Abstract
The mechanism underlying the sleep-inducing effect of oleamide, an endogenous fatty acid amide, was studied in rats. Animals implanted with cerebrocortical and dorsal neck muscle electrodes were monitored continuously by electroencephalograph (EEG) and electromyograph (EMG) for 4 h after i.p. or s.c. injection of drugs. Oleamide induced a dose-dependent increase in slow-wave sleep (SWS), a decrease in wakefulness (W) and sleep latency, but had no effect on rapid-eye-movement sleep (REMS). The oleamide-induced increase in SWS was prevented by 5-HT reuptake inhibitors such as fluoxetine or fenfluramine and by agonists at 5-HT1A receptors such as buspirone or 8-hydroxy-2-(di-N-propylamino) tetralin (8-OH-DPAT). Moreover, the selective 5-HT1A receptor antagonist WAY100635 markedly antagonized the suppression of the oleamide-induced increase in SWS by 8-OH-DPAT. These data provide the first behavioural evidence that the serotonergic system may be involved in the sleep-inducing action of oleamide in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oleamide (cis-9,10-octadecenoamide), a lipid isolated from the cerebrospinal fluid of sleep-deprived cats, induces physiological sleep in rats (Cravatt et al. 1995). Previous studies have demonstrated the sedative and hypnotic function of oleamide. For example, peripherally administered in rats or mice, oleamide decreases sleep latency and wake time and increases total sleep time (Boger et al. 1998a; Mendelson and Basile 1999; Laposky et al. 2001; Huitron-Resendiz et al. 2001). Oleamide also potentiates the hypnotic action of pentobarbitone sodium in mice (Yang et al. 1999) and a behavioural response to a 5-HT2 receptor agonist (Cheer et al. 1999). Although oleamide appears to potentiate the actions of 5-HT on 5-HT2A and 5-HT2C receptors, and to act via an allosteric regulatory site on 5-HT7 receptors in vitro, its mechanism of action is not yet understood fully (Huidobro-Toro and Harris 1996; Mechoulam et al. 1997; Hedlund et al. 1999).
It is well established that the sleep-wakefulness cycle is regulated by the serotonergic system. For example, both the 5HT reuptake inhibitor fluoxetine and the 5HT releaser and uptake inhibitor fenfluramine suppress slow-wave sleep (SWS) and rapid-eye-movement sleep (REMS), but increase wakefulness (W) (Fornal and Radulovacki 1983a; Gao et al. 1992). Buspirone, a partial agonist at 5-HT1A receptors, reportedly increases W and decreases SWS and REMS in rats (Monti and Jantos 1992). 8-Hydroxy-2-(di-N-propylamino) tetralin (8-OH-DPAT), a full agonist at somatodendritic and postsynaptic 5-HT1A receptors, has biphasic effects on the sleep-wakefulness cycle (Lerman et al. 1986; Monti and Jantos 1994; Monti et al. 1995). The present study was designed to investigate the involvement of the serotonergic system in the action of oleamide on sleep in freely moving rats. The study confirmed the sleep-inducing effect of oleamide and demonstrated the influence of changes in 5-HT neurotransmission on the oleamide-induced sleep parameters in rats.
Materials and methods
Animals and surgery
Sixty male Wistar rats weighing 250–350 g were supplied by the Experimental Animal Centre of Shenyang Pharmaceutical University. They were kept under standard conditions (environmental temperature 19±1 °C, 12:12 h light-dark cycle with lights on at 0800 hours). Electrodes for polygraphic recording of the electroencephalogram (EEG) and electromyogram (EMG) were implanted (Fornal and Radulovacki 1983a) under pentobarbitone sodium anaesthesia (40 mg/kg, i.p.). Four stainless-steel electrodes were inserted into the skull above the parietal cortices for subsequent bipolar EEG recordings. Two electrodes were placed over the left hemisphere and two other electrodes at the same position over the right hemisphere (limb area, occipital cortex area). For recording the EMG, two wire electrodes were inserted bilaterally into the dorsal neck muscles. The leads from all electrodes were then affixed to the skull with dental cement. An antibiotic ointment was applied to the incision before suturing to prevent scalp infection. After surgery, animals were housed in individual cages. Experiments were carried out for at least 1 week after electrode implantation. One day before the sleep evaluation, the rats were habituated to a cable system similar to that used in the actual recording. One rat was tested at a time and all rats in a specific drug group were tested once. In addition, animals were carefully handled at this time to minimize the stress involved in subsequent experimental procedures, i.e. drug injection and cable attachment.
Polygraphic recording
On the day of experiment, rats were placed in a cage and were allowed to move freely to some extent. At the start of the recordings, rats received an injection of either saline or drug solutions. All recordings began at 9.00 a.m. and lasted for 4 h. Recordings were made at a chart speed of 25 mm/s using an 8-channel EEG instrument (Nihon Kohden, Japan). The half-amplitude frequency response was 1–35 Hz for the EEG and 30–75 Hz for the EMG.
Experimental protocol
Oleamide (purity better than 98%, synthesized by the Department of Organic Chemistry of Shenyang Pharmaceutical University) was dissolved in corn oil and 25, 50, 100 and 200 mg/kg oleamide were administered by i.p. injection. d,l-Fenfluramine hydrochloride (1 or 3 mg/kg, Huhanaki Oy Leiras, Finland) or fluoxetine hydrochloride (10 or 20 mg/kg, Changzhou Pharmaceutical Factory, China) was dissolved in saline and injected i.p. 10 min before oleamide administration. Buspirone hydrochloride (4 or 8 mg/kg, Huatai Institute for Drug Research, Shenyang, China) or 8-OH-DPAT (0.1 or 0.5 mg/kg, RBI, Natick, Mass., USA) was dissolved in saline and was injected s.c. 10 min before oleamide administration. For antagonism experiments, N-[2-(4-2-methoxyphenyl)-1-piperazinyl)ethyl]-N-(2-pyridynyl)cyclohexanecarboxamide 3HCl (WAY 100635, 0.1 mg/kg, gift from Pharmacia, Nerviano, Italy) was injected s.c. 20 min before 8-OH-DPAT administration. All animal use procedures were in accordance with the Regulations of Experimental Animal Administration issued by the State Committee of Science and Technology of the People’s Republic of China on November 14th, 1988.
Data analysis and statistics
The polygraphic results were analysed visually and classified according to the following critia: W was characterized by a high-frequency, low-amplitude EEG and a relatively high amplitude EMG tone; SWS was a low-frequency, high-amplitude EEG and a relatively low EMG tone; REMS was characterized by a high-frequency, low-amplitude and, except for occasional muscle twitches, an absence of EMG tone. The following definitions of sleep parameters were used. SWS (Wake or REMS): the total time of SWS (Wakefulness or REMS). Mean SWS (Wake or REMS): mean duration of the individual SWS (Wakefulness or REMS). The latency to SWS was defined as the time from injection to the occurrence of the first SWS episode. Results are expressed as means±SEM. All data were analysed by repeated-measures analysis of variance (ANOVA) followed by Duncan’s multiple tests.
Results
Effect of oleamide on sleep
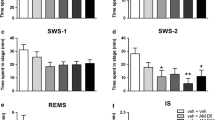
As shown in Table 1, oleamide (50, 100 or 200 mg/kg) significantly increased the total time of SWS, the total sleep time and the mean duration of SWS. It shortened the sleep latency and the total time of W with no effect on REMS. The 100 mg/kg dose had the maximum effect on SWS, the highest dose (200 mg/kg) did not produce any greater effect than 100 mg/kg dose.
Effects of fluoxetine and fenfluramine on the sleep-inducing action of oleamide
Oleamide 50 mg/kg, which produced the smallest observable effect on SWS, was used to study the interactions of oleamide with 5-HT reuptake inhibitors. As shown in Figs. 1 and 2, both fluoxetine (20 mg/kg, i.p.) and fenfluramine (3 mg/kg, i.p.) significantly decreased the total time of SWS. REMS almost disappeared after administration of fluoxetine but did not change significantly after fenfluramine (0.87±0.57 min for the corn oil-treated group, 0.70±0.10 min for the 3 mg/kg fenfluramine-treated group). Pretreatment with fluoxetine or fenfluramine for 10 min before administration of oleamide dose-dependently prevented the increase in SWS induced by oleamide. Moreover, at their lower doses both drugs, which alone had no effect on SWS, significantly suppressed the increase of SWS induced by oleamide.
Effect of fluoxetine on the oleamide-induced increase of slow-wave sleep (SWS) in rats. Animals were implanted chronically with cerebrocortical and dorsal neck muscle electrodes. Fluoxetine (10 or 20 mg/kg, i.p.) was administered alone or 10 min before injection 50 mg/kg oleamide. Animals were then continuously monitored by EEG for 4 h. Means±SEM; n=6; *P<0.05, **P<0.01 vs. vehicle (corn oil); ## P<0.01 vs. oleamide (repeated-measures ANOVA followed by Duncan’s multiple tests)
Effect of fenfluramine on the oleamide-induced increase of SWS in rats. Animals were prepared and monitored as in Fig. 1. Fenfluramine (1 or 3 mg/kg, i.p.) was administered alone or 10 min before injection 50 mg/kg oleamide. . Means±SEM, n=6; **P<0.01, vs. vehicle, ## P<0.01 vs. oleamide (repeated-measures ANOVA followed by Duncan’s multiple tests)
Effects of 5-HT1A agonists on sleep-inducing action of oleamide
Buspirone (4 and 8 mg/kg, s.c.) or 8-OH-DPAT (0.1 and 0.5 mg/kg, s.c.) had no significant effect on SWS and REMS when administered alone (data not shown). As shown in Figs. 3 and 4, however, both drugs produced a dose-dependent decrease in SWS when given in combination with 50 mg/kg oleamide, but did not change REMS significantly (data not shown). 8-OH-DPAT alone or given with oleamide induced behavioural reactions of the 5-HT syndrome in rats, including hind limb abduction and flat body posture. This finding is consistent with other reports (Sorensen et al. 2001).
Effect of buspirone on the oleamide-induced increase of SWS in rats. Animals were prepared and monitored as in Fig. 1. Buspirone (4 or 8 mg/kg, s.c.) was administered alone or 10 min before injection 50 mg/kg oleamide. Means±SEM; n=6; **P<0.01 vs. vehicle, ## P<0.01 vs. oleamide (repeated-measures ANOVA followed by Duncan’s multiple tests)
Effect of 8-hydroxy-2-(di-N-propylamino) tetralin (8-OH-DPAT) on the oleamide-induced increase of SWS in rats. Animals were prepared and monitored as in Fig. 1. 8-OH-DPAT (0.1 or 0.5 mg/kg, s.c.) was administered alone or 10 min before injection 50 mg/kg oleamide. Means±SEM; n=6; **P<0.01 vs. vehicle, ## P<0.01 vs. oleamide (repeated-measures ANOVA followed by Duncan’s multiple tests)
Antagonistic effect of WAY100635 on the suppression of the oleamide-induced increase in SWS by 8-OH-DPAT
WAY100635, a selective and silent 5-HT1A receptor antagonist, had no significant effect on any sleep parameter at 0.1 mg/kg s.c., nor did it have any significant effect on sleep parameters when given 20 min before oleamide or 8-OH-DPAT. It effectively reversed the suppression of the oleamide-induced increase in SWS by 8-OH-DPAT, but had no effect on REMS (data not shown) (Fig. 5).
Antagonistic effect of WAY100635 on the suppression of the oleamide-induced increase of SWS in rats by 8-OH-DPAT. Animals were prepared and monitored as in Fig. 1. WAY100635 (0.1 mg/kg, s.c.) was administered alone or 10 min before injection of oleamide (50 mg/kg, i.p.), 20 min before injection of 8-OH-DPAT (0.1 mg/kg, s.c.) or 20 min before 8-OH-DPAT then followed by injection of oleamide 10 min later. 8-OH-DPAT was administered alone or 10 min before oleamide. Means±SEM; n=6; **P<0.01 vs. vehicle, ## P<0.01 vs. oleamide (repeated-measures ANOVA followed by Duncan’s multiple tests)
Discussion
The present study confirms that oleamide dose-dependently increases SWS in freely moving rats. The major finding is that the 5-HT uptake inhibitor fluoxetine or the 5-HT uptake inhibitor and releaser fenfluramine prevented the increase of SWS induced by oleamide. In addition, the 5-HT1A receptor agonists buspirone or 8-OH-DPAT suppressed the oleamide-induced increase in SWS significantly, this effect being reversed by WAY100635, an antagonist at 5-HT1A receptors.
Both fluoxetine and fenfluramine decreased SWS and REMS, a finding consistent with other reports (Fornal and Radulovacki 1983a, 1983b; Nicholson and Pascoe 1988; Gao et al. 1992). However, neither 10 mg/kg fluoxetine nor 1 mg/kg fenfluramine affected SWS when given alone, but both antagonized the oleamide-induced increase in SWS significantly. Fluoxetine and fenfluramine may induce the increase of 5-HT levels at the synaptic cleft (Pastel and Fernstrom 1987; Hyttel 1994). This increase might finally stimulate serotonergic neurotransmission, which in turn affects the SWS increase induced by oleamide.
However, the duration of SWS in the rats treated with oleamide and fluoxetine or fenfluramine was still longer than in the respective comparison groups treated with the reuptake inhibitor only. This remaining sleep effect may be related to the ability of oleamide to modulate 5-HT receptors as reported (Huidobro-Toro and Harris 1996; Thomas et al. 1997; Boger et al. 1998b).
Identification of multiple serotonergic receptor subtypes and their functions on sleep-wakefulness cycle have been reported in the literature. Administration of buspirone (0.01–4.0 mg/kg) or 8-OH-DPAT (0.375 mg/kg) in rats increases of W and reduces sleep (Lerman et al. 1986; Monti and Jantos 1992; Monti et al. 1995), whereby the effect of 8-OH-DPAT is reversed by (−)pindolol (Monti and Jantos 1994). All these data suggest that activation of 5-HT1A receptors tends to inhibit sleep behaviour. Under our experimental conditions, 8-OH-DPAT (0.1 and 0.5 mg/kg) did not change sleep parameters significantly. Drug effects can be influenced by many factors such as the duration of the period over which sleep was recorded, the animal species and potential SWS ceiling effects (Mistlberger et al. 1983). This discrepancy may be due to the sleep recording time difference (4 h vs. 8 h) and rat strain (Sprague-Dawley vs. Wistar) (Monti and Jantos 1992). Interestingly, co-administration of buspirone or 8-OH-DPAT with oleamide significantly inhibited the SWS increase induced by oleamide. 8-OH-DPAT reportedly inhibits the firing activity of 5-HT neurons, leading to decreased 5-HT neurotransmission (De Montigny and Blier 1984). 8-OH-DPAT thus decreases sleep time since serotonin may have a facilitating role in sleep (Cape and Jones 1998; Sakai and Crochet 2001). Whilst perfusion of oleamide onto oocytes injected with whole brain mouse mRNA does not elicit any membrane currents, oleamide substantially and reversibly potentiates currents elicited by 5-HT (Huidobro-Toro and Harris 1996). Thus the reduction in the duration of SWS after co-administration of oleamide and 8-OH-DPAT may be due to the reduction in the levels of 5-HT and serotonergic neurotransmission.
This result seems to be inconsistent with the observations with 5-HT reuptake inhibitors described above. Although serotonin has been known for many years to play a role in the modulation of sleep, just how and where serotonin exerts this effect is in fact still unknown (Portas et al. 2000). In the present study, the apparent inconsistency is observed between inhibitory role and facilitatory role played by serotonin on oleamide-induced sleep. At least two possible explanations could be suggested. One explanation is that serotonergic modulation might take place through a multitude of post-synaptic receptors that mediate different or even opposite responses. Oleamide reportedly potentiates 5-HT1A and 5-HT2 receptors or modulates 5-HT7 receptor functions (Huidobro-Toro and Harris 1996; Boger et al. 1998b; Thomas et al. 1998) under different conditions. The other possible explanation is that the achievement of a behavioural state induced by oleamide may depend on the complex interaction between the serotonergic and many other neurotransmitters systems. There is substantial evidence that the GABAergic system and cannabinoid-1 (CB1) receptors are involved in the sleep-inducing action of oleamide (Laposky et al. 2001; Coyne et al. 2002; Cravatt et al. 1996). Oleamide is thought to potentiate GABAA receptor function and acts synergistically with sub-threshold doses of triazolam, a benzodiazepine derivative, to reduce sleep latency (Lees et al. 1998; Verdon et al. 2000; Mendelson and Basile 2000). SR141716A, an antagonist at the CB1 receptor, inhibits oleamide-induced sleep and analgesic effects (Pastel and Fernstrom 1987; Mendelson and Basile 1999). Thus, the current data also suggest that appropriate 5-HT neurotransmission is important for oleamide-induced increase in SWS since any disruption of this system may induce its modification.
Although WAY100635 reportedly has no effect on oleamide-induced locomotor, analgesic, hypothermic and anxiolytic actions (Fedorova et al. 2001), it significantly reversed the suppression of oleamide-induced SWS increase by pretreatment with 8-OH-DPAT in the present study. This result suggests that activation of 5-HT1A receptors may mediate the inhibition of oleamide-induced increase in SWS in part. Potentiation of the 5-HT1A receptor response is observed following application of 100 nM oleamide in vitro (Boger et al. 1998b). The oleamide concentration in the CSF of normal rats is ~170 nM, but rises to ~430 nM in rats deprived of sleep for 6 h or more (Basile et al. 1999). Thus the concentrations of oleamide in the CNS are adequate to affect the different serotonin receptors including the 5-HT1A receptor. However, it seems unlikely that oleamide increases SWS by direct potentiation of 5-HT1A receptors, since WAY 100635 neither affected SWS per se nor potentiated oleamide-induced changes of SWS.
In the present study, the normal rats without any treatment showed less SWS and REMS time than normal. The sleep/wakefulness cycle in rats is sensitive to ambient temperature. The higher the temperature, the longer the duration of SWS or REMS (Thomas and Kumar 2000; Obal et al. 1983). The ambient temperature was about 18~20°C in our study, this may be the important reason. Nevertheless, since all groups in our experiments were exposed to the same conditions the present results can be considered credible and compatible.
In conclusion, the increase of 5-HT neurotransmission by fluoxetine or fenfluramine, or activation of 5-HT1A receptors by buspirone or 8-OH-DPAT suppressed the oleamide-induced increase in SWS. These data provide the first behavioural evidence that the serotonergic system may be involved in the sleep-inducing action of oleamide in rats.
References
Basile AS, Hanus L, Mendelson WB (1999) Characterization of the hypnotic properties of oleamide. Neuroreport 10:947–951
Boger DL, Henriksen SJ, Cravatt BF (1998a) Oleamide: an endogenous sleep-inducing lipid and prototypical member of a new class of biological signaling molecules. Curr Pharm Des 4: 303–314
Boger DL, Patterson JE, Jin Q (1998b) Structural requirements for 5-HT2A and 5-HT1A serotonin receptor potentiation by the biological active lipid oleamide. Proc Natl Acad Sci USA 95:4102–4107
Cape EG, Jones BE (1998) Differential modulation of high frequency gamma electroencephalogram activity and sleep-wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J Neurosci 18:2653–2666
Cheer JF, Cadogan AK, Marsden CA, Fone KC, Kendall DA(1999) Modification of 5-HT2 receptor mediated behaviour in the rat by oleamide and the role of cannabinoid receptor. Neurophamarcology 38:533–541
Coyne L, Lees G, Nicholson RA, Zheng J, Neufield KD (2002) The sleep hormone oleamide modulates inhibitory ionotropic receptors in mammalian CNS in vitro. Br J Pharmacol 135:1977–1987
Cravatt BF, Prospero-Garcia O, Siuzdak G, Gilula NB, Henriksen SJ, Boger DL, Lerner RA (1995) Chemical characterization of a family of brain lipids that induce sleep. Science 268:1506–1509
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87
De Montigny C, Blier P (1984) Effects of antidepressant treatments on 5-HT neurotransmission: electrophysiological and clinical studies. Adv Biochem Psychopharmacol 39:223–239
Fedorova I, Hashimoto A, Fecik RA, Hedrick MP, Hanus LO, Boger DL, Rice KC, Basile AS (2001) Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems. J Pharmacol Exp Ther 299:332–342
Fornal C, Radulovacki M (1983a) Sleep suppressant action of fenfluramine in rats: Relation to postsynaptic serotonergic stimulation. J Pharmacol Exp Ther 225:667–674
Fornal C, Radulovacki M (1983b) Sleep suppressant action of fenfluramine in rats: evidence against the involvement of presynaptic serotonergic mechanism. J Pharmacol Exp Ther 225:675–681
Gao B, Duncan WC Jr, Wehr TA (1992) Fluoxetine decreases brain temperature and REM sleep in Syrian hamsters. Psychopharmacology 106:321–329
Hedlund PB, Carson MJ, Sutcliffe JG, Thomas EAl (1999) Allosteric regulation by oleamide of the binding properties of 5-hydroxytryptamine7 receptors. Biochem Pharmacol 58: 1807–1813
Huidobro-Toro JP, Harris RA (1996) Brain lipids that induce sleep are novel modulators of 5-HT receptors. Proc Natl Acad Sci USA 93:8078–8082
Huitron-Resendiz S, Gombart L, Cravatt BF, Henriksen SJ (2001) Effect of oleamide on sleep and its relationship to blood pressure, body temperature, and locomotor activity in rats. Exp Neurol 172:235–243
Hyttel J (1994) Pharmacological characterization of the selective serotonin reuptake inhibitors (SSRI’s). Int Clin Psychopharmacol 9:19–26
Laposky AD, Homanics GE, Basile A, Mendelson WB (2001) Deletion of the GABA(A) receptor beta 3 subunit eliminates the hypnotic actions of oleamide in mice. Neuroreport 12:4143–4147
Lees G, Edwards MD, Hassoni AA, Ganellin CR, Galanakis D (1998) Modulation of GABAA receptors and inhibitory synaptic currents by the endogenous CNS sleep regulator cis-9,10-octadecenoamide (cOA). Br J Pharmacol 124:873–882
Lerman JA, Kaitin KI, Dement WC, Peroutka SJ (1986) The effects of buspirone on sleep in the rat. Neurosci Lett 72:64–68
Mechoulam R, Fride E, Hanus L, Sheskin T, Bisogno T, Di Marzo V, Bayewitch M, Vogel Z (1997) Anandamide may mediate sleep induction. Nature 389:25–26
Mendelson WB, Basile AS (1999) The hypnotic actions of oleamide are blocked by a cannabinoid receptor antagonist. Neuroreport 10:3237–3239
Mendelson WB, Basile AS (2000) Synergistic interaction of the hypnotic effects of oleamide and triazolam (abstract). Sleep 23 (Suppl. 2):A162
Mistlberger RE, Bergamnn BM, Waldenar W, Rechtschaffen A (1983) Recovery sleep following sleep deprivation in intact and suprachiastmatic nuclei-lesioned rats. Sleep 6:217–233
Monti JM, Jantos H (1992) Dose-dependent effects of the 5-HT1A receptor agonist 8-OH-DPAT on sleep and wakefulness in the rat. J Sleep Res 1:169–175
Monti JM, Jantos H (1994) Stereoselective antagonism by the pindolol enantiomers of 8-OH-DPAT-induced changes of sleep and wakefulness Neuropharmacology 33:705–708
Monti JM, Jantos H, Silveira R, Reyes-Parada M, Scorza C (1995) Sleep and waking in 5,7-DHT-lesioned or (−)-pindolol-pretreated rats after administration of buspirone, ipsapirone, or gepirone. Pharmacol Biochem Behav 52:305–312
Nicholson AN, Pascoe PA (1988) Studies on the modulation of the sleep-wakefulness continuum in man by fluoxetine, a 5-HT uptake inhibitor. Neuropharmacology 27:597–602
Obal F Jr, Tobler I, Borbley AA (1983) Effect of ambient temperature on 24 h sleep-wake cycle in normal and caspsaician treated rats. Physiol Behav 30:425–430
Pastel RH, Fernstrom JD (1987) Short-term effects of fluoxetine and trifluoromethylphenylpiperazine on EEG in the rat. Brain Res 436:92–102
Portas CM, Bjorvatn B, Ursin R (2000) Serotonin and the sleep/wake cycle: special emphasis on microdialysis studies. Prog Neurobiol 60:13–35
Sakai K, Crochet S (2001) Differentiation of presumed serotonergic dorsal raphe neurons in relation to behavior and wake-sleep states. Neuroscience 104:1141–1155
Sorensen E, Gronli J, Bjorvatn B, Ursin R (2001) The selective 5-HT1A receptor antagonist p-MPPI antagonizes sleep-waking and behavioral effects of 8-OH-DPAT in rats. Behav Brain Res 121:181–187
Thomas EA, Carson MJ, Neal MJ, Sutcliffe JG (1997) Unique allosteric regulation of 5-HT receptor-mediated signal transduction by oleamide. Proc Natl Acad Sci USA 94: 14115–14119
Thomas EA, Carson MJ, Sutcliffe JG (1998) Oleamide-induced modulation of 5-hydroxytryptamine receptor-mediated signaling. Ann NY Acad Sci 861:183–189
Thomas TC, Kumar VM (2000) Effect of ambient temperature on sleep-wakefulness in normal and medial preoptic are lesioned rats. Sleep Res Online 3:141–145
Verdon B, Zheng J, Nicholson RA, Ganelli CR, Lees G (2000) Stereoselective modulatory actions of oleamide on GABAA receptors and voltage-gated Na+ channels in vitro: a putative endogenous ligand for depressant drug site in CNS. Br J Pharmacol 129:283–290
Yang JY, Wu CF, Song HR (1999) Studies on the sedative and hypnotic effects of oleamide in mice. Arzneim-Forsch/Drug Res 49:663–667
Acknowledgements
This project is partially supported by Liaoning Nature Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, JY., Wu, CF., Wang, F. et al. The serotonergic system may be involved in the sleep-inducing action of oleamide in rats. Naunyn-Schmiedeberg's Arch Pharmacol 368, 457–462 (2003). https://doi.org/10.1007/s00210-003-0843-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-003-0843-z