Abstract
The implementation of the Chemical Weapon Convention (CWC), prohibiting the development, production, storage and use of chemical weapons by 192 nations and the ban of highly toxic OP pesticides, especially class I pesticides according to the WHO classification, by many countries constitutes a great success of the international community. However, the increased interest of terrorist groups in toxic chemicals and chemical warfare agents presents new challenges to our societies. Almost seven decades of research on organophosphorus compound (OP) toxicology was mainly focused on a small number of OP nerve agents despite the fact that a huge number of OP analogues, many of these agents having comparable toxicity to classical nerve agents, were synthesized and published. Only limited physicochemical, toxicological and medical information on nerve agent analogues is available in the open literature. This implies potential gaps of our capabilities to detect, to decontaminate and to treat patients if nerve agent analogues are disseminated and may result in inadequate effectiveness of newly developed countermeasures. In summary, our societies may face new, up to now disregarded, threats by toxic OP which calls for increased awareness and appropriate preparedness of military and civilian CBRN defense, a broader approach for new physical and medical countermeasures and an integrated system of effective detection, decontamination, physical protection and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We are now looking back on more than 160 years of research on highly toxic organophosphorus compounds (OP), a large group of organic phosphorus esters with the basic structure, proposed by Gerhard Schrader in 1937 (Holmstedt 1963), as shown in Fig. 1. Important milestones were the synthesis of tetraethyl pyrophosphate (TEPP) in the mid of the nineteenth century and of a close tabun analogue, N,N′-diethyl tabun, at the beginning of the twentieth century (De Clermont 1854, 1855; Michaelis 1903). Interest in OP as toxic compounds increased immediately before and during World War II. The synthesis of alkyl phosphorofluoridates by Lange and von Krueger and the extensive work by Schrader and colleagues may be considered as the starting point for the development of important OP pesticides but, unfortunately, also of highly toxic OP compounds which were further developed as chemical warfare nerve agents (Lange and von Krueger 1932; Schrader 1951). Further research on OP pesticides led to the discovery of phosphorylated thiocholine derivatives and finally to the development of even more toxic V-agents (Ghosh and Newman 1955; Baldit 1958; Tammelin 1958). The history of OP pesticides and nerve agents was extensively reviewed by various authors and shall not be addressed in more detail (Holmstedt 1963; Kabachnik et al. 1970; Maynard and Beswick 1992; Black 2016).
The intense research on OP started immediately after the end of World War II, and numerous compounds were further developed for use as pesticides (Eto 1974). The lower environmental stability, compared to DDT, and high insecticidal toxicity were major factors for the success of OP pesticides, especially in developing countries (Casida and Durkin 2013). The beneficial effects of widespread use of OP pesticides, among other factors the increase in crop yields and the lowered incidence of vector-borne diseases, are accompanied by a rising insect resistance and a high number of accidental and intentional human poisonings (Eddleston et al. 2002).
The high incidence of suicidal OP pesticide poisoning with more than 250,000 fatal cases per year worldwide (Gunnell et al. 2007) initiated efforts to ban highly toxic OP pesticides. In consequence class I pesticides according to the WHO classification (International Programme on Chemical Safety 2010), e.g., parathion and mevinphos, are banned in most countries (Bertolote et al. 2006). In fact, recent studies provide evidence that this approach may lead to a decrease of OP pesticide-induced human poisonings (Chang et al. 2012; Knipe et al. 2014). However, these regulatory activities have only a limited effect on the ongoing challenge to implement effective strategies for the treatment of human OP pesticide poisoning (Eddleston et al. 2009, 2012). Moreover, the worldwide consumption of OP pesticides is still in the range of multiple kilotons per year (FAO Statistics Division 2013).
The rather accidental discovery of the highly toxic OP tabun by Gerhard Schrader in 1937 initiated extensive and long-lasting programs to identify even more toxic OP and to develop promising candidates as chemical warfare agents (Holmstedt 1963; Maynard and Beswick 1992). This led to large stockpiles of chemical warfare agents, e.g., sarin and VX in the USA, soman and Russian VX (VR) in the former Soviet Union (Szinicz 2005; Maxwell et al. 1997) and at a smaller scale in a number of other countries, notably Iraq and Syria (MacIlwain 1993; Pita and Domingo 2014).
It is noteworthy to mention that, although being fielded, the nerve agents tabun and sarin were not used by Germany during World War II, but repeated homicidal uses of tabun, sarin and VX occurred in later years. During the 1980s nerve agents were deployed against military forces during the Iran–Iraq war and against the civilian population by Iraqi forces (Black et al. 1994; Weimaster et al. 1995; Kadivar and Adams 1991). The deleterious terrorist attacks with sarin in Matsumoto 1994 and Tokyo 1995 as well as assassinations with VX in 1995 in Japan demonstrated the capability of non-state actors to produce and to disseminate nerve agents (Morita et al. 1995; Nozaki et al. 1995a, b). In 2013 attacks with sarin were conducted in Syria including the Ghouta incident in August 2013 with several thousand poisoned and up to 1500 killed humans (Pita and Domingo 2014).
The existence of large chemical warfare agent stockpiles and the fact that a huge number of exposed and intoxicated victims resulted from the repeated homicidal use of these agents convinced the international community to ban chemical warfare agents. The Chemical Weapon Convention (CWC), prohibiting the development, production, storage and use of chemical weapons, entered into force in April 1997 (United Nations Treaty Collection 1997). The CWC is administered by the Organisation for the Prohibition of Chemical Weapons (OPCW), which verifies the observance of the state parties to the convention. At present, more than 97 % of our world countries are members of this treaty which must be considered as an enormous success in the attempt to prevent future use of chemical warfare agents.
This very positive development decreases the likelihood of using chemical warfare agents on a large scale by states and by military forces. However, the use of nerve agents by a terrorist group in Japan and recent incidents in the Middle East give evidence for an increasing interest of non-state actors to get hold of chemical warfare agents and to use such toxic chemicals against the civilian population (Pita and Anadon 2015; European Parliamentary Research Service 2015; Hummel 2016; Hoette 2012). In fact, media reports indicate that the terrorist group Islamic State (ISIS) may have used chemical warfare nerve and blistering agents (Veterans Today 2015; CBC News 2015).
In consequence, we have to face the ongoing risk of accidental or intentional poisoning by OP pesticides and the potentially increasing threat of terrorist use of OP pesticides and nerve agents. This unpleasant situation calls for a closer look on toxicological and medical aspects of OP exposure in an environment with known and potentially new threats.
Toxicology of organophosphorus compounds
The toxicology of organophosphorus compounds was investigated extensively during the last decades by multiple research groups, and the mechanisms of acute toxicity are well defined (cf. Saunders 1957; Holmstedt 1963; O’Brien 1960; Eto 1974; Koelle 1992; Marrs 2007; Watson et al. 2015; Rice 2016). OP toxicology after subacute and chronic exposure as well as the long-term effects of OP poisoning is not within the scope of this paper, and the reader is referred to comprehensive reviews by experts in the field (cf. Krieger 2010; Scott 2007; Eyer 1995; Lotti and Moretto 2005; Abou-Donia 1981; Lohs 1975).
Basic aspects
The main mechanism of toxicologically relevant action of OP pesticides and nerve agents is the covalent binding to the active site serine OH-group at the base of a deep gorge of the pivotal enzyme acetylcholinesterase (AChE; EC 3.1.1.7; Fig. 2). Phosphylation of AChE, which denotes both phosphorylation and phosphonylation, leads to inhibition of its physiological action to hydrolyze the neurotransmitter acetylcholine (Aldridge and Reiner 1972).
Scheme of reactions between an OP and AChE. Incubation of a phosphonate with AChE (EOH) results in a Michaelis-type intermediate and finally in phosphylated AChE (phosphylation). The phosphyl-AChE complex may undergo two post-inhibitory reactions, the spontaneous cleavage of the phosphyl moiety (spontaneous reactivation) and of an alkyl residue leading to irreversible inhibition of AChE (dealkylation, “aging”)
Impaired hydrolysis of acetylcholine results in an overstimulation of muscarinic and nicotinic receptors at nerve–nerve and nerve–organ synapsis of the cholinergic system (Holmstedt 1959). This encompasses the central and vegetative nervous system and neuromuscular junctions. In consequence, OP poisoning may result in a broad spectrum of clinical signs (Fig. 3). The onset, sequence and severity of clinical signs is strongly dependent on the intrinsic toxicity and dose of the OP and the route of exposure (Sidell 2007; Okumura et al. 1996; Peter et al. 2014). Signs of poisoning may develop within minutes after inhalation exposure to OP vapor but may take hours after percutaneous contamination by OP vapor or liquid (Sidell 1974; Okumura et al. 1996; Hamilton et al. 2004; Prinz 1969; Thiermann et al. 2007). Typically, miosis is an early sign after vapor exposure, local sweating and fasciculations after percutaneous exposure and gastrointestinal symptoms after oral intake of an OP (Lee 2003; Rengstorff 1994; Mumford et al. 2008; Goel and Aggarwal 2007).
The phosphyl-AChE complex may undergo two post-inhibitory reactions, spontaneous reactivation and dealkylation (“aging”; Fig. 2). These reactions may have a major impact on the course of intoxication and the effectiveness of a specific class of therapeutic drugs, i.e., AChE reactivators (Jandorf et al. 1955). Aging proceeds extremely rapid with crotylsarin- (t½ < 15 s) and soman-inhibited human AChE (t½ ~ 1–2 min), slower with AChE inhibited by sarin and dimethoxy-OP pesticides (t½ ~ 3 h) and takes up to 40 h in case of AChE inhibition by diethoxy-OP pesticides and the nerve agent VX (Shafferman et al. 1996; Worek et al. 2004; Busker et al. 1991). Spontaneous reactivation, a process which can reverse the toxic OP effects, is negligible with G-type nerve agents (tabun, sarin, soman, cyclosarin), rapid with dimethoxy-OP pesticides (t½ < 1 h) but rather slow with diethoxy-OP pesticides and the nerve agent VX (t½ > 30 h) (Aurbek et al. 2006; Worek et al. 2004).
Besides AChE, being the main target of OP pesticides and nerve agents, other serine esterases, notably butyrylcholinesterase (BChE; EC 3.1.1.8) and carboxylesterase (CaE; EC 3.1.1.1), are inhibited by OP (Moralev and Rozengart 2007; Masson et al. 2009; Maxwell 1992). Inhibition of these enzymes by OP does not result in additional acute toxic effects, but BChE and CaE can serve as endogenous bioscavengers by binding and thus detoxifying a limited amount of incorporated OP (Maxwell et al. 1987; Lenz et al. 2007).
Structural aspects
The extensive and long-lasting work on OP by university and government research laboratories and private companies resulted in the identification of a huge number of compounds (Holmstedt 1963; O’Brien 1960; Eto 1974; Kabachnik et al. 1970; Moralev and Rozengart 2007; Timperley 2015). OP pesticides and nerve agents are based on the generic structure, proposed by Gerhard Schrader in 1937 (Holmstedt 1963; Fig. 1). Combination of different residues (R1 and R2) and leaving groups (X) enables synthesis of an unforeseeable number of derivatives. OP can be divided into different subclasses including phosphates, phosphonates, thiophosphates, phosphoramidates and phosphinates (Table 1; Ballantyne and Marrs 1992), having largely different physicochemical and toxicological properties.

Table 2 shows selected OP pesticides and refers them to the WHO hazard classes, ranging from extremely (class Ia) to slightly hazardous (class III; International Programme on Chemical Safety 2010). An important factor for the human toxicity of OP pesticides is the presence of a P = S versus P = O bond. Thiophosphates have to be metabolized to the respective oxon, e.g., parathion to paraoxon or malathion to malaoxon, to become effective AChE inhibitors (Casida 1956; Eto 1974). This cytochrome P450-mediated reaction underlies large inter-individual variations and may have an essential impact on the individual susceptibility toward OP pesticides bearing a P = S bond (Mutch and Williams 2006; Foxenberg et al. 2007).

The development of highly toxic OP as chemical warfare agents (nerve agents) was focused on a rather small number of agents (Marrs 2007). Stockpiled and in part used nerve agents are the phosphonates sarin, soman and cyclosarin, the phosphoramidate tabun (designated as G-agents) and the phosphonothioates VX and VR. However, numerous nerve agent analogues were under investigation and their structures and in part details on the synthesis are available in the public domain, as can be derived from assigned Chemical Abstract Service registry numbers. By modification of the O-alkyl group a large number of alkylmethylphosphonofluoridates, i.e., sarin analogues, can be generated. A selection of agents is presented in Table 3. Likewise, variations of the O-alkyl groups and amide residues allow the synthesis of a large number of tabun analogues, Table 4 gives a selection of structures. The fundamental work of Tammelin and Ghosh in the early 1950s gave insight into the structure and function of organophosphorylthiocholines (Tammelin 1958; Ghosh and Newman 1955) and led to the presentation of amiton as a highly potent OP pesticide and VX as one of the most toxic nerve agents (Baldit 1958; Sidell 1997). Various VX analogues, e.g., VM and VE, were investigated and selected as chemical warfare agents (Russian VX; VR). Again, modification of the residues R1–R4 allows the synthesis of a huge number of V-agents (Table 5).



The acute human toxicity of OP pesticides and nerve agents can be estimated from the in vitro inhibitory potency toward human and animal AChE, the in vivo toxicity in different animal species and from human studies (Krieger 2010; Maynard and Beswick 1992; Gaines 1969; Rider et al. 1969). In general, the evaluation of OP toxicity is based on data from animal studies. However, one has to be aware of the difficulty to translate these data to humans due to considerable species differences in susceptibility toward OP (International Programme on Chemical Safety 2010; Maynard and Beswick 1992). For obvious reasons, human studies with intentional exposure to OP are limited. In a few trials that were mostly performed decades ago, only sublethal OP doses were used (Sidell 2007; Rider et al. 1969; Hayes 1971). Moreover, relevant data of many highly toxic nerve agent analogues (cf. Tables 3, 4, 5) are not available in the open literature.
The determination of the inhibitory potency of OP toward human AChE in vitro is one option to get an initial insight into the potential toxicity of these compounds. Figure 4 shows the second-order inhibition rate constants relative to sarin of a broad range of pesticides, nerve agents and nerve agent analogues. These data demonstrate the wide range of inhibitory potencies as well as the exceptional high potency of a large number of sarin and VX analogues. It has to be emphasized that these data provide only initial, basic information of the potential to interact with human AChE, being the main target of OP toxicity, but do not necessarily reflect the in vivo toxicity. Comparison of the in vitro inhibitory potency and the estimated human LD50 and LCT50 values of relevant OP nerve agents show an in part substantial difference (Fig. 4, inset) which indicates that other factors are important for the in vivo toxicity of OP. These will be addressed in the following section.

Data are from Aurbek et al. (2006, 2010), Worek et al. (2007), Bartling et al. (2007), Worek et al. (2004, 2009) and from unpublished data. The respective second-order inhibition rate constants, k i, were referred to sarin (k i 4 × 107 M−1min−1; set at 1). The inset presents the relation between the in vitro inhibitory potency and human toxicity estimates referred to sarin (cf. Table 6). GA tabun, GB sarin, GD soman, GF cyclosarin
Inhibitory potency of OP pesticides, nerve agents and nerve agent analogues toward human AChE in vitro
Toxicokinetic and toxicodynamic aspects
The toxicity of OP nerve agents and pesticides is determined by their inhibitory potency toward AChE, the physicochemical properties, the chemical and biological stability and by additives. OP pesticides are marketed and used as formulations containing different organic solvents and emulsifiers, and there is evidence that these co-formulants may increase the toxicity of pesticides (Eddleston et al. 2012), while no information is available on the potential role of stabilizers used in weaponized OP nerve agents (Dacre 1984).
Physicochemical properties, notable volatility, are determining factors for the preferential OP exposure route. Table 6 shows the huge differences in volatility and hydrolytic stability of classical nerve agents, major factors for the largely different human toxicity estimates after vapor and liquid percutaneous exposure (cf. Table 7). The low volatile and persistent nerve agent VX is extremely toxic after skin exposure, while the more volatile G-agents are believed to be 70–500 times less toxic via this exposure route. Hence, knowledge of physicochemical properties of potential threat agents is essential for the assessment of preferential routes of exposure.
Bioavailability, distribution and metabolism of OP nerve agents and to less extent of OP pesticides have been reviewed extensively by multiple authors (cf. Benschop and de Jong 2001; John et al. 2015). These data are essential for physiology-based pharmacokinetic–pharmacodynamic modelling (PBPK-PD) of OP (Benschop and de Jong 2001; John et al. 2015; Timchalk et al. 2007; Sweeney et al. 2006; Covington et al. 2016). Investigation of interactions between OP and blood and tissue constituents, of transfer rates of OP from blood to tissues and of detoxification rates by endogenous enzymes provides important information for the estimation of onset and duration of poisoning after OP incorporation (Fig. 5). Again, research is mainly focused on a small number of nerve agents and pesticides and data on many other pesticides and almost all nerve agent analogues are not available in the open literature. However, existing data on the toxicokinetics of selected G- and V-agents may provide insight into the potential behavior of tabun, sarin and VX analogues in mammalian organisms. There is convincing evidence that tabun, sarin, soman and cyclosarin are detoxified in vivo within a rather short time, i.e., half-life of less than 1 h (Benschop and de Jong 2001; Tenberken et al. 2010; Reiter et al. 2007). In contrast, VX and VR are highly stable in living organisms and limited toxicokinetic data indicate a persistence of toxicologically relevant concentrations for more than 12 h (van der Schans et al. 2003; Reiter et al. 2008, 2015).
The exposure route is decisive for the velocity of agent absorption into the systemic circulation (Benschop and de Jong 2001). Inhalation and intravenous administration, serving as a model for the inhalation route, of G- and V-agents result in a rapid increase of OP concentration in the circulation, while percutaneous exposure to V-agents and OP pesticides is characterized by a delay of several hours until OP can be quantified in blood (Feldmann and Maibach 1974; Reiter et al. 2008; van der Schans et al. 2003; Wester et al. 1983). Hence, percutaneous OP poisoning may result in a rather significant lag time between exposure and onset of clinical signs. These properties are an important factor for the development of appropriate concepts for decontamination and drug treatment (Mikler et al. 2011).
Available data on OP toxicokinetics allow an assumption on the potential persistence of tabun, sarin and VX analogues in vivo. It may be assumed that structural analogues of tabun and sarin should have a short half-life in blood after inhalation exposure (Benschop and de Jong 2001; Jose et al. 2015; Garner and Jones 2014), while VX analogues should persist for prolonged time in the body (van der Schans et al. 2003; Bouchard et al. 2003; Feldmann and Maibach 1974).
Medical aspects
The adequate care of OP casualties requires an initial rapid and effective skin decontamination, especially in case of percutaneous exposure to OP nerve agents and pesticides (Roberts and Maynard 2007; Zilker 2005; Joosen et al. 2013; Chilcott 2007). Immediate skin decontamination can preserve survival and can prevent the occurrence of signs of poisoning. However, the effectiveness of decontamination decreases dramatically with time (Joosen et al. 2013; Braue et al. 2011; Bjarnason et al. 2008; Hamilton et al. 2004). A major disadvantage of commercial skin decontamination kits, e.g., Reactive Skin Decontamination Lotion (RSDL) Kit or M291 Skin Decontamination Kit, is the need to know the exposure location since it is hardly possible to perform whole body decontamination with such kits, and limited information on the effectiveness of commercially available skin decontamination kits against a broader range of OP. In consequence, available skin decontamination kits allow a provisional, nevertheless potentially lifesaving, on-site decontamination which must be followed by timely undressing and whole body decontamination.
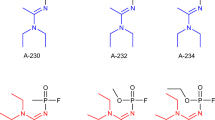
Besides decontamination, treatment with specific antidotes is essential to prevent mortality and incapacitation of OP casualties (Vale et al. 2007; Watson et al. 2015; Thiermann et al. 2016). Since decades, antimuscarinics, primarily atropine, are used as basic therapeutic drugs (McDonough and Shih 2007). Atropine antagonizes the OP effects at muscarinic synapses and can thus reverse or diminish the stimulation of smooth muscles and glands, the decreased heart rate and the impaired function of central respiratory centers. The effect of atropine is only symptomatic and requires appropriate blood and tissue concentrations for prolonged time (Thiermann et al. 2011). Atropine has no therapeutic effect on nicotinic receptors. For that reason and to restore the activity of inhibited AChE atropine has to be supplemented by oximes. The first clinically used pyridinium oxime pralidoxime (2-PAM) was introduced some 60 years ago, followed by the bis-pyridinium oximes obidoxime, trimedoxime (TMB-4) and HI-6 (Eyer and Worek 2007). Up to now, several thousand oxime structures were published and their ability to reactivate OP-inhibited AChE and to counteract the toxic OP effects was investigated in numerous in vitro and in vivo studies. In summary, the available data demonstrate a variable reactivating potency and therapeutic efficacy which is dependent on the structure of the oxime and the OP as well as their concentration (Worek and Thiermann 2013). In general, AChE inhibited by sarin, cyclosarin, VX, VR, CVX and pesticides bearing a dimethoxy or diethoxy residue is susceptible toward reactivation by oximes while AChE inhibited by soman, due to rapid aging, tabun and different pesticides, e.g., fenamiphos and ethoprophos, is rather resistant. Up to now, no broad-spectrum oxime covering the whole spectrum of classical nerve agents and most relevant pesticides was identified. An additional limitation of present oximes, i.e., charged hydrophilic compounds, is the very limited ability to penetrate the blood brain barrier and to reactivate brain AChE (Kalasz et al. 2015). Only very limited information on the ability of oximes to reactivate human AChE inhibited by nerve agent analogues was published. It appears that inhibition by a variety of sarin and VX analogues (Tables 3, 5) results in phosphylated human AChE which can be reactivated by established oximes (Bartling et al. 2007; Worek et al. 2009) while close tabun analogues, N,N-diethyltabun and N,N-di-n-propyltabun (Table 4), turned out to be completely resistant toward reactivation by oximes (Worek et al. 2007, 2015).
The inadequate effectiveness of presently available antidotes initiated intensified research on alternative therapeutic approaches (Table 8). These include non-charged oximes to improve the reactivation of brain AChE, non-oxime reactivators and specifically designed oximes to reactivate OP-inhibited BChE in order to transfer endogenous or injected BChE into a catalytic scavenger (Katz et al. 2015; Mercey et al. 2012; Sit et al. 2014). Ongoing research tries to identify more effective anticonvulsants as replacement of the widely used diazepam and to provide compounds (e.g., antinicotinics) being active at the neuromuscular junction in order to restore OP-impaired neuromuscular function in case of oxime resistance (McDonough 2002; Weissman and Raveh 2008; Ring et al. 2015; Price et al. 2016).
The perception of limited efficacy of standard atropine + oxime therapy, being able to increase survival but hardly to prevent incapacitation, shifted the focus to options directed to avoid the onset of toxic signs. This led to research on small molecules and proteins acting as stoichiometric or catalytic (bio)scavengers which should be able to detoxify incorporated OP before it can reach target tissues (Masson 2015; Nachon et al. 2013; Lenz et al. 2007; Letort et al. 2016; Masson 2016). It could be shown that the prophylactic and therapeutic, i.e., after OP exposure but prior to the onset of signs of poisoning, administration of a small molecule scavenger, an oxime-substituted β-cyclodextrin, the stoichiometric scavenger human BChE and of catalytic enzymes, PON1 and PTE mutants, at appropriate doses almost prevented incapacitation and preserved survival of animals poisoned by selected nerve agents (cyclosarin, VX) (Mumford et al. 2011; Worek et al. 2014a, b, c).
Research on improved therapies of OP poisoning in the last decades was focused almost exclusively on a very limited number of nerve agents (tabun, sarin, soman, cyclosarin, VX) and an even lower number of pesticides. Information on the effectiveness of oximes and other therapeutic options against nerve agent analogues and many pesticides is almost missing in the open literature. Only a small number of studies investigated other OP and give some insight into the potential of present and future therapies (Rice et al. 2015; Cherny et al. 2013; Daczkowski et al. 2015; Goldsmith et al. 2016; Bartling et al. 2007; Worek et al. 2007, 2009).
In consequence, the focus of research activities on a very limited number of OP must be considered as a major issue. The inadequate knowledge of the potential efficacy of standard treatment against poisoning by a large number of OP as well as the missing consideration of these agents for the design of catalytic small molecule and bioscavengers could result in an inadequate therapeutic efficacy of present and future OP therapies.
Impact of changing threats on defense against organophosphorus compounds
The rather well-defined threat to our societies by state actors prior to the implementation of the CWC is going to change fundamentally. Cold war scenarios of chemical war will lose their importance, while asymmetric threats will develop as major issue. Moreover, the increasing interest of terrorist groups in chemical warfare agents may ultimately result in a markedly broader spectrum of threat agents (Pita and Anadon 2015). Although no open source information is available confirming terrorist use of OP nerve agents after the attacks in Japan in 1994 and 1995, there is some evidence that Islamic terrorists may have attacked girl schools in Afghanistan with the OP pesticide malathion (Pita and Anadon 2015). Although not yet verified, the assumed use of the chemical warfare blister agent sulfur mustard by Islamic State terrorists gives a clear indication on the interest and potential capability of this group (European Parliamentary Research Service 2015; OPCW Technical Secretariat 2015). Hence, there is an increasing risk by terrorists to get hold of pesticide stocks or even to synthesize nerve agents or nerve agent analogues on a small scale.
Information on the synthesis of OP pesticides, nerve agents and nerve agent analogues is available in the public domain and gives in part detailed instruction on synthetic procedures (Sartori 1951; Saunders 1957; Schrader 1963; Holmstedt 1951; Black and Harrison 1996; Timperley 2015). OP synthesis by terrorists will depend on intention, chemical skills, technical capabilities and availability of precursors. This may result in the production of OP analogues apart of the classical nerve agents.
Research on defensive procedures against OP nerve agents was focused on a small number of agents in the past decades. Only limited physicochemical, toxicological and medical information on nerve agent analogues is available in the open literature (cf. Aquilonius et al. 1964; Binenfeld 1966; Berry and Davies 1966; Hall et al. 1977; de Jong and Benschop 1988). In consequence, adequate information on the toxicity, chemical and biological stability of nerve agent analogues and the efficacy of antidotes is rather scarce. Moreover, these agents may provoke new challenges for material and skin decontamination and detection. Early detection is fundamental for the initiation of appropriate medical countermeasures. Enzyme-based detection tickets may indicate the dissemination of a broad spectrum of OP and may be suitable for spot detection, while ion mobility spectrometry (IMS) mobile detectors may fail if the respective spectrum is not included into the device database or volatility is too low for sensitive detection (McKone et al. 2000; Mäkinen et al. 2010).
The potentially enlarged spectrum of threat agents has an additional impact on the ongoing development of novel antidotes. The in vitro and in vivo effectiveness of AChE reactivators and catalytic (bio)scavengers is usually tested with a limited number of classical OP nerve agents, and promising candidates may be of inadequate efficacy against nerve agent analogues (Mercey et al. 2012; Worek and Thiermann 2013; Nachon et al. 2013). Only the primary target, human AChE and to less extent human BChE, has the potential to detoxify all OP analogues if their main mechanism of action is cholinesterase inhibition. Hence, cholinesterase-based stoichiometric scavengers, atropine and potentially antinicotinics can be considered as generic antidotes covering the whole spectrum of OP threat agents.
Conclusions
The implementation of the CWC, prohibiting the development, production, storage and use of chemical weapons by 192 nations (United Nations Treaty Collection 1997) and the ban of highly toxic OP pesticides, especially class I pesticides according to the WHO classification (International Programme on Chemical Safety 2010), by many countries constitutes a great success of the international community. However, the increased interest of terrorist groups in toxic chemicals and chemical warfare agents presents new challenges to our societies (Pita and Anadon 2015; European Parliamentary Research Service 2015).
Selection of different residues attached to the central phosphorus atom allows the synthesis of innumerable, potentially toxic OP pesticides, nerve agents and nerve agent analogues (Holmstedt 1963; Timperley 2015). Research on defensive procedures against OP nerve agents was focused on a small number of agents in the past decades. Only limited physicochemical, toxicological and medical information on nerve agent analogues is available in the open literature. This implies potential gaps of our capabilities to detect, to decontaminate and to treat patients if nerve agent analogues are disseminated. Moreover, focus on a limited number of classical nerve agents during research and development of novel medical countermeasures may lead to an inadequate efficacy of new treatment options.
In summary, we may face new, up to now disregarded, threats by toxic OP which calls for increased awareness and appropriate preparedness of military and civilian CBRN defense, a broader approach for new physical and medical countermeasures and an integrated system of effective detection, decontamination, physical protection and treatment.
References
Abou-Donia MB (1981) Organophosphorus ester-delayed neurotoxicity. Annu Rev Pharmacol Toxicol 21:511–548
Aldridge WN, Reiner E (1972) Enzyme inhibitors as substrates—interactions of esterases with esters of organophosphorus and carbamic acids. North-Holland Publishing Company, Amsterdam
Aquilonius SM, Fredriksson SA, Sundwall A (1964) Studies on phosphorylated thiocholine and choline derivatives. I. General toxicology and pharmacology. Toxicol Appl Pharmacol 6:269–279
Aurbek N, Thiermann H, Szinicz L, Eyer P, Worek F (2006) Analysis of inhibition, reactivation and aging kinetics of highly toxic organophosphorus compounds with human and pig acetylcholinesterase. Toxicology 224:91–99
Aurbek N, Herkert NM, Koller M, Thiermann H, Worek F (2010) Kinetic analysis of interactions of different sarin and tabun analogues with human acetylcholinesterase and oximes: is there a structure-activity relationship? Chem Biol Interact 187:215–219
Baldit GL (1958) Amiton-a new acaricide and scalicide. J Sci Food Agr 9:516–524
Ballantyne B, Marrs TC (1992) Overview of the biological and clinical aspects of organophosphates and carbamates. In: Ballantyne B, Marrs T (eds) Clinical and experimental toxicology of organophosphates and carbamates. Butterworth & Heinemann, Oxford, pp 3–14
Bartling A, Worek F, Szinicz L, Thiermann H (2007) Enzyme-kinetic investigation of different sarin analogues reacting with human acetylcholinesterase and butyrylcholinesterase. Toxicology 233:166–172
Benschop HP, de Jong L (2001) Toxicokinetics of nerve agents. In: Somani S, Romano J (eds) Chemical warfare agents: toxicity at low levels. CRC Press, Boca Raton, pp 25–81
Berry WK, Davies DR (1966) Factors influencing the rate of ‘aging’ in a series of alkyl methylphosphonyl-acetylcholinesterases. Biochem J 100:572–576
Bertolote JM, Fleischmann A, Eddleston M, Gunnell D (2006) Deaths from pesticide poisoning: a global response. Br J Psychiatr 189:201–203
Bhattacharjee AK, Marek E, Le HT, Ratcliffe R, DeMar JC, Pervitsky D, Gordon RK (2015) Discovery of non-oxime reactivators using an in silico pharmacophore model of reactivators for DFP-inhibited acetylcholinesterase. Eur J Med Chem 90:209–220
Bierwisch A, Wille T, Thiermann H, Worek F (2016) Kinetic analysis of interactions of amodiaquine with human cholinesterases and organophosphorus compounds. Toxicol Lett 246:49–56
Binenfeld Z (1966) Novi nervni bojni otrovi. Voj Preg 23:40–45
Bjarnason S, Mikler J, Hill I, Tenn C, Garrett M, Caddy N, Sawyer TW (2008) Comparison of selected skin decontaminant products and regimens against VX in domestic swine. Hum Exp Toxicol 27:253–261
Black R (2016) Development, historical use and properties of chemical warfare agents. In: Worek F, Jenner J, Thiermann H (eds) Chemical warfare toxicology, vol 1., Fundamental aspects. The Royal Society of Chemistry, Cambridge, pp 1–28
Black RM, Harrison JM (1996) The chemistry of organophosphorus chemical warfare agents. In: Hartley F (ed) The chemistry of organophosphorus compounds. Wiley, Chichester, pp 781–840
Black RM, Clarke RJ, Read RW, Reid M (1994) Application of gas chromatography–mass spectrometry and gas chromatography–tandem mass spectrometry to the analysis of chemical warfare samples, found to contain residues of the nerve agent sarin, sulphur mustard and their degredation products. J Chromatogr A 662:301–321
Bouchard M, Gosselin NH, Brunet RC, Samuel O, Dumoulin MJ, Carrier G (2003) A toxicokinetic model of malathion and its metabolites as a tool to assess human exposure and risk through measurements of urinary biomarkers. Toxicol Sci 73:182–194
Braue EH, Smith KH, Doxzon BF, Lumpkin HL, Clarkson ED (2011) Efficacy studies of Reactive Skin Decontamination Lotion, M291 Skin Decontamination Kit, 0.5% bleach, 1% soapy water, and Skin Exposure Reduction Paste against chemical warfare agents, part 1: guinea pigs challenged with VX. Cutan Ocul Toxicol 30:15–28
Busker RW, Zijlstra JJ, van der Wiel HJ, Melchers B, van Helden H (1991) Organophosphate poisoning: a method to test therapeutic effects of oximes other than acetylcholinesterase reactivation in the rat. Toxicology 69:331–344
Casida JE (1956) Metabolism of organophosphorus insecticides in relation to their antiesterase activity, stability, and residual properties. J Agric Food Chem 4:772–785
Casida JE, Durkin KA (2013) Anticholinesterase insecticide retrospective. Chem Biol Interact 203:221–225
CBC News (2015) U.S.: Tests show mustard gas traces in ISIS attack. http://www.cbsnews.com/news/ustests-mustard-gas-traces-isis-attack-kurdish-forces-iraq/. Accessed 18 Apr 2016
Chang SS, Lu TH, Eddleston M, Konradsen F, Sterne J, Lin JJ, Gunnell D (2012) Factors associated with the decline in suicide by pesticide poisoning in Taiwan: a time trend analysis, 1987–2010. Clin Toxicol 50:471–480
Cherny I, Greisen P, Ashani Y, Khare SD, Oberdorfer G, Leader H, Baker D, Tawfik DS (2013) Engineering V-type nerve agents detoxifying enzymes using computationally focused libraries. ACS Chem Biol 8:2394–2403
Chilcott RP (2007) Dermal aspects of chemical warfare agents. In: Marrs T, Maynard R, Sidell F (eds) Chemical warfare agents: toxicology and treatment. Wiley, Chichester, pp 409–422
Cohen O, Kronman C, Chitlaru T, Ordentlich A, Velan B, Shafferman A (2001) Effect of chemical modification of recombinant human acetylcholinesterase by polyethylene glycol on its circulatory longevity. Biochem J 357:795–802
Covington TR, Lumley LA, Ruark CD, Clarkson ED, Whalley CE, Gearhart JM (2016) Modeling of organophosphorus chemical warfare nerve agents: a physiologically based pharmacokinetic-pharmacodynamic (PBPK-PD) model of VX. In: Worek F, Jenner J, Thiermann H (eds) Chemical warfare toxicology, vol 1., Fundamental aspects. The Royal Society of Chemistry, Cambridge, pp 213–263
Dacre JC (1984) Toxicology of some anticholinesterases used as chemical warfare agents—a review. In: Brzin M (ed) Cholinesterases. Walter de Gruyter & Co, Berlin, pp 415–426
Daczkowski CM, Pegan SD, Harvey SP (2015) Engineering the organophosphorus acid anhydrolase enzyme for increased catalytic efficiency and broadened stereospecificity on Russian VX. Biochemistry 54:6423–6433
De Clermont P (1854) Chimie organique - note sur la preparation de quelques ethers. Compt Rend Acad Sci 39:338–341
De Clermont P (1855) Mémoire sur les éthers phosphoriques. Ann Chim Phys 44:330–336
De Jong L, Benschop HP (1988) Biochemical and toxicological implications of chirality in anticholinesterase organophosphates. In: Ariens E, van Rensen J, Welling W (eds) Stereoselectivity of pesticides—biological and chemical problems. Elsevier, Amsterdam, pp 109–149
Eddleston M, Karalliedde L, Buckley N, Fernando R, Hutchinson G, Isbister G, Konradsen F, Murray D, Piola JC, Senanayake N, Sheriff R, Singh S, Siwach SB, Smit L (2002) Pesticide poisoning in the developing world—a minimum pesticides list. Lancet 360:1163–1167
Eddleston M, Eyer P, Worek F, Juszczak E, Alder N, Mohamed F, Senarathna L, Hittarage A, Azher S, Jeganathan K, Jayamanne S, von Meyer L, Dawson AH, Sheriff M, Buckley NA (2009) Pralidoxime in acute organophosphorus insecticide poisoning—a randomised controlled trial. PLoS Med 6:e1000104
Eddleston M, Street JM, Self I, Thompson A, King T, Williams N, Naredo G, Dissanayake K, Yu LM, Worek F, John H, Smith S, Thiermann H, Harris JB, Clutton RE (2012) A role for solvents in the toxicity of agricultural organophosphorus pesticides. Toxicology 294:94–103
Eto M (1974) Organophosphorus pesticides: organic and biological chemistry. CRC Press, Cleveland
European Parliamentary Research Service (2015) ISIL/Da’esh and ‘non-conventional’ weapons of terror. http://www.europarl.europa.eu/RegData/etudes/BRIE/2015/572806/EPRS_BRI(2015)572806_EN.pdf. Accessed 18 Apr 2016
Eyer P (1995) Neuropsychopathological changes by organophosphorus compounds—a review. Hum Exp Toxicol 14:857–864
Eyer P, Worek F (2007) Oximes. In: Marrs T, Maynard R, Sidell F (eds) Chemical warfare agents: toxicology and treatment. Wiley, Chichester, pp 305–329
FAO Statistics Division (2013) Pesticide use. http://faostat3.fao.org. Accessed 05 May 2016
Feldmann RJ, Maibach HI (1974) Percutaneous penetration of some pesticides and herbicides in man. Toxicol Appl Pharmacol 28:126–132
Foxenberg RJ, McGarrigle BP, Knaak JB, Kostyniak PJ, Olson JR (2007) Human hepatic cytochrome P450-specific metabolism of parathion and chlorpyrifos. Drug Metab Dispos 35:189–193
Gaines TB (1969) Acute toxicity of pesticides. Toxicol Appl Pharmacol 14:515–534
Garner F, Jones K (2014) Biological monitoring for exposure to methamidophos: a human oral dosing study. Toxicol Lett 231:277–281
Ghosh R, Newman JF (1955) A new group of organophosphorus pesticides. Chem Ind 11
Goel A, Aggarwal P (2007) Pesticide poisoning. Natl Med J India 20:182–191
Goldsmith M, Eckstein S, Ashani Y, Greisen P, Leader H, Sussman JL, Aggarwal N, Ovchinnikov S, Tawfik DS, Baker D, Thiermann H, Worek F (2016) Catalytic efficiencies of directly evolved phosphotriesterase variants with structurally different organophosphorus compounds in vitro. Arch Toxicol. doi:10.1007/s00204-015-1626-2
Gunnell D, Eddleston M, Phillips MR, Konradsen F (2007) The global distribution of fatal pesticide self-poisoning: systematic review. BMC Public Health 7:357
Hall CR, Inch TD, Inns RH, Muir AW, Sellers DJ, Smith AP (1977) Differences between some biological properties of enantiomers of alkyl S-alkyl methylphosphonothioates. J Pharm Pharmacol 29:574–576
Hamilton MG, Hill I, Conley J, Sawyer TW, Caneva DC, Lundy PM (2004) Clinical aspects of percutaneous poisoning by the chemical warfare agent VX: effects of application site and decontamination. Mil Med 169:856–862
Hayes WJ (1971) Studies on exposure during the use of anticholinesterase pesticides. Bull World Health Organ 44:277–288
Hoette TM (2012) Systems analysis of past, present, and future chemical terrorism scenarios. SAND2012-1468, Sandia National Laboratories
Holmstedt B (1951) Synthesis and pharmacology of dimethylamido-ethoxy-phosphoryl cyanide (Tabun) together with a description of some allied anticholinesterase compounds containing the N–P bond. Acta Physiol Scand 25(Suppl. 90):1–120
Holmstedt B (1959) Pharmacology of organophosphorus cholinesterase inhibitors. Pharmacol Rev 11:567–688
Holmstedt B (1963) Structure-activity relationship of organophosphorus anticholinesterase agents. In: Koelle G (ed) Cholinesterases and anticholinesterase agents. Springer, Berlin, pp 428–485
Hummel S (2016) The Islamic State and WMD: assessing the future threat. CTC Sentinel 9:18–21
International Programme on Chemical Safety (2010) The WHO recommended classification of pesticides by hazard and guidelines to classification 2009. World Health Organisation, Geneva
Jackson CJ, Carville A, Ward J, Mansfield K, Ollis DL, Khurana T, Bird SB (2014) Use of OpdA, an organophosphorus (OP) hydrolase, prevents lethality in an African green monkey model of acute OP poisoning. Toxicology 317:1–5
Jandorf BJ, Michel HO, Schaffer NK, Egan R, Summerson WH (1955) The mechanism of reaction between esterases and phosphorus-containing anti-esterases. Disc Faraday Soc 20:134–142
John H, Balszuweit F, Kehe K, Worek F, Thiermann H (2015) Toxicokinetic aspects of nerve agents and vesicants. In: Gupta RC (ed) Handbook of toxicology of chemical warfare agents. Elsevier, Amsterdam, pp 817–856
Joosen M, van der Schans MJ, Kuijpers WC, van Helden H, Noort D (2013) Timing of decontamination and treatment in case of percutaneous VX poisoning: a mini review. Chem Biol Interact 203:149–153
Jose A, Selvakumar R, Peter JV, Karthik G, Fleming DH, Fleming JJ (2015) Estimation of monocrotophos renal elimination half-life in humans. Clin Toxicol 53:629–632
Kabachnik MI, Brestkin AP, Godovikov NN, Michelson MJ, Rozengart EV, Rozengart VI (1970) Hydrophobic areas on the active surface of cholinesterases. Pharmacol Rev 22:355–388
Kadivar H, Adams SC (1991) Treatment of chemical and biological warfare injuries: insights derived from the 1984 Iraqi attack on Majnoon island. Mil Med 156:171–177
Kalasz H, Nurulain SM, Veress G, Antus S, Darvas F, Adeghate E, Adem A, Hashemi F, Tekes K (2015) Mini review on blood–brain barrier penetration of pyridinium aldoximes. J Appl Toxicol 35:116–123
Katz FS, Pecic S, Tran TH, Trakht I, Schneider L, Zhu Z, Ton-That L, Luzac M, Zlatanic V, Damera S, Macdonald J, Landry DW, Tong L, Stojanovic MN (2015) Discovery of new classes of compounds that reactivate acetylcholinesterase inhibited by organophosphates. ChemBioChem 16:2205–2215
Knipe DW, Metcalfe C, Fernando R, Pearson M, Konradsen F, Eddleston M, Gunnell D (2014) Suicide in Sri Lanka 1975–2012: age, period and cohort analysis of police and hospital data. BMC Public Health 14:839
Koelle GB (1992) Pharmacology and toxicology of organophosphates. In: Ballantyne B, Marrs T (eds) Clinical and experimental toxicology of organophosphates and carbamates. Butterworth and Heinemann, Oxford, pp 35–39
Kovarik Z, Katalinic M, Sinko G, Binder J, Holas O, Jung YS, Musilova L, Jun D, Kuca K (2010) Pseudo-catalytic scavenging: searching for a suitable reactivator of phosphorylated butyrylcholinesterase. Chem Biol Interact 187:167–171
Krieger R (2010) Hayes’ handbook of pesticide toxicology. Elsevier, Amsterdam
Lange W, von Krueger G (1932) Über Ester der Monofluorphosphorsäure. Ber Dtsch Chem Ges 65:1598–1601
Lee EC (2003) Clinical manifestations of sarin nerve gas exposure. JAMA 290:659–662
Lenz DE, Yeung D, Smith JR, Sweeney RE, Lumley LA, Cerasoli DM (2007) Stoichiometric and catalytic scavengers as protection against nerve agent toxicity: a mini review. Toxicology 233:31–39
Letort S, Balieu S, Erb W, Gouhier G, Estour F (2016) Interactions of cyclodextrins and their derivatives with toxic organophosphorus compounds. Beilstein J Org Chem 12:204–228
Lohs KH (1975) Delayed toxic effects of chemical warfare agents. SIPRI Monograph. Almqvist & Wiksell, Stockholm
Lotti M, Moretto A (2005) Organophosphate-induced delayed polyneuropathy. Toxicol Rev 24:37–49
MacIlwain C (1993) Study proves Iraq used nerve gas. Nature 363:3
Mäkinen MA, Anttalainen OA, Sillinpää M (2010) Ion mobility spectrometry and its applications in detection of chemical warfare agents. Anal Chem 82:9594–9600
Marrs TC (2007) Toxicology of organophosphate nerve agents. In: Marrs T, Maynard R, Sidell F (eds) Chemical warfare agents: toxicology and treatment. Wiley, Chichester, pp 191–221
Masson P (2015) Catalytic bioscavengers: the new generation of bioscavenger-based medical countermeasures. In: Gupta RC (ed) Handbook of toxicology of chemical warfare agents. Elsevier, Amsterdam, pp 1107–1123
Masson P (2016) Nerve agents: catalytic scavengers as an alternative approach for medical countermeasures. In: Worek F, Jenner J, Thiermann H (eds) Chemical warfare toxicology, vol 2., Management of poisoning. The Royal Society of Chemistry, Cambridge, pp 43–81
Masson P, Carletti E, Nachon F (2009) Structure, activities and biomedical applications of human butyrylcholinesterase. Protein Peptide Lett 16:1215–1224
Maxwell DM (1992) The specificity of carboxylesterase protection against the toxicity of organophosphorus compounds. Toxicol Appl Pharmacol 114:306–312
Maxwell DM, Brecht KM, O’Neill BL (1987) The effect of carboxylesterase inhibition on interspecies differences in soman toxicity. Toxicol Lett 39:35–42
Maxwell DM, Brecht KM, Koplovitz I (1997) Characterization and treatment of the toxicity of O-isobutyl S-[2-(diethylamino)ethyl]methylphosphonothioate, a structural isomer of VX, in guinea pigs. J Am Coll Toxicol 15(Suppl 2):S78–S88
Maynard RL, Beswick FW (1992) Organophosphorus compounds as chemical warfare agents. In: Ballantyne B, Marrs T (eds) Clinical and experimental toxicology of organophosphates and carbamates. Butterworth and Heinemann, Oxford, pp 373–385
McDonough JH (2002) Performance impacts of nerve agents and their pharmacological countermeasures. Mil Psychol 14:93–119
McDonough JH, Shih TM (2007) Atropine and other anticholinergic drugs. In: Marrs T, Maynard R, Sidell F (eds) Chemical warfare agents: toxicology and treatment. Wiley, Chichester, pp 287–303
McKone TE, Huey BM, Downing E, Duffy LM (2000) Strategies to protect the health of deployed U.S. Forces: detecting, characterizing, and documenting exposures. National Academic Press, Washington, DC
Mercey G, Verdelet T, Renou J, Kliachyna M, Baati R, Nachon F, Jean L, Renard PY (2012) Reactivators of acetylcholinesterase inhibited by organophosphorus nerve agents. Acc Chem Res 45:756–766
Michaelis C (1903) Über die organischen Verbindungen des Phosphors mit Stickstoff. Liebigs Ann Chem 326:129–258
Mikler J, Tenn C, Worek F, Reiter G, Thiermann H, Garrett M, Bohnert S, Sawyer TW (2011) Immobilization of Russian VX skin depots by localized cooling: implications for decontamination and medical countermeasures. Toxicol Lett 206:47–53
Moralev SN, Rozengart EV (2007) Comparative enzymology of cholinesterases. International University Line, La Jolla
Morita H, Yanagisawa N, Nakajima T, Shimizu M, Hirabayashi H, Okudera H, Nohara M, Midorikawa Y, Mimura S (1995) Sarin poisoning in Matsumoto, Japan. Lancet 346:290–293
Mumford H, Price ME, Wetherell JR (2008) A novel approach to assessing percutaneous VX poisoning in the conscious guinea-pig. J Appl Toxicol 28:694–702
Mumford H, Price ME, Lenz DE, Cerasoli DM (2011) Post-exposure therapy with human butyrylcholinesterase following percutaneous VX challenge in guinea pigs. Clin Toxicol 49:287–297
Mumford H, Docx CJ, Price ME, Green AC, Tattersall J, Armstrong SJ (2013) Human plasma-derived BuChE as a stoichiometric bioscavenger for treatment of nerve agent poisoning. Chem Biol Interact 203:160–166
Musilek K, Dolezal M, Gunn-Moore F, Kuca K (2011) Design, evaluation and structure–activity relationship studies of the AChE reactivators against organophosphorus pesticides. Med Res Rev 31:548–575
Mutch E, Williams FM (2006) Diazinon, chlorpyrifos and parathion are metabolised by multiple cytochromes P450 in human liver. Toxicology 224:22–32
Myhrer T, Mariussen E, Enger S, Aas P (2015) Supralethal poisoning by any of the classical nerve agents is effectively counteracted by procyclidine regimens in rats. Neurotoxicology 50:142–148
Nachon F, Brazzolotto X, Trovaslet M, Masson P (2013) Progress in the development of enzyme-based nerve agent bioscavengers. Chem Biol Interact 206:536–544
National Research Council—Committee on Toxicology (1997) Review of acute human-toxicity estimates for selected chemical-warfare agents
Nozaki H, Aikawa N, Fujishima S, Suzuki M, Shinozawa Y, Hori S, Nogawa S (1995a) A case of VX poisoning and the difference from sarin. Lancet 346:698–699
Nozaki H, Aikawa N, Shinozawa Y, Hori S, Fujishima S, Takuma K, Sagoh M (1995b) Sarin poisoning in Tokyo subway. Lancet 345:980–981
O’Brien RD (1960) Toxic phosphorus esters—chemistry, metabolism, and biological effects. Academic Press, New York
Okumura T, Takasu N, Ishimatsu S, Miyanoki S, Mitsuhashi A, Kumuda K, Tanaka K, Hinohara S (1996) Report of 640 victims of the Tokyo subway sarin attack. Ann Emerg Med 28:129–135
OPCW Technical Secretariat (2015) Report of the OPCW fact-finding mission in Syria regarding alleged incidents in Marea, Syrian Arab Republic, August 2015
Peter JV, Sudarsan TI, Moran JL (2014) Clinical features of organophosphate poisoning: a review of different classification systems and approaches. Ind J Crit Care Med 18:735–745
Pita R, Anadon A (2015) Chemical weapons of mass destruction and terrorism: a threat analysis. In: Gupta RC (ed) Handbook of toxicology of chemical warfare agents. Elsevier, Amsterdam, pp 55–65
Pita R, Domingo J (2014) The use of chemical weapons in the Syrian conflict. Toxics 2:391–402
Price ME, Docx CJ, Rice H, Fairhall SJ, Poole S, Bird M, Whiley L, Flint DP, Green AC, Timperley CM, Tattersall JE (2016) Pharmacokinetic profile and quantitation of protection against soman poisoning by the antinicotinic compound MB327 in the guinea-pig. Toxicol Lett 244:154–160
Prinz HJ (1969) Eine schwere percutane Vergiftung mit Parathion (E605®). Arch Toxikol 25:318–328
Reiter G, Koller M, Thiermann H, Dorandeu F, Mikler J, Worek F (2007) Development and application of procedures for the highly sensitive quantification of cyclosarin enantiomers in hemolysed swine blood samples. J Chromatogr B 859:9–15
Reiter G, Mikler J, Hill I, Weatherby K, Thiermann H, Worek F (2008) Chromatographic resolution, characterisation and quantification of VX enantiomers in hemolysed swine blood samples. J Chromatogr B 873:86–94
Reiter G, Müller S, Hill I, Weatherby K, Thiermann H, Worek F, Mikler J (2015) In vitro and in vivo toxicological studies of V nerve agents: molecular and stereoselective aspects. Toxicol Lett 232:438–448
Rengstorff RH (1994) Vision and ocular changes following accidental exposure to organophosphates. J Appl Toxicol 14:115–118
Rice H (2016) Toxicology of organophosphorus nerve agents. In: Worek F, Jenner J, Thiermann H (eds) Chemical warfare toxicology, vol 1., Fundamental aspects. The Royal Society of Chemistry, Cambridge, pp 81–116
Rice H, Dalton CH, Price ME, Graham SJ, Green AC, Jenner J, Groombridge HJ, Timperley CM (2015) Toxicity and medical countermeasure studies on the organophosphorus nerve agents VM and VX. Proc R Soc A 471:20140891
Rider JA, Moeller HC, Puletti EJ, Swader JI (1969) Toxicity of parathion, systox, octamethyl pyrophosphoramide, and methyl parathion in man. Toxicol Appl Pharmacol 14:603–611
Ring A, Strom BO, Turner SR, Timperley CM, Bird M, Green AC, Chad JE, Worek F, Tattersall J (2015) Bispyridinium compounds inhibit both muscle and neuronal nicotinic acetylcholine receptors in human cell lines. PLoS ONE 10:e0135811
Roberts G, Maynard RL (2007) Responding to chemical terrorism: operational planning and decontamination. In: Marrs T, Maynard R, Sidell F (eds) Chemical warfare agents: toxicology and treatment. Wiley, Chichester, pp 175–190
Rochu D, Chabriere E, Masson P (2007) Human paraoxonase: a promising approach for pre-treatment and therapy of organophosphorus poisoning. Toxicology 233:47–59
Sartori MF (1951) New developments in the chemistry of war gases. Chem Rev 48:225–257
Saunders BC (1957) Some aspects of the chemistry and toxic action of organic compounds containing phosphorus and fluorine. Cambridge University Press, Cambridge
Schrader G (1951) Die Entwicklung neuer Insektizide auf Grundlage organischer Fluor- und Phosphor-Verbindungen. Monogr Angew Chemie 62:1–62
Schrader G (1963) Die Entwicklung neuer insektizider Phosphorsäure-Ester. Verlag Chemie, Weinheim
Scott L (2007) Nerve agents: low-dose effects. In: Marrs T, Maynard R, Sidell F (eds) Chemical warfare agents: toxicology and treatment. Wiley, Chichester, pp 241–248
Seeger T, Eichhorn M, Lindner M, Niessen KV, Tattersall J, Timperley CM, Bird M, Green AC, Thiermann H, Worek F (2012) Restoration of soman-blocked neuromuscular transmission in human and rat muscle by the bispyridinium non-oxime MB327 in vitro. Toxicology 294:80–84
Shafferman A, Ordentlich A, Barak D, Stein D, Ariel N, Velan B (1996) Aging of phosphylated human acetylcholinesterase: catalytic processes mediated by aromatic and polar residues of the active centre. Biochem J 318:833–840
Shih TM, Rowland TC, McDonough JH (2007) Anticonvulsants for nerve agent-induced seizures: the influence of the therapeutic dose of atropine. J Pharmacol Exp Ther 320:154–161
Sidell FR (1974) Soman and sarin: clinical manifestations and treatment of accidental poisoning by organophosphates. Clin Toxicol 7:1–17
Sidell FR (1997) Nerve agents. In: Sidell F, Takafuji E, Franz D (eds) Medical aspects of chemical and biological warfare. Borden Institute, Washington, DC, pp 129–179
Sidell FR (2007) A history of human studies with nerve agents by the UK and USA. In: Marrs T, Maynard R, Sidell F (eds) Chemical warfare agents: toxicology and treatment. Wiley, Chichester, pp 223–239
Sit RK, Radic Z, Gerardi V, Zhang L, Garcia E, Katalinic M, Amitai G, Kovarik Z, Fokin VV, Sharpless KB, Taylor P (2011) New structural scaffolds for centrally acting oxime reactivators of phosphylated cholinesterases. J Biol Chem 286:19422–19430
Sit RK, Fokin VV, Amitai G, Sharpless KB, Taylor P, Radic Z (2014) Imidazole aldoximes effective in assisting butyrylcholinesterase catalysis of organophosphate detoxification. J Med Chem 57:1378–1389
Sweeney RE, Langenberg JP, Maxwell DM (2006) A physiologically based pharmacokinetic (PB/PK) model for multiple exposure routes of soman in multiple species. Arch Toxicol 80:719–731
Szinicz L (2005) History of chemical and biological warfare agents. Toxicology 214:167–181
Tammelin LE (1958) Organophosphorylcholines and cholinesterases. Ark Kemi 12:287–298
Tenberken O, Mikler J, Hill I, Weatherby K, Thiermann H, Worek F, Reiter G (2010) Toxicokinetics of tabun enantiomers in anaesthetized swine after intravenous tabun administration. Toxicol Lett 198:177–181
Thiermann H, Szinicz L, Eyer P, Felgenhauer N, Zilker T, Worek F (2007) Lessons to be learnt from organophosphorus pesticide poisoning for the treatment of nerve agent poisoning. Toxicology 233:145–154
Thiermann H, Steinritz D, Worek F, Radtke M, Eyer P, Eyer F, Felgenhauer N, Zilker T (2011) Atropine maintenance dosage in patients with severe organophosphate pesticide poisoning. Toxicol Lett 206:77–83
Thiermann H, Aurbek N, Worek F (2016) Treatment of nerve agent poisoning. In: Worek F, Jenner J, Thiermann H (eds) Chemical warfare toxicology, vol 2., Management of poisoning. The Royal Society of Chemistry, Cambridge, pp 1–42
Timchalk C, Busby A, Campbell JA, Needham LL, Barr DB (2007) Comparative pharmacokinetics of the organophosphorus insecticide chlorpyrifos and its major metabolites diethylphosphate, diethylthiophosphate and 3,5,6-trichloro-2-pyridinol in the rat. Toxicology 237:145–157
Timperley CM (2015) Best synthetic methods: organophosphorus (V) chemistry. Elsevier, Amsterdam
United Nations Treaty Collection (1997) Convention on the prohibition of the development, production, stockpiling and use of chemical weapons and on their destruction. https://treaties.un.org/pages/ViewDetails.aspx?src=TREATY&mtdsg_no=XXVI-3&chapter=26&lang=en. Accessed 18 Apr 2016
U.S. Army Chemical School (2005) Potential military chemical/biological agents and compounds. Field Manual 3-11.9. U.S. Army Chemical School, Ft. Leonard Wood, Mo. USA
Vale JA, Rice P, Marrs TC (2007) Managing civilian casualties affected by nerve agents. In: Marrs T, Maynard R, Sidell F (eds) Chemical warfare agents: toxicology and treatment. Wiley, Chichester, pp 249–260
van der Schans MJ, Lander BJ, van der Wiel H, Langenberg JP, Benschop HP (2003) Toxicokinetics of the nerve agent (±)-VX in anesthetized and atropinized hairless guinea pigs and marmosets after intravenous and percutaneous administration. Toxicol Appl Pharmacol 191:48–62
Veterans Today (2015) ISIS stole sarin gas from Lybia & has already used it, Gaddafi's cousin. http://www.veteranstoday.com/2015/12/19/isis-stole-sarin-gas-from-libya-stores-has-already-used-itgaddafis-cousin/. Accessed 18 Apr 2016
Watson A, Opresko D, Young RA, Hauschild V, King J, Bakshi K (2015) Organophosphate nerve agents. In: Gupta RC (ed) Handbook of toxicology of chemical warfare agents. Elsevier, Amsterdam, pp 87–109
Weimaster JF, Beaudry WT, Bossle PC, Ellzy MW, Janes LG, Johnson DW, Lochner JM, Pleva SG, Reeder JH, Rohrbaugh DK, Rosso TE, Szafraniec LJ, Szafraniec LL, Albro TG, Creasey WR, Stuff JR, Smiths PB, Stewart IR (1995) Chemical analysis of environmental samples collected in Iraq: analysis for the presence of chemical warfare agents. J Chem Technol Biotechnol 64:115–128
Weissman BA, Raveh L (2008) Therapy against organophosphate poisoning: the importance of anticholinergic drugs with antiglutamatergic properties. Toxicol Appl Pharmacol 232:351–358
Wester RC, Maibach HI, Bucks AW, Guy RH (1983) Malathion percutaneous absorption after repeated administration to man. Toxicol Appl Pharmacol 68:116–119
Worek F, Thiermann H (2013) The value of novel oximes for treatment of poisoning by organophosphorus compounds. Pharmacol Ther 139:249–259
Worek F, Thiermann H, Szinicz L, Eyer P (2004) Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem Pharmacol 68:2237–2248
Worek F, Aurbek N, Koller M, Becker C, Eyer P, Thiermann H (2007) Kinetic analysis of reactivation and aging of human acetylcholinesterase inhibited by different phosphoramidates. Biochem Pharmacol 73:1807–1817
Worek F, Herkert NM, Koller M, Aurbek N, Thiermann H (2009) Interaction of pentylsarin analogues with human acetylcholinesterase: a kinetic study. Toxicol Lett 187:119–123
Worek F, Seeger T, Goldsmith M, Ashani Y, Leader H, Sussman JL, Tawfik D, Thiermann H, Wille T (2014a) Efficacy of the rePON1 mutant IIG1 to prevent cyclosarin toxicity in vivo and to detoxify structurally different nerve agents in vitro. Arch Toxicol 88:1257–1266
Worek F, Seeger T, Reiter G, Goldsmith M, Ashani Y, Leader H, Sussman JL, Aggarwal N, Thiermann H, Tawfik D (2014b) Post-exposure treatment of VX poisoned guinea pigs with the engineered phosphotriesterase mutant C23: a proof-of-concept study. Toxicol Lett 231:45–54
Worek F, Seeger T, Zengerle M, Kubik S, Thiermann H, Wille T (2014c) Effectiveness of a substituted β-cyclodextrin to prevent cyclosarin toxicity in vivo. Toxicol Lett 226:222–227
Worek F, Herkert NM, Koller M, Thiermann H, Wille T (2015) Application of a dynamic in vitro model with real-time determination of acetylcholinesterase activity for the investigation of tabun analogues and oximes. Toxicol In Vitro 30:514–520
Zilker T (2005) Medical management of incidents with chemical warfare agents. Toxicology 214:221–231
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Worek, F., Wille, T., Koller, M. et al. Toxicology of organophosphorus compounds in view of an increasing terrorist threat. Arch Toxicol 90, 2131–2145 (2016). https://doi.org/10.1007/s00204-016-1772-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1772-1