Abstract
Betulinic acid (BA) is a pentacyclic triterpene found in many plant species, among others in the bark of white birch Betula alba. BA was reported to display a wide range of biological effects, including antiviral, antiparasitic, antibacterial and anti-inflammatory activities, and in particular to inhibit growth of cancer cells. The aim of the study was further in vitro characterization of BA anticancer activity. In this study, we demonstrated a remarkable antiproliferative effect of BA in all tested tumor cell cultures including neuroblastoma, rabdomyosarcoma-medulloblastoma, glioma, thyroid, breast, lung and colon carcinoma, leukemia and multiple myeloma, as well as in primary cultures isolated from ovarian carcinoma, cervical carcinoma and glioblastoma multiforme. Furthermore, we have shown that BA decreased cancer cell motility and induced apoptotic cell death. We also observed decrease of bcl2 and cyclin D1 genes expression, and increase of bax gene expression after betulinic acid treatment. These findings demonstrate the anticancer potential of betulinic acid and suggest that it may be taken into account as a supportive agent in the treatment of cancers with different tissue origin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Betulinic acid (3β-hydroxy-lup-20(29)-en-28-oic acid, BA) is a pentacyclic triterpene found in many plant species, among others in the bark of Betula alba. BA was reported to display several biological effects, including antiviral, antiparasitic, antibacterial and anti-inflammatory activities, and in particular to inhibit growth of cancer cells (Einzhammer and Xu 2004). BA has been shown to be a cytotoxic agent and the growth inhibitor of human melanoma in athymic mice as well as the inducer of apoptosis in neuroblastomas and glioblastomas in vitro (Schmidt et al. 1997; Fulda et al. 1999; Selzer et al. 2000). In vivo, mice bearing ovarian cancer xenografts revealed increased survival time after treatment with BA (Einzhammer and Xu 2004; Zuco et al. 2002). It has been recently demonstrated that BA is also effective against head and neck squamous carcinoma cells (Thurnher et al. 2003; Eder-Czembirek et al. 2005), leukemia cells (Ehrhardt et al. 2004; Raghuvar Gopal et al. 2005) and other cancer cell lines (Zuco et al. 2002; Einzhammer and Xu 2004). Interestingly, normal cells seem to be unaffected by BA treatment. Lack of cytotoxic activity was demonstrated in human astrocytes (Wick et al. 1999), human dermal fibroblasts and peripheral blood lymphoblasts (Einzhammer and Xu 2004; Zuco et al. 2002) as well as in animal studies in the concentration range of 100–500 mg/kg body weight (Zuco et al. 2002; Pisha et al. 1995). Despite intensive investigations, the antiproliferative mechanism of BA has not been fully elucidated at the molecular level. The mode of the action of BA in cancer cells is linked to the activation of mitochondrial intrinsic apoptotic pathway (Einzhammer and Xu 2004; Fulda et al. 1999; Fulda and Debatin 2000) and to perturbations in the cell cycle progression (Einzhammer and Xu 2004). Treatment with BA results in loss of the mitochondrial membrane potential (MMP), followed by release of cytochrome c and apoptotis-inducing factor (AIF), leading to activation of caspases and formation of the apoptosome (Einzhammer and Xu 2004). The release of cytochrome c is determined by the relative amounts of apoptosis-inhibiting Bcl-2 proteins in the outer membrane of the mitochondrion (Adams and Cory 1998). Over-expression of Bcl-2 rescue cancer cells from cytotoxic effects induced by BA (Fulda et al. 1997), showing the importance of Bcl-2 expression for cell death induced by BA. The apoptosis-preventing effect of Bcl-2 is counteracted by the pro-apoptotic protein Bax, and the Bax/Bcl-2 ratios determine the sensitivity to different apoptotic stimuli (Raisova et al. 2001). However, conflicting results exist concerning the role of the Bcl-2 protein family involved in BA-induced apoptosis. The Bcl-2 family proteins (Bax, Bcl-xl, Bcl-2) expression levels after BA treatment were suggested to be tissue-type or even cell line-specific (Einzhammer and Xu 2004).
In the present study, we tested the anticancer potential of BA in a range of tumor cell lines and in primary cultures. The effect of BA on tumor cell proliferation, migration, apoptosis induction as well as on bcl-2, bax and cyclin D1 expression was studied.
Materials and methods
Betulinic acid was purchased from Sigma (Sigma Chemicals, St. Louis, Mo., USA). Stock solutions (10 mM and 100 mM) were prepared in DMSO (Sigma).
Cell lines
Human rhabdomyosarcoma/medulloblastoma (TE671), human neuroblastoma (SK-N-AS), human thyroid carcinoma (FTC 238) and human multiple myeloma (RPMI 8226) were obtained from European Collection of Cell Cultures (Centre for Applied Microbiology and Research, Salisbury, UK). Human Caucasian lung carcinoma (A549), human colon adenocarcinoma (HT 29) and human T-cell leukemia (Jurkat E6.1) were obtained from the Institute of Immunology and Experimental Therapy Polish Academy of Sciences, Wroclaw, Poland. Human breast carcinoma (T47D) was obtained from Department of Human Genetics, Medical University, Lublin, Poland. Rat glioma (C6) was obtained from Department of Neonatology, Charité-Virchow Clinics, Humboldt University, Berlin, Germany. Human skin fibroblasts (HSF) were a laboratory strain established by outgrowth technique from skin explants of young persons.
HT-29, C6 and HSF cell lines were maintained in DMEM culture medium (Sigma). TE671, SK-N-AS, FTC 238 and T47D were grown in 1:1 mixture of DMEM and Nutrient mixture F-12 Ham (Ham’s F-12) (Sigma). The culture medium for A549 cell line consisted of 2:1 mixture of DEMEM/Ham’s F-12. RPMI1640 (Sigma) culture medium was applied for culture of Jurkat E6.1 and RPMI 8226 cell lines. All media were supplemented with 10% FBS (Life Technologies, Karlsruhe, Germany), penicillin (100 u/ml) (Sigma) and streptomycin (100 μg/ml) (Sigma). Cultures were kept at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Primary cultures
Human primary ovarian carcinoma cell culture (HPOC) was established from tumor fragments taken from a 45-year-old women with serous ovarian carcinoma (cystadenocarcinoma, moderately differentiated, G-2). Human primary cervical carcinoma (HPCC) was established from tumor fragments taken from a 68-year-old women with cervical carcinoma diagnosed as squamous cell carcinoma (moderately differentiated, G-2). Human primary glioblastoma multiforme cell culture (HPGBM) was established from tumor fragments taken from a 68-year-old man with glioblastoma multiforme, WHO grade IV. Tumor tissue was mechanically dispersed and plated on 75 cm2 plastic culture flasks (Nunc, Roskilde, Denmark) in a culture medium consisting of alpha-MEM (Life Technologies) supplemented with 10% of FBS, 100 μg/ml penicillin, 100 μg/ml streptomycin and 1% of antibiotic-antimycotic solution (Life Technologies). Cultures were kept at 37°C in a humidified atmosphere of 95% air and 5% CO2. Culture medium was changed every 3 days. After reaching confluency, which took 2–3 weeks, cells were harvested by means of 0.25% Trypsin-EDTA solution and subjected to proliferation assessment.
Cell proliferation assessment
Cells were plated on 96-well microplates (Nunc) at a density of 1×104 (C6, TE671, FTC 238, A549, T47D), 2×104 (Jurkat E6.1, RPMI 8226,) and 3×104 (HT-29, SK-N-AS, HPOC, HPCC, HPGBM). The next day, the culture medium was removed and the cells exposed to serial dilutions of betulinic acid prepared in a fresh medium. In the case of non-adherent cell lines (Jurkat E6.1 and RPMI 8226) compound was added directly to the cell suspension. Cell proliferation was assessed after 96 h by means of MTT assay (Cell proliferation kit I, Roche Diagnostics, Germany) in which the yellow tetrazolium salt (MTT) is metabolized by viable cells to purple formazan crystals. Tumor cells were incubated for 3 h with MTT solution (5 mg/ml). Formazan crystals were solubilized overnight in SDS buffer (10% SDS in 0.01 N HCl) and the product was quantified spectrophotometrically by measuring absorbance at 570 nm wavelength using E-max Microplate Reader (Molecular Devices Corporation, Menlo Park, Calif., USA).
Cytotoxicity assay
A cytotoxicity detection kit based on measurement of lactate dehydrogenase (LDH) activity was applied (Tox-7, Sigma). The assay is based on the reduction of NAD by the action of LDH released from damaged cells. The resulting NADH is utilized for stechiometric conversion of a terazolium dye. The resulting colored compound is measured spectrophotometrically. HSF cells growing on 96-well microplates were subjected to BA (1–100 μM) for 24 h. Culture supernatants were collected and incubated with substrate mixture for 30 min at room temperature in the dark. The reaction was terminated by addition of 1N HCl and the color product was quantified spectrophotometrically at 450 nm wavelength using E-max Microplate Reader.
Cell migration assessment
Tumor cell migration was assessed in wound assay model. Tumor cells (C6, A549, TE671) were plated at 1×106 cells on 4-cm culture dishes (Nunc). Next day, cell monolayer was wounded by pipet tip (P300), the medium and dislodged cells were aspirated and the plates rinsed twice with PBS. Next, the fresh culture medium was applied and the number of cells migrated into the wound area after 24 h was estimated in control and cultures treated with BA (5 μM). Plates were stained with May-Grünwald-Giemsa method. The observation was performed in Olympus BX51 System Microscope (Olympus Optical, Tokyo, Japan) and micrographs were prepared in analySIS software (Soft Imaging System GmbH, Münster, Germany). Cells migrated to the wound area were counted on micrographs and results expressed as a mean cell number migrated to the selected 50 wound areas taken from 4 micrographs.
Assessment of cell death
Measurement of cell death was performed using Cell Death Detection ELISAPLUS kit (Roche Diagnostics, Germany). The assay is based on a quantitative sandwich-enzyme-immunoassay-principle using mouse monoclonal antibodies directed against DNA and histones, respectively. This allows the specific determination of mono- and oligonucleosomes in the cytoplasmatic fraction of cell lysates. A549, HT-29 and HSF cell cultures growing on 96-well microplates were subjected to BA (2.5–25 μM) for 24 h, whereupon supernatants were removed and cells were lysed with 200 μl of lysis buffer for 30 min. Subsequently, cell lysates were centrifuged at 200 g for 10 min and 20 μl of the sample were carefully transferred into the streptavidin-coated 96-well microplate. The immunoreagent (80 μl) containing anti-histone-biotin and anti-DNA-POD mouse monoclonal antibody was added and incubated under gentl shaking (300 rpm) for 2 h at 20°C. The solution was removed by tapping, each plate well was rinsed 3 times with 250 μl of incubation buffer, and finally, 100 μl per well of substrate solution (ATBS) was applied and incubated at RT for 15 min on a plate shaker (250 rpm) until sufficient colour developed. Absorbance was measured at 405 nm wavelength using E-max Microplate Reader.
Western blot analysis
After treatment, cells were harvested and lysed in RIPA buffer (1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 20 mM NaF, 0.5 mM DTT, 1 mM PMSF, and protease inhibitor cocktail in PBS pH 7, 4), than centrifuged 3,000 g for 10 min. For Western blot analysis, supernatants of RIPA cell lysates were solubilized in 3× Laemmli sample buffer (30% glycerol, 3% SDS, 0.19 M Tris-HCl, pH 6.8, 0.015% bromophenol blue, 3% β-mercaptoethanol), than boiled for 5 min at 100°C. Equal amounts of total cellular protein extract were electrophoresed on 10% or 12.5% SDS-PAGE under reducing conditions and transferred by electrobloting onto a nitrocellulose membrane (Schleicher and Schuell, Kassel, Germany). After blocking for 1 h at room temperature (RT) with 5% non-fat milk/TBS/0.1% Tween, membranes were probed overnight at +4°C with the primary antibody as follows: anti-bcl-2 (1:500 in 1%BSA/1% non-fat milk/TBS/0.1% Tween, Santa Cruz Biotechnology, Santa Cruz, Calif.), anti-β actin (Promega, Madison, USA), anti-phospho-ERK1/2, anti-phospho-CREB (Ser133), anti-ERK1/2, anti-phospho-Akt (Ser 473), anti-Akt (1:1000 in 1% BSA/TBS/0,1% Tween, Cell Signaling, Beverly, Mass.), or anti-phosho-CaMKII (Thr 286) (Promega), than incubated with the secondary antibody coupled to horseradish peroxidase (1:5,000 in 1% BSA/TBS/0,1% Tween, Amersham Bioscience, Buckinghamshire, UK), and visualized using enhanced chemiluminescence (Pierce, Rockford, Ill., USA). For stripping, membranes were incubated with stripping buffer (100 mM β-mercaptoethanol, 2% SDS, 62, 5 mM Tris-HCl, pH 6,7) at 50°C for 20 min, than washed, blocked, and probed with the relevant antibody, as described above.
RNA isolation
Total RNA from cancer cells was isolated by acidic phenol/chloroform extraction and DNaseI treated. The concentration of RNA was determined spectrophotometrically, and the integrity of all samples was confirmed by electrophoresis in ethidium bromide-stained 1.0% agarose gels.
Semi-quantitative RT-PCR
500 ng of RNA was reverse transcribed at 42°C (1 h) with 200 U of Moloney murine leukemia virus reverse transcriptase and 2 μM oligo d(T)16-primer (Promega) in 25 μl of reaction mixture. The resulting cDNA (1 μl) was amplified by polymerase chain reaction (PCR). The sequence of oligonucleotide primers used in RT-PCR for each gene and the expected sizes of their RT-PCR products are as follows: human bcl-2 (GenBank BC027258), 5′-ACTTCGCCGAGATGTCCAGC-3′ (sense) and 5′-GGCAGGCATGTTGACTTCAC-3′ (antisense), 405 bp.; cyclin D1 (Genbank NM_053056), 5′-CCTACTTCAAATGTGTGCAG-3′ (sense) and 5′-CCAGGTTCCACTTGAGCTTG -3′ (antisense), 330 bp; bax (Genbank NM_004324), 5′-AAGAAGCTGAGCGAGTGTC-3′ (sense) and 5′- GGCCCCAGTTGAAGTTGC-3′ (antisense), 158 bp; β-actin (Genbank BC013835), 5′-ATCATGAAGTGTGACGTGGAC-3′ (sense) and 5′-AACCGACTGCTGTCACCTTCA-3′ (antisense), 462 bp; and rat bcl-2 (Genbank U34964), 5′-TTATAAGCTGTCACAGAGG-3′ (sense) and 5′-TGAAGAGTTCCTCCACCAC-3′ (antisense), 347 bp; cyclin D1 (Genbank NM_171992), 5′-GTGCAGAGGGAGATTGTGCC-3′ (sense) and 5′-AGGAAGTTGTTGGGGCTGCC-3′ (antisense), 530 bp; β-actin (Genbank V01217), 5′-CCCTAAGGCCAACCGTGAAAAGATG-3′ (sense) and 5′-GAACCGCTCATTGCCGATAGTGATG-3′ (antisense), 433 bp. (PCR cycling conditions are available from the authors on request). Amplified cDNA was subjected to 5% polyacrylamide gel electrophoresis, subsequent silver staining and densitometric analysis with the image analysis program BioDocAnalyze (Whatman Biometra, Göttingen, Germany). The housekeeping gene β-actin was coamplified as an internal control.
Results
The antiproliferative effect of BA was assessed in a range of human tumor cell lines as well as tumor primary cultures. Tumor cells derived from cancers of the nervous system (medulloblastoma/rhabdosarcoma, neuroblastoma and glioma) and peripheral cancers including thyroid carcinoma, colon adenocarcinoma, breast carcinoma, lung carcinoma, leukaemia and multiple myeloma were tested. Additionally, the primary tumor cultures derived from ovarian and cervical carcinoma and glioblastoma multiforme were also applied. Cells were exposed to either culture medium (control), or BA (1–25 μM) for 96 h. Proliferation of all tumor cells was decreased in a concentration-dependent fashion as measured by means of the MTT assay. Threshold concentrations of BA required to elicit antiproliferative effect in established tumor cell lines were as low as 1 and 2.5 μM. Comparison made by means of IC50 (Table 1) showed that the most sensitive cell line to BA was T47D (breast carcinoma) - IC50 - 2.4 μM and HT-29 (colon carcinoma) - IC50 - 2.7 μM (Table 1). HPGBM (glioblastoma multiforme) was the most sensitive primary cell culture exposed to BA (IC50 - 3.9 μM) (Table 1).
To evaluate the effect on normal cell viability, HSF (human skin fibroblasts) cells were subjected to increasing doses of BA (1–100 μM). LDH assay revealed that BA produced modest cytotoxicity in normal HSF cell culture. The significant LDH release after 24-h exposure appeared at 25 μM (not shown).
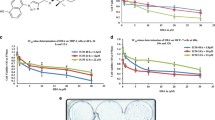
Wound assay revealed that in cultures exposed to BA, significantly fewer cells migrated to the wound area (Fig. 1d). Micrographs showing inhibited glioma C6 cells migration followed BA exposure are presented on Fig. 1a–c.
Effect of betulinic acid on migration of tumor cells in wound assay. Wounded monolayers of tumor cells (C6, TE671, A549) were incubated for 24 h alone or in the presence of betulinic acid (5 μM). Micrographs show wound assay of glioma C6 cells. a Wound, b cell migration after 24 h in control culture and c following exposure to 5 μM of betulinic acid. Magnification 40×. Tumor cells which migrated to the wound area were counted (d). Results are expressed as mean number of cells migrated per field of the wound area ± SEM of 50 measurements; ***, ###, +++ at least p<0.001 vs control, Student’s t-test
The anticancer activity of BA has been linked to the activation of intrinsic apoptotic pathway and perturbations in the cell cycle progression. Indeed, exposure to BA triggered significant apoptotic cell death in A549 cells, as indicated by a dose-dependent increase of immunoreactive cytosolic oligonucleosomal fragments (Fig. 2). In the case of HT-29 cells the significant effect appeared already at 2.5 μM and it was not dose-dependent. Normal cells (HSF) were not sensitive up to 25 μM of BA (not shown).
Therefore, we studied the expression of genes and proteins that could be involved in these processes. Figure 3 presents dose-dependent changes in expression of bcl-2 and bax gene (a,b) and Bcl-2 protein (c,d). T47D, FTC 238, C6, SKNAS, TE671, Jurkat E6.1, RPMI 8226, cells were incubated with 1 μM or 5 μM BA for 24 h, and A549, HT-29 also to 10 μM BA for 24 h. As detected by RT-PCR, all tumor cell lines showed a decrease of bcl-2 expression. Increased dose of BA up to 10 μM did not reduce significantly expression of bcl-2 gene in A549, HT-29 cell lines, when compared with bcl-2 expression after 5 μM BA treatment. The unchanged levels of housekeeping gene β-actin proved specificity of the observed decrease in bcl-2. We observed also a significant increase in bax gene expression, when A549 and HT-29 cells were incubated with different concentration of BA. The increase of bax gene expression was evident at 5 μM BA concentration and was similar at 10 μM concentration (Fig. 3a,b). We also tested, whether the decrease of bcl-2 gene expression after BA treatment is mirrored by decreased Bcl-2 protein expression. A549 and HT-29 cancer cells were treated with 1 μM and 5 μM of BA for 24 h, harvested, lysed with RIPA buffer and subjected for immunobloting. Immunoblot analysis revealed a significant dose-dependent suppression of Bcl-2 protein levels in A549 and HT-29 cells. The total amount of the protein content was unchanged as measured by reblotting the same nitrocellulose membrane with antibody against β-actin protein (Fig. 3c).
Betulinic acid induces dose-dependent changes in expression of bcl-2, bax genes and Bcl-2 protein. A549, HT-29, T47D, FTC 238, C6, SKNAS, TE671, Jurkat E6.1, RPMI 8226, cells were incubated with 1 μM, 5 μM or 10 μM BA, for 24 h. a Representative bands for bcl-2 (all cell lines) and bax (A549, HT-29 cells) detected by RT-PCR in control and BA-exposed cancer cells. The housekeeping gene β-actin was co-amplified as an internal control (lower panels). b Graphic illustration of bcl-2 and bax genes expression after BA treatment in A549 and HT-29 cells (** p<0,01, *** p<0,001). c Graphic illustration of bcl-2 gene expression after BA treatment in the rest of cell lines. d Immunoblot analysis of Bcl-2 proteins from RIPA-lysates of A549 and HT-29 cells, treated with 1 μM or 5 μM BA for 24 h (upper panels). Equal protein loading was confirmed by striping and reprobing the same nitrocellulose membrane with antibody against β-actin protein (lower panels)
To test whether BA may affect expression of genes involved in cell cycle progression. T47D, FTC 238, C6, SKNAS, TE671, Jurkat E6.1, RPMI 8226 cells were incubated with 1 μM or 5 μM BA for 24 h, whereas A549, HT-29 cells were incubated additionally with 10 μM BA for 24 h, and expression of cyclin D1 gene was detected by means of RT-PCR. Decreased expression of cyclin D1 mRNA in all tumor cell lines was detected at 5 μM BA concentrations. 10 μM BA treatment cells did not reduce significantly expression of cyclin D1 gene, compared with 5 μM dose in A549 and HT-29 cells (Fig. 4). The unchanged levels of housekeeping gene β-actin, co-amplified with cyclin D1 gene, proved specificity of the observed decrease in cyclin D1 expression.
Betulinic acid induces dose-dependent changes in expression of cyclin D1 gene. A549, HT-29, T47D, FTC 238, C6, SKNAS, TE671, Jurkat E6.1, RPMI 8226, cells were incubated with 1 μM, 5 μM or 10 μM BA, for 24 h. a Representative bands for cyclin D1 detected by RT-PCR in control and BA-exposed cancer cells. The housekeeping gene β-actin was co-amplified as an internal control (lower panels). b Graphic illustration of cyclin D1 gene expression after BA treatment in A549 and HT-29 cells (** p<0,01). c Graphic illustration of cyclin D1 gene expression after BA treatment in the rest of cell lines
Since bcl-2 and cyclinD1 genes are CREB transcription factor-regulated genes, we also tested CREB-Ser133 phoshorylation status in order to detect BA influence on CREB transcriptional activity. We did not observed any changes in CREB phosphorylation after incubation of A549 and HT-29 cells with 5 μM BA for different periods of time (1, 3, 6, 9 and 24 hours) as analyzed by means of Western Blotting using specific monoclonal antibody against Ser133-phosphorylated CREB (not shown). We were also not able to detect significant changes in ERK1/2, AKT and CaMKII kinases activity (not shown), which have been shown to phosphorylate CREB and promote cell proliferation. Total amounts of the CREB protein, and ERK1/2, Akt, CaMKII kinases remained unchanged after 5 μM BA treatment.
Discussion
BA has been reported to be an anticancer agent with the specificity for neuroectodermal tumors, inducing apoptosis in neuroblastoma cells resistant to doxorubicin and in primary tumor cultures of neuroectodermal origin (Fulda and Debatin 2000). Moreover, it was also active against medulloblastoma and glioblastoma cell lines, and against primary tumor cells isolated from patients with medulloblastoma and glioblastoma, but not cytotoxic against murine neurons in vitro (Fulda et al. 1999). Our study revealed that BA exert an antiproliferative and pro-apoptotic activity against different cancer cell lines of neuroectodermal and non-neuroectodermal origin, as well as in primary tumor cell cultures. Initial studies reported that BA treatment induced apoptosis in cell lines of neuroectodermal and non-neuroectodermal origin (Einzhammer and Xu 2004; Pisha et al. 1995; Fulda et al. 1997). Recently, a pro-apoptotic activity of BA against other tumor cell lines was described. Treatment with BA induced apoptosis in human myelogenous leukemia (K562), human breast carcinoma (SKBR) and colon carcinoma (Colo-205) cell lines (Basu et al. 2004; Raghuvar Gopal et al. 2005). Moreover, promyelocytic leukemia (HL-60) cell line was also very sensitive to BA treatment with IC50 equal to 5.7 μM (Poon et al. 2004). It was shown that about 65% of primary pediatric acute leukemias were more sensitive to BA than to standard therapeutics measures (Ehrhardt et al. 2004). It was also found that BA inhibited growth of ovarian carcinoma (A2780), cervix carcinoma (A431) and small cell lung carcinoma (POGB), independently of their p53 status and histotype (Zuco et al. 2002). In our experiments, we did not observe any tumor specificity of BA antiproliferative activity. However, analysis of IC50 showed differences in tumor cell sensitivity. BA was shown to induce apoptosis in tumor cells by activation of mitochondria and release of apoptogenic factors (Fulda et al. 1999). The BA-induced apoptosis can be reversed by overexpression of the anti-apoptotic protein Bcl-2 (Fulda and Debatin 2005).
In this study, we demonstrated that BA acts as an effective anticancer agent by inducing growth arrest and apoptosis in concentration-dependent manner. Apoptosis was accompanied by a down-regulation of bcl-2 and cyclin D1 genes. Our results are in contradiction to other data, showing that BA did not affect the levels of Bcl-2 proteins in neuroblastoma (Fulda et al. 1997) and tongue squamous cell carcinoma cell lines (Thurnher et al. 2003). However, Ji et al. (2002) reported decreased bcl-2 expression in human leukemia HL-60 cells after treatment with BA derivative −23-hydroxbetulinic acid. It is possible that the decrease of bcl-2 and cyclin D1 genes expression is cell line specific, as has been suggested by Einzhamer and Xu (2004). Raisova et al. (2001) demonstrated importance of Bax/Bcl-2 ratio in resistance for CD95-mediated apoptosis in melanoma cells. In their experimental model, a Bax/Bcl-2 ratio >1.0 was characteristic for sensitive melanoma cells. Furthermore, BA was able to bypass the mitochondrial blockade in CD95-resistant melanoma cells and induces apoptosis in both CD95-resistant and sensitive cells, independent of Bax/Bcl-2 ratio. Our preliminary results showed increase of bax and decrease of bcl-2 genes expression after BA treatment in A549 and HT-29 cell lines, which change significantly bax/bcl-2 ratio in treated cells. Since decrease of Bax and increase of Bcl-2 expressions are correlated with chemoresistant phenotype of cancer cells and serves as a negative prognostic marker (Chan and Yu 2004), the opposite effect after BA treatment observed in our study could contribute to the BA-induced apoptosis.
The exact mechanism by which the BA might regulate the expression of bcl-2 and cyclin D1 genes remains unclear since CREB phosphorylation status remain unchanged, as well as total amounts of CREB proteins after BA treatment.
Our findings suggest possible involvement of bcl-2 and cyclin D1 in apoptosis induction and growth inhibition mediated by BA in cancer cells, but this issue requires further study.
From the clinical point of view, it is important that anticancer agents exert antiproliferative activity in tumor cells without affecting normal cells. Aggressive chemotherapy very often causes severe side effects, which are partially a consequence of impairment of normal cells functions. In our experiments, BA exhibited low cytotoxicity against normal skin fibroblasts when measured by sensitive, LDH release test. This is in agreement with Zuco et al. 2002), who showed that BA discriminated between normal and tumor cells and was selectively cytotoxic for tumor cells.
Targeting tumor metastasis has the highest priority in cancer therapy, since metastatic disease and not local tumor growth determines the mortality of patients. Migration of tumor cells across blood vessels is of crucial importance in the initiation of metastatic disease. We detected that BA at low concentration of 5 μM significantly inhibited migration of glioma (C6), lung carcinoma (A549) and medulloblastoma (TE671) cells. Given the fact that nontoxic doses of BA can significantly inhibit proliferation and migration of tumor cells, it can be suggested that it may be applied as a prophylactic treatment measure, especially in patients with cancer risk development.
Betulinic acid proved to be not toxic in vivo. It can be applied at doses up to 500 mg/kg of body weight (Sarek et al. 2003). Moreover, the compound is relatively inexpensive since it is abundantly available from white birch bark. Taking into account the low toxicity in fibroblasts, lack of toxicity towards neurons and melanocytes (Fulda and Debatin 2000; Bernard et al. 2001), as well as remarkable antiproliferative activity in a wide range of tumor cells, it may be expected that it will be shortly accepted as a candidate for adjuvant therapy in the treatment of human cancers.
References
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281:1322–1326
Basu S, Ma R, Boyle PJ, Mikulla B, Bradley M, Smith B, Basu M, Banerjee S (2004) Apoptosis of human carcinoma cells in the presence of potential anti-cancer drugs. III. Treatment of Colo-205 and SKBR3 cells with: cis-platin, tamoxifen, melphalan, betulinic acid, L-PDMP, L-PPMP, and GD3 ganglioside. Glycoconj J 20:563–577
Bernard P, Scior T, Didier B, Hibert M, Berthon JY (2001) Etnopharmacology and bioinformatic combination for leads discovery: application to phospholipase A2 inhibitors. Phytochemistry 58:865–874
Chan SL, Yu VC (2004) Proteins of the bcl-2 family in apoptosis signalling: from mechanistic insights to therapeutic opportunities. Clin Exp Pharmacol Physiol 31:119–128
Eder-Czembirek C, Czembirek C, Erovic BM, Selzer E, Turhani D, Vormittag L, Thurnher D (2005) Combination of betulinic acid with cisplatin-different cytotoxic effects in two head and neck cancer cell lines. Oncol Rep 14:667–671
Ehrhardt H, Fulda S, Fuhrer M, Debatin KM, Jeremias I (2004) Betulinic acid-induced apoptosis in leukemia cells. Leukemia 8:1406–1412
Einzhammer DA, Xu ZQ (2004) Betulinic acid: a promising anticancer candidate. IDrugs 4:359–373
Fulda S, Debatin K (2000) Betulinic acid induces apoptosis through a direct effect on mitochondria in neuroectodermal tumors. Med Pediatr Oncol 35:616–618
Fulda S, Debatin KM (2005) Sensitization for anticancer drug-induced apoptosis by betulinic Acid. Neoplasia 7:162–170
Fulda S, Friesen C, Los M, Scaffidi C, Mier W, Benedict M, Nunez G, Krammer PH, Peter ME, Debatin KM (1997) Betulinic acid triggers CD95 (APO-1/Fas)- and p53-independent apoptosis via activation of caspases in neuroectodermal tumors. Cancer Res 57:4956–4964
Fulda S, Jeremias J, Steiner HH, Pietsch T, Debatin KM (1999) Betulinic acid: a new cytotoxic agent against malignant brain tumors. Int J Cancer 82:435–441
Ji ZN, Ye WC, Liu GG, Hsiao WL (2002) 23-Hydroxybetulinic acid-mediated apoptosis is accompanied by decreases in bcl-2 expression and telomerase activity in HL-60 Cells. Life Sci 72:1–9
Litchfield JT, Wilcoxon F (1949) A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 96:99–113
Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown DM, et al (1995) Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med 1:1046–1051
Poon KH, Zhang J, Wang C, Tse AK, Wang CK, Fong WF (2004) Betulinic acid enhances lalpha, 25-dihydroxyvitamin D3-induced differentiation in human HL-60 promyelocytic leukemia cells. Anticancer Drugs 15:619–624
Raghuvar Gopal DV, Nakar AA, Badrinath Y, Mishra KP, Joshi DS (2005) Betulinic acid induces apoptosis in human chronic myelogenous leukemia (CML) cell line K-562 without altering the levels of Bcr-Abl. Toxicol Lett 15:343–351
Raisova M, Hossini AM, Eberle J, Riebeling C, Wieder T, Sturm I, Daniel PT, Orfanos CE, Geilen CC (2001) The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol 117:333–340
Sarek J, Klinot J, Dzubak P, Klinotova E, Noskova V, Krecek V, Korinkova G, Thomson JO, Janostakova A, Wang S, Parson S, Fischer PM, Zhelev NZ, Hajduch M (2003) New lupane derived compounds with pro-apoptotic activity in cancer cells: synthesis and structure-activity relationships. J Med Chem 46:5402–5415
Schmidt ML, Kuzmanoff KL, Ling-Indeck L, Pezzuto JM (1997) Betulinic acid induces apoptosis in human neuroblastoma cell lines. Eur J Cancer 33:2007–2012
Selzer E, Pimental E, Wacheck V, Schlegel W, Pehamberger H, Jansen B, Kodym R (2000) Effects of betulinic acid alone and in combination with irradiation in human melanoma cells. J Invest Dermatol 114:935–942
Thurnher D, Turhani D, Pelzmann M, Wannemacher B, Knerer B, Formanek M, Wacheck V, Selzer E (2003) Betulinic acid: a new cytotoxic compound against malignant head and neck cancer cells. Head Neck 25:732–740
Wick W, Grimmel C, Wagenknecht B, Dichgans J, Weller M (1999) Betulinic acid-induced apoptosis in glioma cells: A sequential requirement for new protein synthesis, formation of reactive oxygen species, and caspase processing. J Pharmacol Exp Ther 289:1306–1312
Zuco V, Supino R, Righetti SC, Cleris L, Marchesi E, Gambacorti-Passerini C, Formelli F (2002) Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett 175:17–25
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rzeski, W., Stepulak, A., Szymański, M. et al. Betulinic acid decreases expression of bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cells. Naunyn-Schmied Arch Pharmacol 374, 11–20 (2006). https://doi.org/10.1007/s00210-006-0090-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-006-0090-1