cut acute adverse (neurotoxic) effects with respect to motor coordination impairment in the chimney test, and its TD50 value denoted experimentally was 957.8 (723.9–1,267.3) mg/kg (Fig. 1b). Noteworthy, GBP at 600 mg/kg produced a 25% impairment of motor coordination in mice, whereas the drug at 1,400 mg/kg exerted a 75% motor deficit in mice challenged with the chimney test (Table 2). In the case of TPM, the drug produced motor coordination deficits in mice at doses ranged between 500 mg/kg (12.5% impairment) and 800 mg/kg (75% impairment); hence, the log-probit analysis of DRRC for TPM allowed the calculation of its TD50 value, which was 640.4 (554.3–739.9) mg/kg (Table 2; Fig. 1b). The protective index (as a ratio of TD50 and ED50 values) for GBP was 1.50, whereas that for TPM was 12.94.
Brain AED concentrations
Total brain AED concentrations were evaluated for TPM and GBP coadministered at the fixed ratio of 1:1 from the MES test. The brain concentrations of GBP, administered singly at 117.4 mg/kg, were 20.95 ± 4.01 μg/ml and did not differ significantly from those evaluated for the mixture of GBP (117.4 mg/kg) with TPM (9.1 mg/kg), which were 23.08 ± 3.85 μg/ml. Similarly, GBP (117.4 mg/kg) coadministered with TPM (9.1 mg/kg) did not affect the brain TPM concentrations. In this case, the brain concentrations of TPM (injected singly at 9.1 mg/kg) were 2.01 ± 0.28 μg/ml, whereas those for the two-drug mixture at the fixed ratio of 1:1 amounted to 2.11 ± 0.26 μg/ml.
Isobolographic assessment of interactions between GBP and TPM
The mixture of GBP and TPM at the fixed ratio of 1:1 exerted supraadditive (synergistic) interaction in the MES test in mice. The ED50 mix for this fixed-ratio combination was 126.45 mg/kg, whereas the corresponding ED50 add values were 257.5 mg/kg (for the lower ED50 add) and 430.6 mg/kg (for the upper ED50 add; Table 3). In this case, the ED50 mix (i.e., protecting a 50% of animals against MES-induced seizures) was substantially reduced by 51% as compared to the theoretically presumed lower ED50 add (at P < 0.001; Table 3, Fig. 2a). In contrast, GBP combined with TPM at the fixed ratio of 1:1 exerted additive interaction in the chimney test in mice. The TD50 mix for the mixture of GBP + TPM at the fixed ratio of 1:1 was 891.9 mg/kg, whereas the TD50 add values amounted to 461.3 mg/kg (for the lower TD50 add) and 1,137.0 mg/kg (for the upper TD50 add; Table 4, Fig. 2b).
Isobolograms showing interactions between GBP and TPM against MES-induced seizures (a) and chimney test (b) in mice. The median effective doses (ED 50 ) and median toxic doses (TD 50 ) for GBP and TPM are shown plotted graphically on the x- and y-axes, respectively. The lower and upper isoboles of additivity represent the curves connecting the ED50 or TD50 values for GBP and TPM administered alone. The dotted line starting from the point (0,0) corresponds to the fixed-ratio of 1:1 for the combination of GBP with TPM. The points A′ and A″ depict the theoretically calculated ED50 add or TD50 add values for both lower and upper isoboles of additivity. The point A represents the experimentally derived ED50 mix or TD50 mix value for total dose of the mixture expressed as proportions of GBP and TPM that produced 50% anticonvulsant effects in the MES test in mice or impairment of motor coordination in 50% of the animals in the chimney test. The sum of x and y coordinates, for each point placed on the isobologram (A, A′, A″), corresponds to the respective ED50 or TD50 values. On the graph, the SE values are presented as horizontal and vertical error bars for every ED50 or TD50 value. a The ED50 mix value is placed significantly below the area of additivity bounded by two isoboles of additivity indicating synergistic interactions between GBP and TPM in the MES test in mice ([triple asterisk]P < 0.001, Student’s t test). b The experimentally derived TD50 mix is placed into the area of additivity suggesting the additive interaction between TPM and GBP in the chimney test in mice
Discussion
The objective of this study was to characterize the anticonvulsant and acute adverse effect (neurotoxic) profiles as well as to determine the exact types of interactions between GBP and TPM in the MES and chimney tests in mice using the type I isobolographic analysis. Because TPM and GBP did not have their DRRCs parallel in both the MES and chimney tests (Fig. 1a,b), the modified isobolographic analysis based on different regression line slopes was used in this study (Tallarida 2006, 2007).
The experimentally derived ED50 value of GBP (administered separately, i.p., 60 min before the MES test) was 638.5 mg/kg, and markedly differed from that denoted by White et al. (2002), which was 78.2 mg/kg. At present, it is difficult to explain this discrepancy in the determination of ED50 value in the MES test in mice after systemic (i.p.) administration of GBP. The results of this study are partly in agreement with those documented by Dalby and Nielsen (1997), who had reported the lack of the antiseizure activity of GBP up to 300 mg/kg in the MES test. Moreover, it has been previously documented that GBP at 75 and 100 mg/kg considerably elevated the threshold for electroconvulsions in mice from 7.2 mA (control) to 9.0 mA (GBP at 75 mg/kg) and 9.7 mA (GBP at 100 mg/kg), respectively (Luszczki et al. 2003a). In light of the above-mentioned facts, the ED50 value of GBP denoted by White et al. (2002) seems to be incidentally low.
Results presented herein indicate clearly that GBP interacted supraadditively (synergistically) with TPM in terms of suppression of MES-induced seizures in mice. The observed interaction between TPM and GBP against MES-induced seizures is generally in agreement with our previous findings showing that GBP interacts synergistically with a number of conventional and newer AEDs in the MES test in mice (Borowicz et al. 2002; Luszczki et al. 2003a, 2005a). It is worth mentioning that previously performed studies were based on type II isobolographic analysis because GBP was considered virtually ineffective in the MES test. However, as reported herein, it was possible to determine its ED50 value against MES-induced seizures. Therefore, the present study was based on the type I isobolographic analysis for nonparallel DRRCs. Moreover, with type I isobolographic analysis for different regression line slopes, it was found that both AEDs exert the additive interaction with respect to their acute adverse effect potentials (motor coordination impairment) in the chimney test. Additionally, no significant changes in total brain concentrations of both AEDs were identified in this study, demonstrating the lack of pharmacokinetic interaction between GBP and TPM in the MES test. Calculations of protective index values for the AEDs administered alone indicated that there was a narrow range between the doses of GBP offering anticonvulsant protection in the MES test and those producing motor coordination impairment in animals. In the case of TPM, its TD50 value (assessed in the chimney test) considerably differed from the ED50 value of the drug in the MES test, and thus, TPM displays a wide gap between the anticonvulsant doses and those producing motor coordination deficits in mice. From a preclinical point of view, the AEDs should have their protective index values above 5, which guarantee their safety and tolerability in the clinical setting (Löscher et al. 1991). Nevertheless, the classification of AEDs’ efficacy, based on their protective index values in preclinical studies, is not a unique criterion for the clinical application of AEDs.
Another crucial problem deserves more attention and should be discussed here. It is accepted that synergistic interactions offered by AEDs in combinations allow the reduction of drug doses comprised their mixtures (Schmidt 1996). Undoubtedly, any decrease in both drug doses in the mixture may considerably reduce or even eliminate undesired side effects, closely associated with two-AED therapy in the clinical setting (Perucca 1995). Generally, in the case of synergistic interaction, both AEDs can be given in reduced doses without any loss of their anticonvulsant efficacy, and thus, this modified two-drug therapy may substantially ameliorate the patients’ quality of lives by reducing unwanted adverse effects (Perucca 1995; Deckers et al. 2000).
Considering thoroughly preclinical profiles of TPM and GBP administered alone and the synergistic interaction observed in the present study, one could suppose that the combination of TPM with GBP should also be efficacious in epileptic patients refractory to the monotherapeutic use of these AEDs. Generally, the combinations found to be synergistic in the MES test were also synergistic in humans, protecting refractory patients against seizure attacks. More detailed discussion concerning the efficacy of combined AED therapy in both preclinical and clinical conditions has been presented elsewhere (Stephen and Brodie 2002; Luszczki and Czuczwar 2004a; Luszczki et al. 2005b).
Summing up, synergistic cooperation of both AEDs in suppressing MES-induced seizures, additive interaction with respect to motor coordination impairment in the chimney test, and lack of pharmacokinetic interactions between TPM and GBP make the combination of these AEDs of pivotal importance for patients refractory to the monotherapeutic use of both AEDs. From a preclinical point of view, this profitable AED combination deserves more attention and further clinical verification to provide reliable evidence about its efficacy in epileptic patients. This study, for the first time, describes a practical application of isobolographic analysis of interaction for drugs with different (nonparallel) regression line slopes.
Abbreviations
- AED:
-
antiepileptic drug
- DRRC:
-
dose–response relationship curve
- GBP:
-
gabapentin
- MES:
-
maximal electroshock seizure test
- TPM:
-
topiramate
References
Bartoszyk GD, Meyerson M, Reimann W, Satzinger G, von Hodenberg A (1986) Gabapentin. In: Meldrum BS, Porter RJ (eds) New anticonvulsant drugs. Libbey, London, pp 147–163
Berenbaum MC (1989) What is synergy? Pharmacol Rev 41:93–141 (erratum published in 1989, Pharmacol Rev 41:422)
Boissier JR, Tardy J, Diverres JC (1960) Une nouvelle methode simple pour explorer l’action tranquilisante le test de la cheminee. Med Exp 3:81–84 (Basel)
Borowicz KK, Swiader M, Luszczki J, Czuczwar SJ (2002) Effect of gabapentin on the anticonvulsant activity of antiepileptic drugs against electroconvulsions in mice—an isobolographic analysis. Epilepsia 43:956–963
Dalby NO, Nielsen EB (1997) Comparison of the preclinical anticonvulsant profiles of tiagabine, lamotrigine, gabapentin and vigabatrin. Epilepsy Res 28:63–72
Deckers CLP, Czuczwar SJ, Hekster YA, Keyser A, Kubova H, Meinardi H, Patsalos P, Renier WO, van Rijn CM (2000) Selection of antiepileptic drug polytherapy based on mechanism of action: the evidence reviewed. Epilepsia 41:1364–1374
Grabovsky Y, Tallarida RJ (2004) Isobolographic analysis for combinations of a full and partial agonist: curved isoboles. J Pharmacol Exp Ther 310:981–986
Greco WR, Bravo G, Parsons JC (1995) The search for synergy: a critical review from response surface perspective. Pharmacol Rev 47:331–385
Leach JP (2000) Antiepileptic drugs: safety in numbers? Seizure 9:170–178
Litchfield JT, Wilcoxon F (1949) A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 96:99–113
Loewe S (1953) The problem of synergism and antagonism of combined drugs. Arzneimittelforschung 3:285–290
Luszczki JJ, Czuczwar SJ (2003) Isobolographic and subthreshold methods in the detection of interactions between oxcarbazepine and conventional antiepileptics—a comparative study. Epilepsy Res 56:27–42
Luszczki JJ, Czuczwar SJ (2004a) Preclinical profile of combinations of some second-generation antiepileptic drugs: an isobolographic analysis. Epilepsia 45:895–907
Luszczki JJ, Czuczwar SJ (2004b) Isobolographic profile of interactions between tiagabine and gabapentin: a preclinical study. Naunyn-Schmiedeberg’s Arch Pharmacol 369:434–446
Luszczki JJ, Czuczwar SJ (2006) Biphasic characteristic of interactions between stiripentol and carbamazepine in the mouse maximal electroshock-induced seizure model: a three-dimensional isobolographic analysis. Naunyn-Schmiedeberg’s Arch Pharmacol 374:51–64
Luszczki JJ, Swiader M, Parada-Turska J, Czuczwar SJ (2003a) Tiagabine synergistically interacts with gabapentin in the electroconvulsive threshold test in mice. Neuropsychopharmacology 28:1817–1830
Luszczki JJ, Borowicz KK, Swiader M, Czuczwar SJ (2003b) Interactions between oxcarbazepine and conventional antiepileptic drugs in the maximal electroshock test in mice: an isobolographic analysis. Epilepsia 44:489–499
Luszczki JJ, Czuczwar M, Kis J, Krysa J, Pasztelan I, Swiader M, Czuczwar SJ (2003c) Interactions of lamotrigine with topiramate and first-generation antiepileptic drugs in the maximal electroshock test in mice: an isobolographic analysis. Epilepsia 44:1003–1013
Luszczki JJ, Andres MM, Czuczwar SJ (2005a) Synergistic interaction of gabapentin and oxcarbazepine in the mouse maximal electroshock seizure model—an isobolographic analysis. Eur J Pharmacol 515:54–61
Luszczki JJ, Ratnaraj N, Patsalos PN, Czuczwar SJ (2005b) Pharmacodynamic and pharmacokinetic interaction studies with loreclezole and felbamate, lamotrigine, topiramate and oxcarbazepine in the mouse maximal electroshock seizure model. Epilepsia 46:344–355
Luszczki JJ, Andres MM, Czuczwar P, Cioczek-Czuczwar A, Ratnaraj N, Patsalos PN, Czuczwar SJ (2006a) Pharmacodynamic and pharmacokinetic characterization of interactions between levetiracetam and numerous antiepileptic drugs in the mouse maximal electroshock seizure model: an isobolographic analysis. Epilepsia 47:10–20
Luszczki JJ, Ratnaraj N, Patsalos PN, Czuczwar SJ (2006b) Isobolographic analysis of interactions between loreclezole and conventional antiepileptic drugs in the mouse maximal electroshock-induced seizure model. Naunyn-Schmiedeberg’s Arch Pharmacol 373:169–181
Löscher W, Nolting B (1991) The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. IV. Protective indices. Epilepsy Res 9:1–10
Löscher W, Wauquier A (1996) Use of animal models in developing guiding principles for polypharmacy in epilepsy. Epilepsy Res Suppl 11:61–65
Löscher W, Fassbender CP, Nolting B (1991) The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. II. Maximal electroshock seizure models. Epilepsy Res 8:79–94
Perucca E (1995) Pharmacological principles as a basis for polytherapy. Acta Neurol Scand Suppl 162:31–34
Porreca F, Jiang Q, Tallarida RJ (1990) Modulation of morphine antinociception by peripheral [Leu5]enkephalin: a synergistic interaction. Eur J Pharmacol 179:463–468
Schmidt D (1996) Modern management of epilepsy: Rational polytherapy. Bailliere’s Clin Neurol 5:757–763
Shank RP, Gardocki JF, Vaught JL, Davis CB, Schupsky JJ, Raffa RB, Dodgson SJ, Nortey SO, Maryanoff BE (1994) Topiramate: preclinical evaluation of structurally novel anticonvulsant. Epilepsia 35:450–460
Sills GJ, Butler E, Thompson GG, Brodie MJ (2004) Pharmacodynamic interaction studies with topiramate in the pentylenetetrazol and maximal electroshock seizure models. Seizure 13:287–295
Stephen LJ, Brodie MJ (2002) Seizure-freedom on more than one antiepileptic drug. Seizure 11:349–351
Tallarida RJ (2000) Drug synergism and dose-effect data analysis. CRC, Boca Raton, FL
Tallarida RJ (2006) An overview of drug combination analysis with isobolograms. J Pharmacol Exp Ther 319:1–7
Tallarida RJ (2007) Interactions between drugs and occupied receptors. Pharmacol Ther 113:197–209
White HS, Woodhead JH, Wilcox KS, Stables JP, Kupferberg HJ, Wolf HH (2002) Discovery and preclinical development of antiepileptic drugs. In: Levy RH, Mattson RH, Meldrum BS, Perucca E (eds) Antiepileptic drugs, 5th edn. Williams & Wilkins, Philadelphia, pp 36–48
Acknowledgements
I would like to express my gratitude to Prof. Ronald J. Tallarida (Temple University School of Medicine, Philadelphia, USA) for his stimulating hints and help during the preparation of the isobolograms. I would also like to thank to Mr. Wojciech Zgrajka (Institute of Agricultural Medicine, Lublin, Poland) for the skillful determination of the brain concentrations of GBP. This study was supported by a grant (DS 345/2003–2005) from the Medical University of Lublin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luszczki, J.J. Isobolographic analysis of interaction between drugs with nonparallel dose–response relationship curves: a practical application. Naunyn-Schmied Arch Pharmacol 375, 105–114 (2007). https://doi.org/10.1007/s00210-007-0144-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-007-0144-z