Abstract
TRPM2 is a Ca2+-permeable non-selective cation channel that uniquely is activated by intracellular ADP-ribose. To date, only one pharmacological blocker of this channel, namely flufenamic acid (FFA), has been described. Here we demonstrate, using patch clamp electrophysiology, that the antifungal imidazoles clotrimazole and econazole inhibit ADP-ribose-activated currents in HEK-293 cells expressing recombinant human TRPM2 (hTRPM2). For both compounds, all concentrations in a range from 3 μM to 30 μM produced an essentially complete inhibition of the TRPM2-mediated current. The rate of current antagonism was dependent on the concentration applied, with higher concentrations producing faster block. In addition, decreasing extracellular pH accelerated inhibition of TRPM2 by both clotrimazole and econazole; extracellular alkalisation produced the converse effect. Additional experiments indicated hTRPM2 activation was required for the antagonism of either compound to develop, and that neither compound blocked from the intracellular face of the plasma membrane. ADP-ribose-activated whole-cell and single-channel currents in the rat insulinoma cell-line CRI-G1 were also antagonised by clotrimazole. Contrary to the observations made with hTRPM2, antagonism in CRI-G1 cells could be largely reversed following clotrimazole removal. These experiments suggest that imidazole antifungals may be useful tool antagonists for future studies of TRPM2 function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Molecular cloning has identified more than 20 mammalian members of the transient receptor potential (TRP) family. Until recently these channels have been subdivided into three major subgroups the TRPVs (the vanilloid receptor family), the TRPCs (the so-called short TRPs), and the TRPMs (the so-called long TRPs; Clapham et al. 2003). The recent identification of a channel known as ANKTM1 (a mustard oil and cinnamide receptor; Story et al. 2003; Jordt et al. 2004), which does not easily sit in any of the other three TRP groupings, has lead to the addition of a fourth group dubbed TRPAs (which currently only contains ANKTM1; Bandell et al. 2004).
With the possible exception of TRPV1, for which a rapidly growing number of agonists and antagonists have been described, the pharmacology of TRP channels is largely undeveloped. We have become particularly interested in one member of the latter family, known as TRPM2. This channel is most abundantly expressed in the brain, within which it exhibits a widespread expression pattern (Smith et al. 2003; Kraft et al. 2004). In addition, TRPM2 is expressed by neutrophil granulocytes (Heiner et al. 2003) and insulinoma cell-lines such as the CRI-G1 cells (Herson et al. 1999; Inamura et al. 2003). Expression of TRPM2 in a range of host cells yields a voltage-independent, Ca2+-permeable, non-selective cation conductance activated by intracellular ADP-ribose (Perraud et al. 2001). TRPM2-mediated currents can also be activated by extracellular hydrogen peroxide in a manner that is antagonised by inhibitors of the enzyme poly-ADP-ribopolymerase (PARP) (Fonfria et al. 2004). This latter observation suggests that ADP-ribose levels, and consequently TRPM2 gating, may be modulated by cellular redox status via the regulation of enzyme pathways that synthesis and metabolise NADH, NAD+, ADP-ribose and its cyclical analogue cyclic ADP-ribose (see Wilson et al. 2001). Similar H2O2 and ADP-ribose-activated currents can be observed in CRI-G1 cells and other cells that endogenously express TRPM2 such as cultured rat striatal neurones (KH unpublished observations).
As described above, ADP-ribose activates TRPM2 through binding to an intracellular site. Activity of TRPM2 depends strictly on the presence of extracellular Ca2+, thus removal of extracellular Ca2+ or its replacement with Ba2+ completely inhibits channel activity. However, like most other TRP channels, the range of pharmacological agents that can be used to antagonise TRPM2 is very limited. In addition to the above-mentioned effects of PARP blockers, it has been shown that a hydrogen peroxide-mediated depolarisation of striatal neurones, which may be due to activation of TRPM2, can be blocked by the free radical scavenger DMTU (Smith et al. 2003). As far as directly acting compounds are concerned, the only TRPM2 antagonist described to date is the arylaminobenzoate analgesic flufenamic acid. This molecule produces a voltage-independent, activity-dependent block that seems to rapidly convert from a reversible to an irreversible form (Hill et al. 2004).
Clotrimazole (1-[(2-chlorophenyl)diphenylmethyl]-1H-imidazole) and econazole (1-[2-[4-chlorophenyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole) are structurally related imidazole antifungal agents, first synthesised in the 1960s and used widely in the clinical and veterinary treatment of fungal infestation (for a review see Fromtling 1988). The mechanism of action is believed to be via inhibition of sterol 14-α-demethylase, a microsomal cytochrome P450-dependent enzyme system. This leads to impaired synthesis of ergosterol and accumulation of 14-α-methylsterols, the latter of which may impair membrane structure and inhibit respiratory function and consequently fungal growth.
In addition to their antimycotic actions, both clotrimazole and econazole have been reported to interact with a range of mammalian ion channels. Notably econazole inhibits store-operated/capacitive Ca2+ entry in a number of systems, a process that is thought to intimately involve TRP channels. Indeed, econazole has been shown to inhibit a member of the TRPV family namely TRPV5 (EcaC1) with an IC50 around 1 μM (Nilius et al. 2001). Another widely reported action of both clotrimazole and econazole is inhibition of both small and intermediate Ca2+-activated K+ channels. For example, antagonism of the IK channel, the molecular correlate of the Gardos channel of red blood cells, occurs with an IC50 of around 150 nM for clotrimazole and 2.5 μM for econazole (Jensen et al. 1998). Clotrimazole also is reported to inhibit ATP-gated K+ channels in pancreatic cells (Jager et al. 2004), the transient outward current of ventricular myocytes (Hernandez-Benito et al. 2001) and L-type voltage gated Ca2+ channels (Fearon et al. 2000; Thomas et al. 1999). In addition, clotrimazole is reported to be neuroprotective, an activity ascribed to inhibition of NMDA receptors (Isaev et al. 2002). Given the TRP inhibition described above and that blockers of TRPM2 have been proposed as potential neuroprotectants we have examined the inhibition of human recombinant TRPM2 by clotrimazole and econazole.
Materials and methods
Cell culture
Human embryonic kidney (HEK293) cells expressing tetracycline-inducible flag-tagged TRPM2 (TRPM2-HEK293 cells, Perraud et al. 2001) were grown in tissue culture flasks in a minimum essential medium (MEM) supplemented with non-essential amino acids, 10% foetal calf serum and 0.2 mM l-glutamine. The incubator was maintained at 37°C and contained 95% air and 5% CO2. For patch-clamp experiments cells were removed from the flasks and replated onto poly-lysine coated glass coverslips. TRPM2 expression was then induced by incubating cells for 24 h with culture medium supplemented with 1 μg/ml tetracycline.
Rat insulinoma CRI-G1 cells (ECACC Clone Ref. No. 87052701) were cultured in Dulbecco’s minimum essential medium (DMEM) supplemented with 10% foetal calf serum and 2 mM l-glutamine and maintained under 95% air and 5% CO2 at 37°C. Cells were used for patch-clamp experiments 1 day after plating onto poly-lysine-coated glass coverslips.
Electrophysiology
The whole-cell and outside-out variants of the patch clamp technique were used to characterise TRPM2-mediated currents. All experiments were performed at room temperature using an Axopatch-200B amplifier (Axon Instruments, Inc., Foster City, CA, USA). Patch pipettes of 2–5 MΩ resistance were fabricated from borosilicate glass capillaries. Experiments were performed and analysed using the pClamp 9 software suite (Axon Instruments, Inc.).
During whole-cell recordings cells were usually voltage-clamped at −15 mV, which is close to the zero current potential of control HEK-293 cells. The recorded cell was continuously perfused from a commercially available rapid solution exchange device (Warner Instruments, Hamden, CT, USA). Series resistances were <10 MΩ and were compensated by 75–85%. The extracellular solution always contained (mM) NaCl 140, KCl 10, MgCl2 2 and HEPES-NaOH 10. This was supplemented with either 1 mM CaCl2 (to permit TRPM2 gating) or 1 mM BaCl2 (to inhibit TRPM2 activity). For most experiments this extracellular solution was adjusted to pH 7.4 with NaOH, however, for analysis of the pH-dependence of drug action, identical solutions were also prepared at pH 8.5 and pH 6.2 (clotrimazole) or pH 8.0 and pH 6.2 (econazole).
Recording pipettes were filled with the following solution (mM): KGluconate 140, NaCl 5; MgCl2 2, EGTA 0.5, HEPES-KOH 10, pH 7.3. To produce TRPM2 activation ADP-ribose was added to the pipette solution at a concentration of 300 μM. In one series of experiments, designed to address site of action, clotrimazole or econazole was added to the pipette solution at a concentration of 30 μM. Outside-out patches were also studied; these were formed by rapidly moving the recording electrode away from a cell immediately after entering the whole-cell configuration. For outside-out patches an electrode solution consisting of (mM) CsCl 140, MgCl2 0.6, EGTA 0.5, HEPES-CsOH pH 7.3 supplemented with 60 μM or 100 μM ADP-ribose was used.
All membrane potentials in whole-cell recordings were corrected for a liquid junction potential of 15 mV. In all experiments membrane current was filtered at 2 kHz (4-pole Bessel characteristic filter) and sampled continuously at 5 kHz or 10 kHz. All data are presented as mean ± SEM.
Source of key reagents
ADP-ribose was obtained from Sigma (St Louis, MO, USA) and prepared as a stock solution in 10 mM HEPES/KOH buffer at pH 7.4 and frozen in aliquots at −20°C. Clotrimazole and econazole (nitrate salt, also known as spectazole) were obtained from Sigma and prepared as a fresh stock solution in DMSO each day. This was subsequently diluted to the required concentrations in the extracellular solution. The DMSO concentration did not exceed 0.1% and the solvent had no effect on membrane currents at this concentration. All cell culture media were obtained from Invitrogen (Paisley, UK).
Results
Clotrimazole antagonism of recombinant human TRPM2
As previously reported inducing expression of TRPM2 in HEK293 cells lead to the appearance of a large non-selective cation current that was strictly dependent on the presence of ADP-ribose on the intracellular side of the membrane. In whole-cell recordings at −15 mV this current had a mean amplitude of −1.17±0.06 nA. In isolated patch-recordings TRPM2 activity was apparent as distinctive long duration, large conductance, openings. Due to its strict dependence on extracellular Ca2+, this ADP-ribose-activated TRPM2-mediated current was rapidly eliminated by replacement of extracellular Ca2+ with Ba2+ (data not shown).
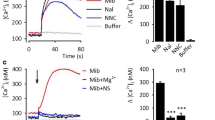
Figure 1a illustrates a typical whole-cell recording of a TRPM2-mediated current and its antagonism by clotrimazole. At the start of the trace, the whole-cell configuration was entered using an ADP-ribose containing pipette solution. After a short delay, a large inward current developed which exhibited little or no desensitization over a period of some 30 s. As shown, subsequent application of 30 μM clotrimazole completely eliminated this current, in a seemingly irreversible fashion. The block produced by clotrimazole, typically arose after a short delay. This delay is not a feature of the perfusion system, because it is not seen for either antagonism produced by replacement of extracellular Ca2+ with Ba2+ or application of flufenamic acid (Hill et al. 2004).
Clotrimazole causes an irreversible antagonism of hTRPM2. a A typical recording of a large ADP-ribose-induced inward current in a hTRPM2-HEK293 cell and its inhibition by 30 μM clotrimazole. The whole-cell configuration was entered at the time indicated by the arrow labelled WC. The cell was voltage clamped at −15 mV and the zero current level is indicated in this and all other figures by the dashed line annotated with I=0. Note the delay before the block starts to develop and the irreversible nature of the antagonism. b Pooled data for the concentration dependence of the clotrimazole-mediated block of TRPM2-mediated currents. The light grey bars plot the extent of the equilibrium block produced at each concentration tested, whereas the black bars quantify the lack of recovery observed 3 min after clotrimazole washout. c A graph plotting the relationship between the reciprocal of time required to produce half-maximal inhibition of the hTRPM2 current and the clotrimazole concentration on a logarithmic scale. d Pooled data illustrating the extent of block produced (grey bars) and its reversibility after a 3-min wash-out period (black bars) for three different exposure times (20, 60 and 120 s) to 30 μM clotrimazole. Note that unlike data previously published for flufenamic acid, inhibition of TRPM2 short compound exposures do not produce reversible block.
At 3, 5, 10 and 30 μM mean inhibition of the ADP-ribose-induced inward current by clotrimazole was always >90%. Furthermore, the antagonism produced was consistently irreversible in nature, with no significant current recovery being observed following removal of any of the concentrations of clotrimazole tested (Fig. 1b). In contrast, between 1 μM and 30 μM clotrimazole the rate of current antagonism was concentration-dependent, with a more rapid block being observed at higher compound concentrations. For example, the time to half block at 1 μM clotrimazole was 310±58 s but was 54±13 s at 5 μM clotrimazole. The relationship between antagonist concentration and rate of current decline is illustrated in Fig. 1c which plots the reciprocal of time to 50% inhibition against the concentration of clotrimazole on a logarithmic scale. It was impossible to determine the level of equilibrium block at a concentration of 1 μM or less because of the slow rate of block combined with the fact that prolonged activation of TRPM2 routinely compromises cell integrity, presumably through a combination of intracellular Ca2+ and Na+ overload.
Additional properties of clotrimazole-mediated block
The properties of the block produced by clotrimazole are somewhat reminiscent of the pharmacological profile of the only other TRPM2 blocker described to date, flufenamic acid (Hill et al. 2004) a molecule structurally unrelated to clotrimazole. This molecule produces irreversible antagonism of hTRPM2, with a blocking rate that is dependent on antagonist concentration. An additional facet of the action of flufenamic acid (FFA) is that its irreversible block is preceded by a period of reversible block. Experimentally, this is manifest as a reversible block of current for short applications of FFA but irreversible block of current for more prolonged antagonist exposures (Hill et al. 2004). We tested if this was also the case for clotrimazole. To investigate this possibility we applied the compound to a number of cells at 30 μM for precisely 20, 60 or 120 s before removing it again. We then measured, for each experiment the extent of block produced during the period of compound exposure and the amount of current recovered after 180 s in a drug-free solution. As shown in Fig. 1d, the block induced during the compound exposure (grey bars) was always completely non-recoverable (black bars); thus, unlike the actions of FFA at TRPM2, clotrimazole does not seem to produce a reversible antagonism that rapidly converts to an irreversible antagonism.
We wondered if we could generate a significant reversal of the clotrimazole-mediated inhibition of TRPM2 if we inhibited the functionality of the channel during the clotrimazole washout period. To do this, we blocked the channel in the usual fashion and then switched to a clotrimazole free solution in which Ca2+ had been replaced with Ba2+. After 120 s in these conditions, which would stop TRPM2 opening even if the clotrimazole block had reversed, we switched to the Ca2+-containing, clotrimazole-free bath solution to test if any TRPM2-mediated current had been recovered. In four such experiments, no increase in current was observed on adding back Ca2+, indicating that the block by clotrimazole failed to reverse in absence of extracellular Ca2+ (data not shown).
The imidazole ring of clotrimazole is mostly uncharged at normal physiological pH allowing it to effectively cross membranes. This raised the possibility that the short delay observed before clotrimazole-mediated block started to develop (e.g. Fig. 1a) might reflect a need for clotrimazole to cross the plasma membrane. We tested this by applying clotrimazole intracellularly via the recording pipette. A typical example of such an experiment is shown in Fig. 2a. It is clear that the ADP-ribose-activated current still developed when a high concentration of clotrimazole (30 μM) was applied to the inside of the cell. Furthermore, this current was maintained at a steady level under these conditions for at least 250 s. Subsequent extracellular application of the same concentration of clotrimazole rapidly eliminated the steady inward current. Figure 2b summarises a number of such experiments and illustrates that the magnitude of the ADP-ribose induced current was not different between cells with (n=7) or without (n=13) intracellular clotrimazole. Additionally, the inward current in the former group was completely blocked by extracellular application of clotrimazole (n=7). These experiments strongly suggest that clotrimazole blocks from the extracellular side.
Clotrimazole acts from the extracellular site of the channel. a An example recording where both 300 μM ADP-ribose and 30 μM clotrimazole were present in the pipette solution. Whole-cell access was obtained at the time shown by the arrow labelled WC and a robust TRPM2-mediated current develops. After 180 s, 30 μM clotrimazole was applied extracellularly and produced antagonism of the ADP-ribose-activated, TRPM2-mediated inward current. The cell was voltage-clamped at −15 mV and the zero current level is indicated by the line annotated with I=0. b Pooled data comparing the peak amplitude of ADP-ribose-induced currents in control cells (i.e. without clotrimazole in the intracellular solution, left hand bar) and cells with 30 μM intracellular clotrimazole (centre bar). There was no statistically significant difference between these two groups, indicating a lack of TRPM2 antagonism from the intracellular side. In contrast, extracellular application of 30 μM clotrimazole (right bar), led to a complete abolition of the ADP-ribose-induced current.
Inhibition of TRPM2 by FFA (a weak acid) is speeded by acidic extracellular pHs. Therefore, we examined how similar changes in extracellular pH affected the ability of clotrimazole (a weak base) to block TRPM2. Data from such experiments plotting both the extent (white bars) and time to 50% block (black bars) produced by 30 μM clotrimazole at three different pH levels are shown in Fig. 3. It is clear, that like FFA (Hill et al. 2004), the rate of TRPM2 block by clotrimazole is greatly speeded by acidification and retarded by alkalisation. Extracellular acidification to pH 6 alone has no detectable effects on channel function (Hill et al. 2004). In addition acidifying the cells interior to pH 6 did not alter the properties of clotrimazole-mediated block (data not shown).
The rate of clotrimazole-mediated antagonism of TRPM2 is enhanced at acid pH levels. A graph plotting the time taken (left-hand axis, black bars) for 30 μM clotrimazole to produce half-maximal block at the three different extracellular pHs. Also plotted for the same cells (right-hand axis, white bars) is the mean steady-state decrease in hTRPM2-mediated current.
Block of TRPM2 by FFA requires the channel to be activated. We tested if this was also the case for clotrimazole. To do this we performed an experiment using a protocol like that illustrated in Fig. 4a. We first formed a cell-attached recording in bathing medium in which Ca2+ had been replaced by Ba2+ (a manoeuvre that eliminated any possibility of channel activation). Whilst still in the cell-attached mode, we then applied 30 μM clotrimazole for 2 min (a concentration and duration of exposure that produces an irreversible, >95% block of active TRPM2, Fig. 1b). Clotrimazole was then washed out in the absence of extracellular Ca2+ for 3 min, and the whole-cell configuration was established with ADP-ribose in the pipette. After a short period extracellular Ca2+ was applied, allowing any unblocked TRPM2 channels to rapidly activate (Fig. 4b). A similar control protocol was applied which lacked the exposure to clotrimazole during the cell-attached phase. The magnitude of the inward current observed on entering the whole-cell configuration was indistinguishable when control cells (−1.23±0.14 nA, n=13) and those pre-exposed to clotrimazole in the cell-attached configuration were compared (−1.36±0.24 nA, n=5, Fig. 4c). Furthermore, in the cells that had been preincubated with clotrimazole, the large current that was produced on entering the whole-cell configuration was completely blocked by a subsequent application of clotrimazole (Fig. 4c, n=5). These data suggest that clotrimazole only blocks active TRPM2 channels.
Inhibition of hTRPM2 by clotrimazole depends on channel activation. a A schematic illustration of the experimental method used to test whether the block of TRPM2 by clotrimazole requires channel activation. A cell-attached recording was made with a pipette containing ADP-ribose and extracellular Ca2+ was removed (and replaced with Ba2+). The absence of extracellular Ca2+ and the lack of intracellular ADP-ribose both served to stop TRPM2 activation. Clotrimazole (Cltz) was then applied for 120 s at 30 μM, a concentration and duration of application suitable to completely and reversibly inhibit hTRPM2 reliably (see Fig. 1). This was then washed off for 120 s before Ca2+ was added back and the whole-cell configuration entered (thus allowing ADP-ribose to enter the cytoplasm). After waiting a suitable period for any ADP-ribose-activated current to appear, clotrimazole was reapplied at 30 μM to block any remaining TRPM2-mediated current. Experiments like that in the schematic illustration and where first clotrimazole addition was excluded were compared. b A typical current trace from the type of protocol shown in a. The data shown correspond to the period of the experiment highlighted by the dashed box in a. Note the presence of a large ADP-ribose-induced inward current despite the prior incubation with clotrimazole in the absence of channel activation. c A graph comparing the amplitude of ADP-ribose-induced currents in 13 control cells (i.e. cells not preincubated with clotrimazole, left bar) with five experiments (middle bar) in which the cell was exposed to 30 μM clotrimazole in the absence of extracellular calcium (i.e. protocol as shown in a). The graph also plots the amount of current remaining in the five cells preincubated with clotrimazole following the second application of clotrimazole during the whole-cell phase of the recording (right bar).
Econazole inhibition of human TRPM2
We also examined a second imidazole anti-fungal agent, the tricyclic econazole, for its actions at TRPM2. In many ways this compound mirrored the actions of clotrimazole. Firstly, it produced a block of TRPM2 current that was consistently preceded by a short delay (Fig. 5a). Secondly, at concentrations between 3 μM and 30 μM econazole produced an irreversible >90% block of ADP-ribose induced current (Fig. 5b). Thirdly, the time to 50% antagonism was strongly concentration-dependent with a mean half time of block >200 s at 3 μM (Fig. 5c). Regarding this third point, for any given drug concentration, clotrimazole consistently blocked 2–3 times faster than econazole. The rate of block by 30 μM econazole (which is like clotrimazole a weak base) was speeded by extracellular acidification (time to half block at pH 6 was 10.4±1.3 s [n=9] vs. 33.2±5.4 s [n=4] at pH 7.4) and correspondingly slowed by alkalisation (time to half block at pH 8 was 42.2±4.4 s [n=5]). The magnitude of block was greater than 92% at all three pHs (Fig. 5d). Finally, like clotrimazole-mediated TRPM2 antagonism, but unlike FFA block, irreversible antagonism by econazole did not follow a period of reversible antagonism and could not be produced by intracellular application of the compound (data not shown).
Properties of econazole-mediated antagonism of hTRPM2. a An example voltage-clamp recording at −15 mV of an ADP-ribose-activated current and its complete antagonism by an extracellular application of 10 μM econazole. Note the failure of the block to reverse in the subsequent 3-min wash-out period. The zero current level is indicated by the dashed line. b Summary data for the concentration dependence of econazole-mediated block (grey bars) at four concentrations. Also plotted for each concentration is the current amplitude following a 3-min wash-out period (black bars). c A graph plotting the relationship between the reciprocal of time required to produce half-maximal inhibition of the hTRPM2 current and the applied concentration of econazole plotted on a logarithmic scale. d A graph plotting the time to 50% inhibition (black bars) and the extent of equilibrium antagonism (grey bars) produced by econazole at three different extracellular pHs.
Clotrimazole-mediated inhibition of ADP-ribose-activated currents in rat insulinoma cells
Insulinoma cell-lines such as the rat CRI-G1 cell express TRPM2 mRNA and possess a robust ADP-ribose-activated current that shares many features with the currents produced by expression of recombinant TRPM2. Accordingly, Hill et al. (2004) report that this current is blocked by FFA. In marked contrast to the block of hTRPM2 by this compound, however, antagonism of the ADP-ribose-gated currents in the insulinoma cell-line is almost fully reversible. Consequently we tested the ability of clotrimazole to block the ADP-ribose-activated current in a CRI-G1 cell. Figure 6a illustrates a typical recording where a large (∼1 nA), maintained, inward current developed after entering the whole-cell configuration. This current was blocked by extracellular application of 10 μM clotrimazole with a time to half block of 62±11 s, similar to the rate of block of hTRPM2 (see Fig. 1b). Removal of clotrimazole leads to the reappearance of the inward current over a period of about 60 s. Following full recovery of the current, it could be totally re-antagonised by a second clotrimazole application. Figure 6b plots pooled data from five such experiments showing that 10 μM clotrimazole produced >90% current inhibition and that >70% of the blocked current was recovered on washout.
Inhibition of a TRPM2-like current in a rat insulinoma cell line. a Example of ADP-ribose-induced currents in a CRI-G1 insulinoma cell line. Application of 10 μM clotrimazole produced a complete inhibition of the current that could be reversed on compound wash-out. A subsequent second clotrimazole application reinhibited the ADP-ribose-induced current. b A graph plotting pooled data from five recordings describing the extent of clotrimazole (10 μM) block of CRI-G1 TRPM2-like currents. The substantial reversibility of this effect is also illustrated.
In a final series of experiments, we examined the actions of clotrimazole on TRPM2 channels in outside-out patches isolated from CRI-G1 cells. Whole-cell recordings were initiated with 60 μM or 100 μM intracellular ADP-ribose and outside-out patches formed immediately by standard procedures. In these patches the activation of a large conductance channel, mean slope conductance 95±17 pS (n=4), with long open times soon became apparent (Fig. 7a). These channels appeared very similar to those previously described in studies of recombinant TRPM2 (Perraud et al. 2001) or to NAD+-ribose and ADP-ribose-activated channels in pancreatic beta cell lines (Inamura et al. 2003). In many patches, the number of simultaneously active channels rapidly rose to between approximately 15 and 30, producing a substantial macroscopic current, within which clear channel openings and closings could still be discerned. A typical example of such a recording is shown in Fig. 7b. This macroscopic current, and its associated channel noise, could be completely and reversibly blocked by 10 μM clotrimazole. We were also able to isolate a few patches in which a smaller number (i.e. 4–7) of ADP-ribose-activated channels were present. Although this channel activity exhibited a tendency to run down with time, a clear block of single channel openings by 10 μM clotrimazole could be elicited (Fig. 7c).
Clotrimazole inhibition of ADP-ribose-activated channels in outside-out patches isolated from CRI-G1 cells. a Left, single channel activity in an outside-out patch exposed to 60 μM ADP-ribose at its intracellular face. Epochs recorded at five different membrane potentials are shown. Right, timebase-expanded traces from the same recording illustrating single-channel transitions at four different membrane potentials. At −60, −40 and −20 mV the upward transitions correspond to closures of a high open probability channel. Downward transitions at +20 mV represent equivalent closures at +20 mV. b Currents from an outside-out patch in which approximately 20 ADP-ribose-activated channels are open at one time generating a fluctuating inward current. This current was blocked reversibly and repeatably by application of clotrimazole (10 μM) to the extracellular side. c A demonstration of the complete inhibition of ADP-ribose-induced single channel transitions by clotrimazole (10 μM). The recording is from an outside-out patch isolated from a CRI-G1 cell and containing about seven TRPM2-like channels. The patch was held at −60 mV except at the point illustrated by the asterisk where the patch potential was briefly depolarised to 0 mV.
Very similar ADP-ribose-activated channels and macroscopic responses were also observed in isolated patches taken from the hTRPM2 expressing HEK-293 cell-line. These hTRPM2-mediated responses, however, rapidly ran-down irrespective of whether the outside-out or inside-out configuration was used. For example, in outside-out patches hTRPM2 channel activity declined to zero with a time constant of 106±26 s. Despite several experimental manoeuvres we were unable to inhibit this rundown which was significantly fast to preclude our attempts to study the detailed pharmacology of TRPM2 in detached patches.
Discussion
We have demonstrated that two related imidazole anti-fungal agents, namely clotrimazole and econazole, are effective inhibitors of recombinant hTRPM2 channels and the TRPM2-like channel present in the rat pancreatic cell-line CRI-G1. This is the second class of small molecules shown to inhibit these channels, following on from the recent report of their inhibition by the non-steroidal anti-inflammatory agent flufenamic acid (Hill et al. 2004). Although the inhibition by neither class of agent is selective for TRPM2, inhibition of a given current by both types of molecule, particularly with appropriate kinetics and use-dependence, would be much better pharmacological evidence for its mediation by TRPM2 than inhibition by either agent alone.
The block produced by clotrimazole and econazole shares a number of features with that elicited by flufenamic acid, despite the unrelated structural nature of these two compound classes. For example, the block of human recombinant channels by both types of agent is irreversible and requires the channels to be activated in order for block to develop. The clotrimazole- and econazole-mediated inhibition exhibit a clear delay before starting to develop. This is not due to perfusion system delays since the antagonism produced by removal of extracellular Ca2+ or application of flufenamic acid (Hill et al. 2004) are close to instantaneous. It is tempting to speculate that the clear delay in the block produced by the imidazole compounds shares some mechanistic parallels with the short-lived reversible component of flufenamic acid-mediated inhibition we have recently described (Hill et al. 2004).
TRMP2 is the only ion channel known to be activated by ADP-ribose. Consequently, the robust ADP-ribose-activated current in CRI-G1 cells, and other insulinoma cells, is believed to arise from channels containing TRPM2 subunits. Furthermore, as shown here the single channels activated by ADP-ribose in these cells are very similar to those generated by expression of recombinant TRPM2 (Perraud et al. 2001; K.H., unpublished data). Accordingly, the ADP-ribose-activated current in CRI-G1 cells, which we previously demonstrated to be flufenamic acid sensitive, was blocked by both clotrimazole and econazole. In addition, as seen with flufenamic acid this antagonism was freely reversible, contrasting with the irreversible block of hTRPM2 expressed in HEK-293 cells. The reason for this difference between the recombinant human channel and the current in CRI-G1 cells is not well understood. It may represent differences in the amino acid sequence of the human and rat TRPM2 channels. An alternative possible explanation is that the TRPM2 channels in CRI-G1 cells are heteromeric, containing other TRP subunits, and it is this heteromeric nature that gives rise to the reversible nature of block by both the imidazoles and fenamates.
Using electrophysiology we were unable to determine an IC50 for TRPM2 inhibition by either clotrimazole or econazole. We encountered a similar problem in our study of flufenamic acid, in both cases this difficulty arose from the relatively slow kinetics of channel inhibition. The rate of TRPM2 current antagonism is proportional to the concentration of compound applied, as one would expect for a simple drug-receptor interaction, however, even concentrations that completely block the ADP-ribose response can take minutes to produce their block. Consequently, concentrations that would produce 50% equilibrium block are likely to take ten or more minutes to approach this level of antagonism. Unfortunately, such prolonged periods of TRPM2 activation invariably compromise cell viability. We can be fairly sure the clotrimazole IC50 would be sub-micromolar because experiments using 1 μM clotrimazole reached 50% block after ∼5 min (Fig. 1c) and 3 μM produced essentially complete current blockade (Fig. 1b). We have no reason to suspect econazole is significantly less potent than clotrimazole although it blocks slightly more slowly at any given concentration, although both imidazoles block considerably faster than flufenamic acid at an equivalent concentration.
As well as their classical action at sterol 14-α-demethylase, both clotrimazole and econazole are known to produce actions at a range of ion channels (see Introduction). Indeed, independent of its antifungal properties, and as a result of its actions on IK channels, clotrimazole has been in clinical trials for the treatment of sickle cell disease (see Brugnara 2003) and diarrhoea. Clotrimazole has also been suggested to ameliorate the symptoms of rheumatoid arthritis. Another important feature of the pharmacology of antifungal azoles is their reported, but often overlooked, activity at calmodulin (Hegemann et al. 1993; McNeil et al. 1993). This latter aspect of the pharmacology of clotrimazole and econazole may play some role in their actions at TRPM2 which, like many other TRPs is a highly Ca2+ sensitive channel (McHugh et al. 2003). Thus, these imidazoles are far from selective inhibitors of TRPM2. Despite this, we feel that, with due consideration of their other known actions, clotrimazole and econazole may be useful tools in the study of TRPM2 in appropriate experimental systems.
Changing the extracellular pH produced substantial effects on the inhibition of TRPM2 by both imidazoles. For both econazole and clotrimazole applied at 30 μM, acidification substantially speeded the rate of current inhibition. The contrary effect was observed with alkalisation. Although the imidazoles are weak bases losing their charge at acidic pH, their pH-dependence of TRPM2 antagonism mirrors the situation seen with the weak acid flufenamic acid. It seems that the enhanced block at acidic pH does not reflect a need for the imidazoles to cross the lipid bilayer in order to access an intracellular binding site, as neither econazole nor clotrimazole seem to block TRPM2 when introduced into the cytoplasm. One possible explanation for the observed pH dependence is that flufenamic acid and the imidazoles share a common binding pocket, the conformation of which is more favourable at acidic pH. Neither agent exhibits a strong chemical reactivity at either acidic or neutral pH.
The presence of TRPM2 in the brain and insulinoma cells raises questions regarding its function in vivo. As a result of its activation by species produced by oxidative stress and its substantial Ca2+ permeability, TRPM2 is a good candidate for a channel that triggers cellular responses to changes in oxidative load. An extension of this is the consideration that TRPM2 is a “suicide channel” causing cells to overload with Ca2+ and become irreversibly compromised under conditions of oxidative stress. A consequence of this latter hypothesis is that TRPM2 antagonists may be able to rescue cell populations from cell death triggered by compromised oxidative metabolism, such as occurs in conditions such as ischaemic stroke and Parkinson’s disease. In this regard clotrimazole has been shown to be neuroprotective in vitro (Isaev et al. 2002). This activity was completely ascribed to an inhibition of NMDA receptors; however, a reassessment of this conclusion may be warranted in the light of the data present here. H2O2-activated (Smith et al. 2003) or ADP-ribose activated (K.H. and A.R., unpublished observations) TRPM2-like channels can be found in cultured neurones, and consequently one might expect their inhibition to contribute to any neuroprotective efficacy of clotrimazole.
The role of TRPM2 channels in pancreatic beta cells is presently unclear. Indeed, although a feature of insulinomas, functional channels have not as yet been reported in native beta cells. Micromolar concentrations of clotrimazole have been reported to alter insulin secretion in two studies. In the first (Chan et al. 1997), clotrimazole was reported to produce an insulinotropic action that was temperature-concentration and glucose-concentration dependent but not dependent on extracellular Ca2+, probably ruling out a role of TRPM2. In a second study no effect on control glucose stimulated insulin release was apparent, but a clotrimazole and miconazole inhibited insulin secretion that had been potentiated by inhibition of nitric oxide production with L-NAME (Lajoix et al. 2001). To determine if this action in any way reflects a contribution of TRPM2 activity to L-NAME-mediated enhancement of insulin release it will probably require the development of a much more specific TRPM2 antagonist.
A better understanding of the role played by TRPM2 in any particular tissue may come from a better understanding of the factors controlling the levels of intracellular ADP-ribose. An appropriate membrane associated enzymatic activity has been reported in the brain although the specific enzyme responsible has not been isolated (Ceni et al. 2003). Interestingly, the activity of the enzyme can be enhanced by GTP and GTP-γ-S, suggesting the production of ADP-ribose may be under the control of a G-protein and therefore perhaps can be receptor regulated.
To conclude, we have demonstrated that the antifungal imidazoles clotrimazole and econazole are effective antagonists of human TRPM2 channels and ADP-ribose-activated currents in rat CRI-G1 cells. The observed antagonism exhibits a number of interesting features, many of which parallel recent observations made with the only other TRPM2 blocker described to date, flufenamic acid. It is hoped that these molecules will have some utility in further probing the roles of TRPM2 and the development of new, more selective, TRP channel antagonists.
References
Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857
Brugnara C (2003) Sickle cell disease: from membrane pathophysiology to novel therapies for prevention of erythrocyte dehydration. J Pediatr Hematol Oncol 25:927–933
Ceni C, Muller-Steffners H, Lund F, Pochon N, Schweitzer A, de Waard M, Schuber F, Villaz M, Moutin M-J (2003) Evidence for an intracellular ADP-ribosyl cyclase/NAD+-glycohydrolase in brain from CD38-deficient mice. J Biol Chem 278:40670–40678
Chan SLF, Pallet AL, Morgan NG (1997) Clotrimazole and efaroxan stimulate insulin secretion by different mechanisms in rat pancreatic islets. Naunyn-Schmiedebergs Arch Pharmacol 356:763–768
Clapham DE, Montell C, Schultz G, Julius D (2003) International union of pharmacology. XLIII. Compendium of voltage-gated ion channels: transient receptor potential channels. Pharmacol Rev 55:591–596
Fearon IM, Ball SG, Peers C (2000) Clotrimazole inhibits the recombinant human cardiac L-type Ca2+ channel alpha 1C subunit. Br J Pharmacol 129:547–554
Fonfria E, Marshall ICB, Benham CD, Boyfield I, Brown JB, Hill K, Hughes JP, Skaper SD, McNulty S (2004) TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol 143:186–192
Fromtling RA (1988) Overview of medically important antifungal azole derivatives. Clin Microbiol Rev 1:187–217
Hegemann L, Toso SM, Lahijani KI, Webster GF, Uitto J (1993) Direct interaction of antifungal azole-derivatives with calmodulin: a possible mechanism for their therapeutic activity. J Invest Dermatol 100:343–346
Heiner I, Eisfeld J, Halaszovich CR, Wehage E, Jungling E, Zitt C, Luckhoff A (2003) Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem J 371:1045–1053
Hernandez-Benito MJ, Macianskiene R, Sipido KR, Flameng W, Mubagwa K (2001) Suppression of transient outward potassium currents in mouse ventricular myocytes by imidazole antimycotics and by glybenclamide. J Pharmacol Exp Ther 298:598–606
Herson PS, Lee K, Pinnock RD, Hughes J, Ashford ML (1999) Hydrogen peroxide induces intracellular calcium overload by activation of a non-selective cation channel in an insulin-secreting cell line. J Biol Chem 274:833–841
Hill K, Benham CD, McNulty S, Randall AD (2004) Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology 47:450–460
Inamura K, Sano Y, Mochizuki S, Yokoi H, Miyake A, Nozawa K, Kitada C, Matsushime H, Furuichi K (2003) Response to ADP-ribose by activation of TRPM2 in the CRI-G1 insulinoma cell line. J Membr Biol 191:201–207
Isaev NK, Stelmashook EV, Dirnagl U, Andreeva NA, Manuhova L, Vorobjev VS, Sharonova IN, Skrebitsky VG, Victorov IV, Katchanov J, Weih M, Zorov DB (2002) Neuroprotective effects of the antifungal drug clotrimazole. Neuroscience 113:47–53
Jager H, Dreker T, Buck A, Giehl K, Gress T, Grissmer S (2004) Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol Pharmacol 65:630–638
Jensen BS, Strobaek D, Christophersen P, Jorgensen TD, Hansen C, Silahtaroglu A, Olesen SP, Ahring PK (1998) Characterization of the cloned human intermediate-conductance Ca2+-activated K+ channel. Am J Physiol 275:C848–C856
Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427:260–265
Kraft R, Grimm C, Grosse K, Hoffmann A, Sauerbruch S, Kettenmann H, Schultz G, Harteneck C (2004) Hydrogen peroxide and ADP-ribose induce TRPM2-mediated calcium influx and cation currents in microglia. Am J Physiol Cell Physiol 286:C129–C137
Lajoix A-D, Reggio H, Chardes T, Peraldi-roux S, Tribillac F, Roye M, Dietz S, Broca C, Manteghetti M, Ribes G, Wollheim CB, Gross R (2001) A neuronal isoform of nitric oxide synthase expressed in pancreatic β-cells controls insulin secretion. Diabetes 50:1311–1323
McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ (2003) Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem 278:11002–11006
MacNeil S, Dawson RA, Crocker G, Tucker WF, Bittiner B, Singleton JG, Hunter T, Tierney DF (1993) Antiproliferative effects on keratinocytes of a range of clinically used drugs with calmodulin antagonist activity. Br J Dermatol 128:143–150
Nilius B, Prenen J, Vennekens R, Hoenderop JG, Bindels RJ, Droogmans G (2001) Pharmacological modulation of monovalent cation currents through the epithelial Ca2+ channel ECaC1. Br J Pharmacol 134:453–462
Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM (2001) ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411:595–599
Smith MA, Herson PS, Lee K, Pinnock RD, Ashford ML (2003) Hydrogen-peroxide-induced toxicity of rat striatal neurones involves activation of a non-selective cation channel. J Physiol 547:417–425
Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–829
Thomas GP, Karmazyn M, Zygmunt AC, Antzelevitch C, Narayanan N (1999) The antifungal antibiotic clotrimazole potently inhibits L-type calcium current in guinea-pig ventricular myocytes. Br J Pharmacol 126:1531–1533
Wilson HL, Dipp M, Thomas JM, Lad C, Galione A, Evans AM (2001) Adp-ribosyl cyclase and cyclic ADP-ribose hydrolase act as a redox sensor. A primary role for cyclic ADP-ribose in hypoxic pulmonary vasoconstriction. J Biol Chem 276:11180–11188
Acknowledgements
The authors thank Dr A. Scharenberg (University of Washington) for the kind gift of the HEK293-TRPM2 cell line. K.H. is in receipt of EU Framework V Postdoctoral Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hill, K., McNulty, S. & Randall, A.D. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn-Schmiedeberg's Arch Pharmacol 370, 227–237 (2004). https://doi.org/10.1007/s00210-004-0981-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-004-0981-y