Abstract
Bisphenol A (BPA), one of the most frequently detected emerging pollutants in the environment, has been implicated in adverse effects in male and female reproduction at extremely low concentrations. This study aimed to investigate the effects and potential mechanism of BPA on mouse ovarian follicular development and female germline stem cells (FGSCs). Female CD-1 adult mice were administered gradient concentrations of BPA (12.5, 25, and 50 mg/kg/day) by intraperitoneal injection. We found that the number of atretic ovarian follicles was significantly increased at high BPA concentrations. Additionally, the numbers of primordial follicles, primary follicles, and corpus luteum (CL) were significantly reduced at high BPA concentrations. Interestingly, the number of FGSCs was remarkably reduced in BPA-treated ovaries. Furthermore, the increased apoptotic rate of FGSCs in vitro was triggered by BPA accompanied by increased BPA concentrations. To investigate the mechanism of BPA in ovarian follicular development, 193 differentially expressed proteins were identified in BPA-treated ovaries by the isobaric tags for relative and absolute quantification-coupled 2D liquid chromatography-mass spectrometry technique. A total of 106 proteins were downregulated and 85 proteins were upregulated. Among these proteins, the apoptosis-related protein SAFB-like transcriptional modulator (SLTM) was remarkably upregulated, and this result was consistent with western blotting. Taken together, our results suggest that an ovarian follicular development, especially, the development of primordial follicles, primary follicles, and the CL, is inhibited by high BPA concentrations, and the ovarian follicle atresia is initiated by BPA through upregulated expression of SLTM. Furthermore, BPA induces apoptosis of cultured FGSCs. The effect of BPA on ovarian follicular development and FGSCs, especially the effect on FGSCs, suggests a novel mechanism of how BPA causes female infertility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is recognized as a xenobiotic estrogenic compound that is widely used in the manufacture of polycarbonate plastics and epoxy resins, such as food and drink containers, dental sealants, and baby bottles. BPA can be released from polycarbonate and resins with exposure to light, aging, heating, and contact with either acidic or basic compounds, especially during the process of cleaning. Therefore, an increasing amount of research has been performed on the effects of BPA on human health. Various studies have shown that BPA is present in blood, saliva, human tissues, and in 95% of human urine samples (Calafat et al. 2005, 2008; Ouchi and Watanabe 2002).
As an endocrine-disrupting chemical, BPA exposure results in reproductive developmental disorders. In males, BPA exposure causes dysfunctional spermatogenesis, an increased number of apoptotic cells within the seminiferous tubules, variations in hormone and steroidogenic enzyme levels, reduced sperm counts and sperm motility, and damage to sperm DNA (Peretz et al. 2014). In females, maternal BPA exposure during gestation causes early onset of puberty, weight gain changes, early vaginal opening, ovarian morphological abnormalities (Honma et al. 2002), and primordial follicle loss in offspring (Manikkam et al. 2013). Neonatal exposure to BPA in castor oil at 500 µg/50 µL alters ovarian morphology, showing a large number of cysts (Fernandez et al. 2010). Administration of BPA to pregnant mice at 0.1–1000 µg/kg on days 9–16 of gestation results in a significant increase in ovarian cysts in BPA-treated groups (Newbold et al. 2009). Additionally, BPA induces aneuploidy in maturing oocytes (Hunt et al. 2003). Therefore, female reproduction, especially ovarian follicular development, is disrupted by BPA. Although studies have shown that BPA adversely affects ovarian follicular development, there is some controversy regarding the effects of different BPA concentrations. Therefore, this study investigated the effect of different BPA concentrations on ovarian follicular development, and proteomics was used to further examine the potential mechanism of BPA on the ovaries.
Successfully isolated female germline stem cells (FGSCs) offer important opportunities for understanding human oogenesis, delaying menopause, treating infertility, and providing a new strategy for preserving fertility (Ding et al. 2016; Li et al. 2017; Wu et al. 2017; Zhang et al. 2011; Zou et al. 2009). Proliferation, differentiation, and apoptosis of FGSCs are regulated by strict mechanisms in vivo. Dysfunction of FGSCs is caused by disorder of homeostasis of FGSCs. Whether BPA affects FGSCs is unclear, and whether proliferation, differentiation, and apoptosis of FGSCs are regulated by BPA is unknown. Therefore, this study examined the role of BPA in FGSCs.
Materials and methods
Ethical approval
All procedures with animals were approved by the Institutional Animal Care and Use Committee of Shanghai, and were performed in accordance with the National Research Council Guide for Care and Use of Laboratory Animals.
Mice and treatments
Six weeks old female CD-1 mice (20–25 g body weight) were purchased from Linchang Company (China, Shanghai), and allowed to acclimate to the facility for at least 5 days before use. Food and water were provided ad libitum. Temperature was maintained at 22 ± 1 °C. Mice were subjected to 12-h light–dark cycles. The mice were randomly assigned to four groups with three mice in each group as follows. (1) The control group comprised mice that were administered the diluent dimethylsulfoxide (DMSO, Sigma-Aldrich). (2) The 12.5 mg/kg-treated group comprised mice that were administered 12.5 mg/kg BPA (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). (3) The 25 mg/kg-treated group comprised mice that were administered 25 mg/kg BPA. (4) The 50 mg/kg-treated group comprised mice that were administered 50 mg/kg BPA. For providing an equivalent volume for each mouse each day, BPA was dissolved in DMSO with the concentrations of 10, 20, and 40 mg/ml, and BPA was consecutively administered by intraperitoneal (ip) injection for 10 days.
Ovarian follicle count
Mice were euthanized by carbon dioxide and killed by cervical dislocation. The ovaries were collected, followed by fixation in 4% paraformaldehyde, and the ovaries were embedded in paraffin as described previously (Yang et al. 2013). The paraffin-embedded ovaries were cut into serial sections and stained using hematoxylin and eosin (HE). The ovarian follicles were counted as described previously (Tilly 2003; Wang et al. 2012).
Immunofluorescence staining
The mice were injected with 200 µg 5-ethynyl-2′-deoxyuridine (EDU) (Invitrogen Life Sciences, Carlsbad, CA, USA) by ip on the second day after ten consecutive days of BPA. 2 days later, the ovaries were collected and fixed in 4% paraformaldehyde. The ovaries were embedded in paraffin according to previous studies (Yang et al. 2013) and then the paraffin-embedded ovaries were cut into 6-µm-thick section. Immunofluorescence staining was performed according to previous study with a small modification (Lee et al. 2016). Briefly, after removal of paraffin, sections were washed twice with 3% bovine serum albumin (BSA). The sections were then permeated by 0.5% Triton X-100 for 20 min at room temperature, washed twice with 3% BSA, and incubated with the Click-iT®Plus reaction cocktail for 30 min at room temperature. The sections were then washed once with 3% BSA and counterstained with Hoechst 33342 at room temperature. The sections were washed twice with PBS, incubated with 0.5% trypsin in PBS for 20 min at 37 °C, and washed twice with PBS for 10 min. Blocking with 10% goat serum in PBS was performed for 20 min at 37 °C and then incubation with an anti-DDX4 antibody in PBS (1:200, ab13840; Abcam, Cambridge, MA, USA) at 4 °C overnight. After incubation, sections were washed twice with PBS for 10 min and incubated with secondary antibodies in PBS for 30 min at 37 °C (1:200; Proteintech, Wuhan, China). Finally, sections were washed twice with PBS for 10 min and mounted on slides for fluorescence microscopy.
Culture of FGSCs in vitro
The mouse FGSC line was cultured in vitro according to our previous study (Wang et al. 2014; Xie et al. 2014; Zou et al. 2009). Briefly, the culture medium for FGSCs was minimum essential medium alpha (Invitrogen Life Sciences) supplemented with 10% fetal bovine serum (Life Technologies), including 30 mg/ml pyruvate (Amresco, VWR International, LLC, Lardner, PA, USA), 2 mM l-glutamine (Amresco), 50 mM β-mercaptoethanol (Sigma Chemical Co., St. Louis, MO, USA), 6 mg/ml penicillin (Amresco), 1 mM nonessential amino acids (Invitrogen Life Sciences), 20 ng/ml mouse epidermal growth factor (Pepro Tech), 10 ng/ml mouse basic fibroblast growth factor (PeproTech), 10 ng/ml mouse glial cell line-derived neurotrophic factor (Pepro Tech), and 10 ng/ml mouse leukemia inhibitory factor (Santa Cruz Biotechnology, CA, USA). The SIM-6-thiogunania-oualiain cell line (ATCC, Manassas, VA, USA) was used as the feeder for FGSC culturing.
Cell proliferation assay
FGSCs were seeded into 96-well plates and incubated with different concentrations of 0, 50, 100, and 150 µM BPA for 24, 48 and 72 h. After treatments, CCK8 solution (10 µl) (Genomeditech, Shanghai, China) was added to each well. FGSCs were then cultured at 37 °C for 1 h. Absorption values were measured at a wave length of 450 nm with a Bio-Tek microplate reader (Bio-Tek Instruments, Thermo Fisher Scientific, Winooski, VT, USA).
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling assay
The apoptotic rates of ovarian sections and cultured FGSCs were detected by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL), and the procedure was performed using an Apoptosis Detection Kit (Vazyme Biotech Co., Ltd., Nanjing, China) according to the manufacturer’s instructions. After TUNEL reaction mixture for 1 h, the sections and FGSCs were washed twice with PBS for 10 min and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) for 5 min. Washing was then performed three times with ddH2O. A confocal laser scanning microscope (Leica, TSP8) was used to photograph sections and cells, and the apoptotic rate was analyzed using IPP 6.0 Software.
Flow cytometry
FGSCs were cultured in vitro and treated with 150 µM BPA for 48 h. Cells were collected and annexin V/propidium iodide (PI) (Thermo Fisher Scientific) staining was performed to detect the apoptotic rate according to the manufacturer’s instructions. Briefly, cells were washed once with PBS and 1 × Binging Buffer, and incubated with fluorochrome-conjugated annexin V for 15 min at room temperature. Cells were then washed with 1 × Binging Buffer and resuspended. Finally, cells were incubated with PI staining solution for 15 min and analyzed by flow cytometry (BD FACSCalibur machine, BD Biosciences).
Western blotting
The ovaries were lysed with protein lysis buffer, and protein concentrations were measured using the bicinchoninic acid (BCA) assay. The procedure of western blotting was performed according to a previous study with a small modification (Yang et al. 2015). Briefly, a total of 20 µg protein was separated in 10% w/v sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a PVDF membrane. Subsequently, the membranes were blocked with 5% nonfat milk in TBST buffer (1M Tris–HCl, 0.15 M NaCl, and 0.05% Tween-20) at room temperature for 1 h. The proteins were incubated with primary antibodies (1:400; LifeSpan BioScience, Inc., USA) in 5% nonfat milk in TBST buffer at room temperature for 2 h and washed three times with TBST for 10 min. This was followed by incubation with secondary antibody (1:2000; Proteintech) in 5% nonfat milk in TBST buffer at room temperature for 2 h, and washing three times with TBST for 10 min. Finally, the images were scanned with a chemiluminescence imaging system (ProteinSimple, Santa Clara, CA, USA). The densitometry of the bands was analyzed by Quantity One software. The gray value ratio of target protein represented the relative expression levels.
Proteomics
The total protein of control and BPA-treated ovaries was extracted with protein lysis buffer, and the concentration of protein was quantified by the BCA assay. To determine whole proteome expression, isobaric tags for relative and absolute quantification (iTRAQ)-coupled 2D liquid chromatography-mass spectrometry (LC–MS/MS) were performed at Majorbio (Shanghai, China) according to a standard procedure (Xu et al. 2014; Zhang et al. 2017; Zheng et al. 2017).
Statistical analyses
All experiments were replicated at least three times for each group. The data are presented as the mean ± SEM. Data were analyzed with ANOVA followed by the Fisher’s least significant difference test with SPSS software (Version 13.0; SPSS, Inc., Chicago, IL, USA). Differences were considered significant at p < 0.05.
Results
Morphological observation of BPA-treated ovaries
Ovarian morphology of the control, 12.5, 25, and 50 mg/kg BPA-treated groups was examined by HE staining (Fig. 1a). The numbers of primordial follicles, primary follicles, antral follicles, corpus luteum, and atretic follicles were counted (Fig. 1b–f). We found that the number of antral follicles in BPA-treated groups was lower than that in the control group, but this was not significantly different. The numbers of primordial follicles, primary follicles, and corpus luteum were significantly lower in the 25 and 50 kg/kg BPA-treated groups compared with the control group (p < 0.05). However, the number of atretic follicles was significantly higher in the 50 kg/kg BPA-treated group compared with the control group (p < 0.05).
Ovarian phenotype of BPA-treated mouse ovaries. a Ovarian histology of BPA-treated ovaries with various BPA concentrations. Control group; 12.5 mg/kg BPA-treated mouse ovaries; 25 mg/kg BPA-treated mouse ovaries; 50 mg/kg BPA-treated mouse ovaries. b–f The number of ovarian follicles and corpus luteum in different groups. The number of antral follicles in the BPA-treated groups was lower than that in the control group, but this was not significant. The numbers of primordial follicles, primary follicles and corpus luteum were significantly lower in the 25 and 50 mg/kg BPA-treated groups compared with the control group (p < 0.05). However, the number of atretic follicles was significantly higher in the 50 mg/kg BPA-treated group compared with control group (p < 0.05). CL corpus luteum; AF antral follicle; AtrF: atretic follicle. Bar 50 µm. b Number of primordial follicles; c number of primary follicles; d number of antral follicles; e number of atretic follicles; f number of corpus luteum. *p < 0.05, **p < 0.01, ***p < 0.001
BPA exposure increases apoptosis of ovarian follicles
After TUNEL assay, we found that the apoptotic signal was positive in granulosa cells and oocytes (Fig. 2a). The number of apoptotic cells per section in the 50 mg/kg BPA-treated group was significantly higher than that in the control group (Fig. 2b, p < 0.01).
TUNEL apoptosis assay in 50 mg/kg BPA-exposed mouse ovaries. a Apoptosis was detected by TUNEL. The apoptotic signal was positive in granulosa cells and oocytes. Bar: 50 µm. b The number of apoptotic cell per section in controls and 50 mg/kg BPA-treated mouse ovaries. The number of apoptotic cells per section in the 50 mg/kg BPA-treated group was significantly higher than that in the control group (p < 0.01). *p < 0.05, **p < 0.01, ***p < 0.001
FGSCs are decreased in BPA-treated ovaries
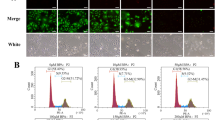
FGSCs were identified by dual immunostaining for DDX4 and EDU labeling (Zhang and Wu 2016; Zhou et al. 2014). FGSCs were located in the cortical surface of ovaries immediately below the epithelium (Fig. 3a). Furthermore, the number of FGSCs was significantly lower in the BPA groups compared with the control group (p < 0.05, Fig. 3b).
Effect of BPA on FGSCs in the ovaries. a Identification of FGSCs by dual immunofluorescence of DDX4 (green) and EDU (red). DAPI (blue) staining was also performed on the same sample. Control group: no BPA; BPA group: 50 mg/kg BPA-treated mouse ovaries. Arrows indicate FGSCs. Bar 50 µm. b Number of FGSCs in the two groups. The number of FGSCs was significantly lower in the BPA groups compared with the control group (p < 0.05). *p < 0.05, **p < 0.01, ***p < 0.001
BPA induces apoptosis of FGSCs in vitro
The effect of 50, 100, and 150 µM BPA on viability of FGSCs was determined with the CCK8 kit. FGSC viability was detected at 24, 48, and 72 h. The 50 and 100 µM BPA-treated groups showed no significant difference in the number of FGSCs compared with the control group. However, the number of FGSCs was significantly lower in the 150 µM BPA-treated group at 48 and 72 h (p < 0.05) (Fig. 4a). Subsequently, apoptosis of FGSCs was detected by TUNEL at 50, 100, and 150 µM BPA at 48 h (Fig. 4b). We found that the apoptotic rate in the 150 µM BPA-treated group was significantly higher than that in the other groups (p < 0.05), and that in the 100 µM BPA-treated group was significantly higher than that in the control and 50 µM BPA-treated groups (p < 0.05). Furthermore, flow cytometry showed that the apoptotic rate in the 150 µM BPA-treated group was significantly higher than that in the control group (p < 0.01, Fig. 4c).
Effect of BPA on FGSCs in vitro. a Cellular viability of FGSCs was detected by CCK8 staining. The 50 and 100 µM BPA-treated groups showed no significant difference in the number of FGSCs compared with the control group. The number of FGSCs was significantly lower in the 150 µM BPA-treated group at 48 and 72 h (p < 0.05) than in the control group. b Apoptosis was detected by TUNEL. The apoptotic rate in the 150 µM BPA-treated group was significantly higher than that in the other groups (p < 0.001). The apoptotic rate in the 100 µM BPA-treated group was significantly higher than that in the control and 50 µM BPA-treated groups (p < 0.05). Bar: 50 µm. c The apoptotic rate of 150 µM BPA-treated FGSCs. The apoptotic rate in 150 µM BPA-treated FGSCs was significantly higher than that in the control group (p < 0.01). *p < 0.05, **p < 0.01, ***p < 0.001
Proteomics analysis of BPA-treated ovaries
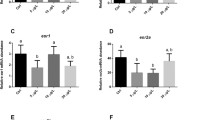
To further identify the related pathways and direct target proteins in BPA-treated ovaries, iTRAQ-coupled 2D LC–MS/MS was used to analyze differentially expressed proteins (DEPs). Hierarchical clustering of DEPs showed that DEP expression patterns were different between the control group and BPA-treated groups (Fig. 5a). DEPs were screened in a volcano plot. We found 193 DEPs, and 85 were upregulated and 106 were downregulated (Fig. 5b). Gene ontology (GO) results suggested that 23 subgroups of DEPs were involved in the biological process, 17 subgroups were involved in the cellular component, and 16 were involved in molecular function (Fig. 6). Subgroups of gene ontology were further analyzed by pie chart and were classified into three types based their participation in biological process (BP), cell components (CC) and molecular function (MF) with level 2 (Supplementary data, Fig. S1), level 3 (Supplementary data, Fig. S2), and level 4 (Supplementary data, Fig. S3). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis showed that 193 DEPs were distributed in nine pathways (Supplementary data, Fig. S4). The KEGG pathway involved in the metabolism process was analyzed by interactive Pathways Explorer (Supplementary data, Fig. S5). Information on two pathways of bisphenol degradation and retinol metabolism is shown in Supplementary data, Fig. S6 and Fig. S7. The relationship of the 193 DEPs was analyzed (Fig. 7a). One apoptosis-associated protein, scaffold attachment factor-like transcription modulator (SLTM) was identified (Fig. 7b). This protein might be closely related to BPA-induced ovarian follicle atresia.
Results of hierarchical clustering and a volcano plot of BPA-treated ovaries. a Hierarchical clustering shows that DEP expression patterns are different between the control group and BPA-treated groups. b DEPs were screened in a volcano plot. This plot shows that 193 DEPS were identified, and 85 were upregulated and 106 were downregulated
BPA upregulates the apoptotic protein SLTM
To further evaluate whether SLTM protein was regulated by BPA-treated ovaries, SLTM protein in BPA-treated ovaries was detected by western blot (Fig. 8a). SLTM protein expression in BPA-treated ovaries was higher than that in control ovaries (p < 0.05, Fig. 8b).
Discussion
As a widespread environmental contaminant, BPA has been investigated for many years regarding its toxicity in the female and male reproductive systems. Previous studies have reported that BPA can function as an endocrine disruptor in vivo and in vitro, and can interact with estrogen receptor, androgen receptor, and thyroid hormone receptor (Kitamura et al. 2005). Xenoestrogens play a hormone-like role by the two mechanisms of genomic and non-genomic pathways (Heldring et al. 2007; Nadal et al. 2000). An effect of BPA on the ovaries has been reported by several studies, but the results are controversial, and this may be attributed to the dosage of BPA. The mechanism of BPA on the ovaries is not fully understood.
Our study showed that the numbers of primordial follicles, primary follicles, and corpus luteum were significantly decreased in a dose-dependent manner, and atretic follicles were increased. Furthermore, our TUNEL results further suggested that BPA accelerated apoptosis of ovaries and inhibited ovarian follicular development, which is consistent with previous studies (Peretz et al. 2011). Therefore, the development of the ovary was inhibited by BPA exposure during the pre-pubertal period. Follicle development-related genes may play certain roles in this process (Li et al. 2014). In a previous study, female newborn rats were exposed to 50 mg/kg BPA, and the number of corpus luteum was reduced, but the number of multioocyte follicles was increased (Adewale et al. 2009). Exposing neonatal mice to BPA results in an increased rate of ovarian hemorrhagic cysts, abnormal numbers of antral follicles, and decreased corpus luteum (Newbold et al. 2007; Suzuki et al. 2002). Therefore, ovarian follicular development and atresia are affected by BPA exposure and this is dependent on the dosage of BPA.
Apoptosis of male germ cells and somatic cells is induced by BPA (Qi et al. 2014; Urriola-Munoz et al. 2014; Xie et al. 2016). Additionally, apoptosis of female germ cells and ovarian somatic cells is initiated by BPA, and BPA induces apoptosis of oocytes (Wang et al. 2016). BPA exposure during in vitro oocyte maturation results in dose-dependent alterations in embryonic developmental rates, the apoptotic rate, sex ratio, and gene expression (Ferris et al. 2016). BPA also induces apoptosis of human luteinized membrana granulosa cells (Mansur et al. 2017) and mouse granulosa cells (Xu et al. 2002). However, the effect of BPA on stem cells is largely unknown, especially the effect of BPA on FGSCs. Additionally, whether apoptosis of FGSCs is triggered by BPA is unknown. Surprisingly, the number of FGSCs in BPA-treated ovaries in our study was remarkably reduced compared with normal mice. FGSCs were also cultured in vitro and treated with different BPA concentrations. We found that proliferation of FGSCs was remarkably decreased accompanied by increased BPA concentrations and treatment time. Consistent with the results of proliferation, apoptosis of FGSCs was gradually increased accompanied by an increase in BPA concentrations. These results were confirmed by flow cytometry analysis.
To further examine the mechanism of BPA on the ovaries, we performed ovarian proteomic analysis. A total of 191 DEPs were identified, and 85 proteins were upregulated and 106 proteins were downregulated. All DEPs were analyzed, and one apoptosis-related protein, SLTM, was identified. Protein expression of SLTM was significantly increased by BPA intervention. Additionally, overexpression of SLTM results in generalized inhibition of transcription and induces apoptosis in cultured cells (Chan et al. 2007). Therefore, BPA-induced ovarian dysfunction might be attributed to upregulation of the apoptosis-associated protein SLTM. The effect of BPA on ovarian follicular development and FGSCs suggests a novel mechanism of BPA-induced female infertility. Additionally, our results could be useful for new methods of preventing and curing of pollutants, especially BPA-induced female infertility.
Abbreviations
- BPA:
-
Bisphenol A
- FGSCs:
-
Female germline stem cells
- CL:
-
Corpus luteum
- DMSO:
-
Dimethylsulfoxide
- EDU:
-
5-Ethynyl-2′-deoxyuridine
- BSA:
-
Bovine serum albumin
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- PI:
-
Propidium iodide
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- LC–MS/MS:
-
Liquid chromatography–mass spectrometry
- DEPs:
-
Differentially expressed proteins
- GO:
-
Gene ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
References
Adewale HB, Jefferson WN, Newbold RR, Patisaul HB (2009) Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod 81(4):690–699 https://doi.org/10.1095/biolreprod.109.078261
Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL (2005) Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 113(4):391–395
Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL (2008) Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 116(1):39–44 https://doi.org/10.1289/ehp.10753
Chan CW, Lee YB, Uney J, Flynn A, Tobias JH, Norman M (2007) A novel member of the SAF (scaffold attachment factor)-box protein family inhibits gene expression and induces apoptosis. Biochem J 407(3):355–362. https://doi.org/10.1042/BJ20070170
Ding X, Liu G, Xu B, Wu C, Hui N, Ni X, Wang J, Du M, Teng X, Wu J (2016) Human GV oocytes generated by mitotically active germ cells obtained from follicular aspirates. Sci Rep 6:28218. https://doi.org/10.1038/srep28218
Fernandez M, Bourguignon N, Lux-Lantos V, Libertun C (2010) Neonatal exposure to bisphenol A and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect 118(9):1217–1222. https://doi.org/10.1289/ehp.0901257
Ferris J, Mahboubi K, MacLusky N, King WA, Favetta LA (2016) BPA exposure during in vitro oocyte maturation results in dose-dependent alterations to embryo development rates, apoptosis rate, sex ratio and gene expression. Reprod Toxicol 59:128–138 https://doi.org/10.1016/j.reprotox.2015.12.002
Heldring N, Pike A, Andersson S et al (2007) Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87(3):905–931. https://doi.org/10.1152/physrev.00026.2006
Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T (2002) Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol 16(2):117–122
Hunt PA, Koehler KE, Susiarjo M et al (2003) Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr Biol 13(7):546–553
Kitamura S, Suzuki T, Sanoh S et al. (2005) comparative study of the endocrine-disrupting activity of Bisphenol A and 19 related compounds. Toxicol Sci 84(2):249–259 https://doi.org/10.1093/toxsci/kfi074
Lee Y, Kim KH, Yoon H et al. (2016) RASD1 knockdown results in failure of oocyte maturation. Cell Physiol Biochem 40(6):1289–1302. https://doi.org/10.1159/000453182
Li Y, Zhang W, Liu J et al. (2014) Prepubertal bisphenol A exposure interferes with ovarian follicle development and its relevant gene expression. Reprod Toxicol 44:33–40 https://doi.org/10.1016/j.reprotox.2013.09.002
Li X, Ao J, Wu J (2017) Systematic identification and comparison of expressed profiles of lncRNAs and circRNAs with associated co-expression and ceRNA networks in mouse germline stem cells. Oncotarget 8(16):26573–26590. https://doi.org/10.18632/oncotarget.15719
Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK (2013) Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PloS One 8(1):e55387 https://doi.org/10.1371/journal. pone.0055387
Mansur A, Israel A, Combelles CM et al (2017) Bisphenol-A exposure and gene expression in human luteinized membrana granulosa cells in vitro. Human Reprod (Oxf Engl) 32(2):409–417. https://doi.org/10.1093/humrep/dew316
Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B (2000) Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci USA 97(21):11603–11608 https://doi.org/10.1073/pnas.97.21.11603
Newbold RR, Jefferson WN, Padilla-Banks E (2007) Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol 24(2):253–258. https://doi.org/10.1016/j.reprotox.2007.07.006
Newbold RR, Jefferson WN, Padilla-Banks E (2009) Prenatal exposure to bisphenol A at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect 117(6):879–885. https://doi.org/10.1289/ehp.0800045
Ouchi K, Watanabe S (2002) Measurement of bisphenol A in human urine using liquid chromatography with multi-channel coulometric electrochemical detection. J Chromatogr B Anal Technol Biomed Life Sci 780(2):365–370
Peretz J, Gupta RK, Singh J, Hernandez-Ochoa I, Flaws JA (2011) Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci 119(1):209–217. https://doi.org/10.1093/toxsci/kfq319
Peretz J, Vrooman L, Ricke WA et al (2014) Bisphenol A and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect 122(8):775–786. https://doi.org/10.1289/ehp.1307728
Qi S, Fu W, Wang C et al. (2014) BPA-induced apoptosis of rat Sertoli cells through Fas/FasL and JNKs/p38 MAPK pathways. Reprod Toxicol 50:108–116. https://doi.org/10.1016/j.reprotox.2014.10.013
Suzuki A, Sugihara A, Uchida K et al (2002) Developmental effects of perinatal exposure to bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reprod Toxicol 16(2):107–116
Tilly JL (2003) Ovarian follicle counts–not as simple as 1, 2, 3. Reproductive biology and endocrinology: RB&E 1:11
Urriola-Munoz P, Lagos-Cabre R, Moreno RD (2014) A mechanism of male germ cell apoptosis induced by bisphenol-A and nonylphenol involving ADAM17 and p38 MAPK activation. PloS One 9(12):e113793. https://doi.org/10.1371/journal.pone.0113793
Wang Y, Chang Q, Sun J et al (2012) Effects of HMG on revascularization and follicular survival in heterotopic autotransplants of mouse ovarian tissue. Reprod Biomed 24(6):646–653. https://doi.org/10.1016/j.rbmo.2012.02.025
Wang H, Jiang M, Bi H et al (2014) Conversion of female germline stem cells from neonatal and prepubertal mice into pluripotent stem cells. J Mol Cell Biol 6(2):164–171. https://doi.org/10.1093/jmcb/mju004
Wang T, Han J, Duan X et al (2016) The toxic effects and possible mechanisms of Bisphenol A on oocyte maturation of porcine in vitro. Oncotarget 7(22):32554–32565. https://doi.org/10.18632/oncotarget.8689
Wu C, Xu B, Li X et al. (2017) Tracing and characterizing the development of transplanted female germline stem cells in Vivo. Mol Therapy 25(6):1408–1419. https://doi.org/10.1016/j.ymthe.2017.04.019
Xie W, Wang H, Wu J (2014) Similar morphological and molecular signatures shared by female and male germline stem cells. Sci Rep 4:5580. https://doi.org/10.1038/srep05580
Xie M, Bu P, Li F et al (2016) Neonatal bisphenol A exposure induces meiotic arrest and apoptosis of spermatogenic cells. Oncotarget 7(9):10606–10615. https://doi.org/10.18632/oncotarget.7218
Xu J, Osuga Y, Yano T et al (2002) Bisphenol A induces apoptosis and G2-to-M arrest of ovarian granulosa cells. Biochem Biophys Res Commun 292(2):456–462. https://doi.org/10.1006/bbrc.2002.6644
Xu DD, Deng DF, Li X et al (2014) Discovery and identification of serum potential biomarkers for pulmonary tuberculosis using iTRAQ-coupled two-dimensional LC–MS/MS. Proteomics 14(2–3):322–331. https://doi.org/10.1002/pmic.201300383
Yang Y, Lin P, Chen F et al. (2013) Luman recruiting factor regulates endoplasmic reticulum stress in mouse ovarian granulosa cell apoptosis. Theriogenology 79(4):633–639.e1–3 https://doi.org/10.1016/j.theriogenology.2012.11.017
Yang Y, Chen J, Wu H et al (2015) The Increased Expression of Connexin and VEGF in Mouse Ovarian Tissue Vitrification by Follicle Stimulating Hormone. BioMed Res Int 2015:397264. https://doi.org/10.1155/2015/397264
Zhang C, Wu J (2016) Production of offspring from a germline stem cell line derived from prepubertal ovaries of germline reporter mice. Mol Human Reprod 22(7):457–464 https://doi.org/10.1093/molehr/gaw030
Zhang Y, Yang Z, Yang Y et al (2011) Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol 3(2):132–141. https://doi.org/10.1093/jmcb/mjq043
Zhang Y, Pan J, Huang X et al (2017) Differential effects of a post-anthesis heat stress on wheat (Triticum aestivum L.) grain proteome determined by iTRAQ. Sci Rep 7(1):3468. https://doi.org/10.1038/s41598-017-03860-0
Zheng X, Xie Z, Wang S, Lin P (2017) Determination of the protein expression profiles of Propsilocerus akamusi (Tokunaga) Malpighian tubules response to cadmium stress by iTRAQ coupled LC–MS/MS. J Proteomics 164:85–93. https://doi.org/10.1016/j.jprot.2017.05.017
Zhou L, Wang L, Kang JX et al. (2014) Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol Human Reprod 20(3):271–281. https://doi.org/10.1093/molehr/gat081
Zou K, Yuan Z, Yang Z et al (2009) Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol 11(5):631–636. https://doi.org/10.1038/ncb1869
Acknowledgements
This work was supported by National Natural Science Foundation of China (81720108017), National Basic Research Program of China (2017YFA0504201), and Shanghai Jiao Tong University Medicine-Engineering Fund (YG2017ZD11).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, X., Tian, G.G., Yu, B. et al. Effects of bisphenol A on ovarian follicular development and female germline stem cells. Arch Toxicol 92, 1581–1591 (2018). https://doi.org/10.1007/s00204-018-2167-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-018-2167-2