Abstract
Zearalenone (ZEN), a mycotoxin with high estrogenic activity in vitro and in vivo, is a widespread food contaminant that is commonly detected in maize, wheat, barley, sorghum, rye and other grains. Human exposure estimates based on analytical data on ZEN occurrence in various food categories and food consumption data suggest that human exposure to ZEN and modified forms of ZEN may be close to or even exceed the tolerable daily intake (TDI) derived by the European Food Safety Authority (EFSA) for some consumer groups. Considering the inherent uncertainties in estimating dietary intake of ZEN that may lead to an under- or overestimation of ZEN exposure and consequently human risk and current lack of data on vulnerable consumer groups, there is a clear need for more comprehensive and reliable exposure data to refine ZEN risk assessment. Human biomonitoring (HBM) is increasingly being recognized as an efficient and cost-effective way of assessing human exposure to food contaminants, including mycotoxins. Based on animal and (limited) human data on the toxicokinetics of ZEN, it appears that excretion of ZEN and its major metabolites may present suitable biomarkers of ZEN exposure. In view of the limitations of available dietary exposure data on ZEN and its modified forms, the purpose of this review is to provide an overview of recent studies utilizing HBM to monitor and assess human exposure to ZEN. Considerations are given to animal and human toxicokinetic data relevant to HBM, analytical methods, and available HBM data on urinary biomarkers of ZEN exposure in different cohorts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zearalenone (ZEN) is a phenolic resorcyclic acid lactone mycotoxin produced by several Fusarium species (mainly F. graminearum, but also F. culmorum, F. cerealis, F.equiseti and F. semitectum) that grow on crops in temperate and warmer climate zones. This mycotoxin accumulates in grains mainly at preharvest but also postharvest under poor storage conditions. ZEN is a widespread contaminant that is commonly detected in maize, wheat, barley, sorghum, rye and other grains. Grain-based products such as grains for human consumption, grain milling products, breakfast cereals, fine bakery wares and snacks, infant foods, corn germ oil and wheat germ oil, bread, rolls and pasta are considered as main sources of human exposure to ZEN (EFSA 2011). While ZEN exhibits low acute toxicity, long-term exposure to ZEN may present a health risk due to its high estrogenic activity. The EFSA Panel on Contaminants in the Food Chain derived a tolerable daily intake (TDI) for ZEN of 0.25 μg/kg b.w. based on a NOEL of 10 μg/kg b.w. per day for estrogenic effects in female pigs (reddened/swollen vulva and cervix, increased uterus weight) as the most sensitive endpoint of ZEN toxicity and an uncertainty factor of 40 (4 for interspecies differences in toxicokinetics and 10 for interhuman variability) (EFSA 2011).
The most recent and detailed assessment of dietary exposure to ZEN in Europe is based on analytical data on ZEN occurrence in food provided by 19 European countries and food consumption data at the individual consumer level collected from national dietary surveys recorded in the EFSA Comprehensive European Food Consumption Database (EFSA 2011). In total, 28 different dietary surveys carried out in 17 different European countries were taken into account to estimate mean (average consumption in total population) and high (95th percentile food consumption in total population) chronic dietary exposure to ZEN for different age and consumer groups. Exposure estimates within age classes varied across European countries. In toddlers, for example, higher dietary exposure levels for both average and high consumers were recorded in Germany as compared to Belgium and the Netherlands (EFSA 2011). In adults, slightly lower levels of exposure were observed in Finland, Sweden and Germany as compared to Italy and the UK (EFSA 2011). Preliminary analyses indicated no significant gender differences in ZEN exposure. Exposure to ZEN was found to be higher in young consumers (infants, toddlers, and children) as compared to adults and elderly, which was explained by higher food intake per kg b.w. in younger age groups (EFSA 2011). The highest exposure estimates were calculated for the age group of toddlers (12–36 months), in which mean exposure to ZEN across survey studies ranged from 9.3 to 100 ng/kg b.w. per day [based on minimum lower bound (LB) and maximum upper bound (UB) scenarios, respectively], whereas the 95th percentile dietary exposure ranged from 24 to 277 ng/kg b.w. per day (EFSA 2011). In adults, dietary exposure estimates ranged from 2.4 to 29 ng/kg b.w. per day for the average consumer and 4.7–54 ng/kg b.w. per day for high consumers (EFSA 2011). Based on this exposure assessment, EFSA concluded that estimates of chronic dietary exposure to ZEN “based on the available occurrence data are below or in the region of the TDI for all age groups and not a health concern” (EFSA 2011).
However, it needs to be emphasized that there are inherent uncertainties in estimating chronic dietary exposure to ZEN that may lead to an under- or overestimation of ZEN exposure and consequently human risk. The currently available occurrence data, which were mainly from grains and grain-based food, do not cover all foods in which ZEN may be present. For instance, limited data indicate the presence of ZEN in soy and soy-based foods, which may present a significant source of ZEN exposure for vegetarians. Regional differences in ZEN occurrence in food were not considered, as the majority of the occurrence data were obtained from samples collected in Germany, France and Slovakia (EFSA 2011). Use of LB occurrence data obtained by assigning a value of zero to samples below the limit of detection/limit of quantitation (LOD/LOQ) may cause underestimation of exposure/risk, whereas use of UB occurrence data obtained by assigning the numerical value of LOD to values reported as <LOD, and LOQ to values reported as <LOQ overestimates exposure/risk. Special consumer groups such as vegetarians or consumers with high intake of maize products (e.g., patients suffering from celiac disease) are not adequately represented in this assessment. Importantly, modified mycotoxins such as α- and β-zearalenol (α- and β-ZEL), sulfate- and glucose-conjugates of ZEN and ZELs (ZEN-14-Sulf, ZEN-14-Glc, β-ZEL-14-Glc, α-ZEL-14-Glc), which are neither routinely screened for nor regulated by legislation, commonly co-occur with ZEN in a variety of grain-based foodstuffs and may contribute to human exposure to ZEN (De Boevre et al. 2013). Limited analyses of ZEN and ZELs conjugates in raw cereals and cereal-based products suggest that the sum of ZEN modifications present in food may be up to 100 % with respect to ZEN (De Boevre et al. 2013; EFSA 2014a). The presence of α-ZEL and its conjugates presents a particular concern as this ZEN modification exhibits even higher estrogenic activity as parent ZEN (Frizzell et al. 2011; Molina–Molina et al. 2014). Based on the very limited occurrence data and the assumption that modified mycotoxins exhibit a similar toxicity profile and potency as their parent compounds, EFSA added 100 % to the previous ZEN exposure estimates to account for the presence of modified ZEN (EFSA 2014a). Using this pragmatic approach, the UB 95th percentile exposure to the sum of modified and parent ZEN exceeded the TDI in the age group of infants and toddlers by 1.7- and 2.2-fold, respectively (EFSA 2014a). Given that current human exposure estimates for ZEN and its modified forms (Rychlik et al. 2014) are close to or even exceed the TDI, there is a clear need for more comprehensive and refined exposure and toxicity data to reduce some of the uncertainties associated with the current exposure assessment and to provide more information on vulnerable consumer groups.
Human biomonitoring (HBM) is increasingly being recognized as an efficient and cost-effective way of assessing human exposure to food contaminants, including mycotoxins (Choi et al. 2014). HBM utilizing validated biomarkers of exposure covers exposure from all sources, thereby reducing uncertainties related to occurrence and consumption rates. HBM can be used to establish population reference ranges and identify vulnerable consumer groups and individuals with higher exposures. It can demonstrate regional and temporal variability and trends within a population. HBM requires a sufficiently sensitive and validated analytical test method for accurate measurement of a biomarker (e.g., the parent compound and/or a metabolite) that correlates with external dose. For HBM, easily accessible biological matrices such as urine or blood are used, with urine being preferred in field studies for several reasons including the noninvasive sampling and thus higher acceptance by study participants (Solfrizzo et al. 2011). Yet, which biomarker or matrix is most suitable ultimately depends on the toxicokinetic profile of the compound under investigation. Thus, a detailed understanding of the compounds toxicokinetics, including major routes of metabolism and excretion in humans, is needed, particularly for “translation” of HBM biomarker data into daily intake estimates. In view of the limitations of available (dietary/external) exposure data on ZEN and its modified forms, the purpose of this review is to provide an overview of recent studies utilizing HBM to monitor and assess human exposure to ZEN. Considerations are given to animal and human toxicokinetic data relevant to HBM, analytical methods, and available HBM data on urinary biomarkers of ZEN exposure in different cohorts.
Toxicokinetics of ZEN
Absorption, distribution, metabolism and excretion of ZEN
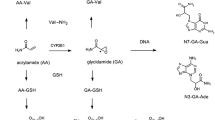
Studies in animals indicate rapid and extensive absorption of ZEN (e.g., 80–85 % in pigs) from the gastrointestinal tract (Biehl et al. 1993). Oral bioavailability is, however, low (<10 % in poultries and rats) due to extensive presystemic metabolism (Shin et al. 2009; Devreese et al. 2015). ZEN is distributed to various tissues including kidney, liver, adipose tissue and estrogen target tissues such as the uterus, testes and ovarian follicles. ZEN undergoes enterohepatic circulation as evidenced by significantly reduced elimination half-lives in bile-cannulated rats and pigs as compared to intact animals (Biehl et al. 1993; Shin et al. 2009). Biotransformation of ZEN occurs primarily via two major pathways: (1) enzymatic reduction in ZEN via 3α- and 3β-hydroxysteroid dehydrogenases (HSDs) to α- and β-zearalenol (α- and β-ZEL), and (2) uridine diphosphate-glucuronosyltransferase (UGT) and sulfotransferase (SULT) dependent conjugation of ZEN and its reduced metabolites with glucuronic acid and sulfate (Fig. 1). A minor metabolic pathway involves cytochrome P450 (CYP) mediated oxidation to produce aliphatic C6/8-hydroxylated and aromatic C13/15-hydroxylated ZEN (Fig. 1). The major route of excretion in rats is via feces (55 vs. 15–20 % in urine) (Fitzpatrick et al. 1988), whereas in pigs ~50 and 7–22 % of the total administered dose were recovered in urine and feces, respectively (Biehl et al. 1993). Urinary excretion occurs in the conjugated and unconjugated forms of ZEN and its reductive metabolites, whereby reported metabolite pattern and excretion rates vary considerably between species (Mirocha et al. 1981), and also between age groups (Danicke et al. 2005; Gambacorta et al. 2013). A comparative study on urinary metabolite pattern in different animal species revealed β-ZEL-14-GlcA as the major urinary excretion product in lactating cow, whereas free and conjugated ZEN accounted for more than 90 % of urinary ZEN in female prepubertal rats (Mirocha et al. 1981). In pigs, conjugated and unconjugated ZEN and α-ZEL are the predominant urinary metabolites (Mirocha et al. 1981; Danicke et al. 2005; Gambacorta et al. 2013), although different ratios between parent ZEN and its reduced metabolite α-ZEL are reported in piglets versus adult pigs, presumably due to higher activity of 3α-hydroxysteroid dehydrogenase in adults (Danicke et al. 2005; Gambacorta et al. 2013).
Urinary biomarkers of ZEN exposure: studies in pigs
In a recent study designed to validate urinary biomarkers of exposure for multiple mycotoxins, groups of piglets (n = 4 per group) were administered a single feed bolus of mixtures of deoxynivalenol (DON), aflatoxin B1 (AFB1), fumonisin B1 (FB1), ochratoxin A (OTA) and ZEN at different dose levels. Concentrations of ZEN in feed were below and up to twofold the guidance values reported in Recommendation 2006/576/EC and corresponded to doses of 0.68, 1.94, 2.38, 5.66 µg ZEN/kg b.w. (Gambacorta et al. 2013). Urine was collected from individual animals the day before dosing (control) and for 24 h after administration of the contaminated diet. Urine samples were subjected to β-glucuronidase/sulphatase treatment to hydrolyze conjugated metabolites, cleanup by immunoaffinity columns and solid-phase extraction, and analyzed by a multi-biomarker LC–MS/MS method which covers ZEN and its reduced metabolites α-ZEL and β-ZEL along with DON, de-epoxy-DON (DOM-1), AFM1, FB1 and OTA. ZEN and α-ZEL were detected and quantified in all postdose urine samples, whereas β-ZEL was only found in piglets given the highest dose of ZEN (Gambacorta et al. 2013). Based on 24-h urinary volume and urinary concentration of each analyte, mean percentage of ingested mycotoxin excreted as biomarker in 24-h urine were calculated. For ZEN, 36.8 % of the administered dose were excreted in urine within 24 h as ZEN and α-ZEL, with 28.4 % excreted as the parent compound ZEN and 8.3 % as α-ZEL (ratio ZEN/α-ZEL = 3:1). Considering the longer urine collection period, it is not surprising that these values are higher than 8-h excretion rates reported in previous studies in which ~15 % of ingested ZEN were excreted within 8 h as ZEN and α-ZEL (Olsen et al. 1985; Zollner et al. 2002). Linear regression analysis revealed a good correlation between ingested ZEN doses and urinary ZEN and α-ZEL excretion in 24 h post-dose urine (linear dose–response correlation coefficients for ZEN and α-ZEL: r = 0.71, p < 0.0001 and r = 0.77, p < 0.0001, respectively) in the piglet study (Gambacorta et al. 2013).
Although no data on urinary excretion rates are available, studies in mature gilts also support a linear relationship between dietary ZEN intake and urinary ZEN and its metabolites over a wide dose range. Gutzwiller et al. (2014) used a commercial ELISA kit for ZEN (with relative cross-reactivity of 42 % for α-ZEL) to determine urine concentrations as “ZEN equivalents” (ZEN plus 42 % of α-ZEL present) in gilts treated with ZEN at daily doses of 0, 5, 10, 20 and 40 µg/kg b.w. for 7 days. In this study, which used dose levels ranging from below the NOEL of 10 µg/kg b.w. in immature gilts to doses exceeding the LOEL of 20 µg/kg b.w., linear dose–response correlation coefficients were ≥0.90 (Gutzwiller et al. 2014).
Using hydrolysis of conjugates by β-glucuronidase/sulphatase, immunoaffinity cleanup and HPLC with fluorescence detection, Danicke et al. (2005) observed linear dose-related increases in the concentrations of ZEN, α-ZEL and β-ZEL in urine (and bile) of gilts given ZEN at 0.06, 1.5, 4.0 and 6.3 µg/kg b.w. (i.e., same dose range as in Gambacorta et al. 2013) for 35 days (Danicke et al. 2005). Consistent with piglet data, urinary concentrations of β-ZEL were low compared to ZEN and α-ZEL. Interestingly, however, 1.3-fold higher concentrations of α-ZEL compared to ZEN were found in urine of mature gilts (Danicke et al. 2005). This contrasts results obtained in piglets, in which the ratio of ZEN/α-ZEL in urine was 3:1 (Gambacorta et al. 2013), and may reflect more extensive metabolism of ZEN to α-ZEL via steroid-inducible 3α-hydroxysteroid dehydrogenase in adult pigs.
Taken together, studies in pigs indicate (1) a linear relationship between ZEN intake and urinary concentrations of ZEN and its reduced metabolites and (2) sufficiently high urinary excretion rates of ZEN and α-ZEL to permit calculation of probable daily intakes (PDI) based on urinary biomarker concentrations. Importantly, however, age-dependent differences in the extent of biotransformation of ZEN may result in different ratios in the excretion of ZEN and α-ZEL. Therefore, relying on a single biomarker may not adequately reflect ZEN exposure in different age groups. From these data in pigs, it appears that monitoring the sum of ZEN and α-ZEL in urine may be most appropriate to derive exposure estimates.
Urinary biomarkers of ZEN exposure: studies in humans
There is very limited data on toxicokinetics of ZEN in humans. Warth et al. (2013) monitored urinary excretion of ZEN in a single male volunteer exposed to ZEN at a dose of 0.167 µg/kg b.w. (i.e., 66 % of the TDI of 0.25 μg/kg b.w.) via a naturally contaminated diet for four consecutive days (Warth et al. 2013a). Following enzymatic hydrolysis, the concentration of total ZEN (as the sum of conjugated and unconjugated ZEN) was measured in 24-h urine samples collected before, during and after ZEN exposure. ZEN was reported to be present mainly in the form of its glucuronide (ZEN-14-GlcA). During ZEN exposure, concentrations of total ZEN in 24-h urine samples ranged from 0.30–0.59 ng/ml (mean 0.39 ng/ml), which corresponded to an excretion rate of 7.0–13.2 % (mean 9.4 %) of the administered dose within 24 h. However, α- and β-ZEL were not covered by this analysis. In an older study, excretion of ZEN and its metabolites was analyzed in urine of an adult male volunteer given a single (high!) oral dose of 100 mg ZEN (Mirocha et al. 1981). HPLC with UV and fluorescence detection was used to quantify ZEN and α- and β-ZEL with and without enzymatic hydrolysis. The glucuronides of parent ZEN and α-ZEL were reported to be the major excretion products that were present at about equal concentrations followed by β-ZEL. From the concentrations of urinary metabolites (ZEN, α- and β-ZEL), Metzler et al. (2010) estimated that 10–20 % of the administered dose (100 mg) were excreted within 24 h.
From these studies in single individuals, it appears that urinary excretion of ZEN, α-ZEL and possibly also β-ZEL is sufficiently high to facilitate biomonitoring studies in humans. Results of cohort studies, reviewed in the next section, support this view. However, there is a clear need to further validate ZEN and its metabolites as urinary biomarkers of ZEN exposure by determining urinary excretion rates—including dose–response relationships and potential gender- and age-related differences—in a larger number of individuals.
HBM studies
Analytical methods
Various analytical approaches have been used for the analysis of ZEN and its metabolites in human urine. Most authors employ LC–MS/MS methods—often designed as multi-biomarker methods—but there are also studies which rely on immunoassays or high-performance liquid chromatography (HPLC) with fluorescence detection, reviewed by (Warth et al. 2013b) and references therein. LC–MS/MS-based methods can be broadly grouped in two categories: a) direct “dilute and shoot” approaches without prior enzymatic hydrolysis, which minimize sample cleanup and utilize commercial reference standards for ZEN and its major urinary metabolites (e.g., α-ZEL, β-ZEL) and in house synthesized standards, for glucuronide conjugates (e.g., ZEN-14-GlcA, α-ZEL-14-GlcA, β-ZEL-14-GlcA), and b) indirect approaches which determine total ZEN (and α- and β-ZEL) after enzymatic deconjugation with β-glucuronidase/sulphatase and subsequent sample cleanup with enrichment of analytes by immunoaffinity and/or solid-phase extraction. Indirect analytical methods using enzymatic hydrolysis and immunoaffinity cleanup are generally more sensitive than the “dilute-and-shoot” approaches which results in a higher percentages of positive samples in HBM studies as exemplified by rather different frequencies of detecting ZEN biomarkers in European cohorts (Tables 1, 2). Analysis of urines of a South African cohort analyzed by both approaches also demonstrate significant differences between both methods, with 2 versus 98 % of samples tested positive using a direct versus an indirect approach (Shephard et al. 2013). These results are not surprising considering the big difference of the amount of urine equivalent injected in the LC–MS/MS apparatus by using the two approaches. For the South African cohort, the amounts of urine equivalent injected were 0.5 and 300 µl for direct and indirect approaches, respectively. Moreover, the indirect method involves β-glucuronidase/sulphatase hydrolysis that produces an increase in the parent toxin concentrations, thus improving the measurement of low concentrations of mycotoxin biomarkers.
Biomonitoring studies using direct approaches without enzymatic hydrolysis to determine urinary ZEN and its metabolites
There are several studies utilizing direct LC–MS/MS-based urinary multi-biomarker approaches to assess exposure of the most common mycotoxins, including ZEN, in various populations around the world (Table 1). Gerding et al. (2014, 2015) employed a “dilute and shoot” LC–MS/MS method capable of monitoring 23 analytes (mycotoxins and their metabolites) to investigate mycotoxin exposure patterns in cohorts from Germany, Bangladesh and Haiti. In 101 urine samples from German adults, DON-GlcA was found to be the most abundant urinary mycotoxin metabolite, which was present in 82 % of all urine samples (Gerding et al. 2014, 2015). In contrast, ZEN was detected in only 4 % of urine samples in the form of ZEN-14-GlcA, but the concentration in urine was below the limit of quantitation (LOQ). The parent compound ZEN and its metabolites α- and β-ZEL and α-and β-ZEL-14-GlcA were not detected in any of these urines (Gerding et al. 2014). In a subsequent study, neither ZEN nor its metabolites were found at detectable levels in urine samples collected from healthy adults of another German cohort (n = 50) and in Bangladeshi adults (n = 95). In the same study (Gerding et al. 2014, 2015), 4 out of 142 urine samples (3 %) provided by healthy adults and children from Haiti contained α-ZEL, with a mean concentration of 1.46 ± 1.02 ng/ml. Based on the urinary excretion rate of 8.3 % for α-ZEL in pigs (Gambacorta et al. 2013) and assuming a mean daily urine excretion of 1.5 L and an average body weight of 60 kg, Gerding et al. (2015) calculated mean and maximum probable daily intakes (PDI) of ZEN of 0.03 and 0.75 µg/kg b.w., respectively. Based on these exposure estimates, it was concluded that exposure to ZEN exceeded the TDI of 0.2 µg/kg b.w. previously set by the Scientific Committee on Food (SCF) (SCF 2000) in 2 out of 142 volunteers from Haiti.
Huybrechts et al. (2014) developed a direct LC–MS/MS method capable of measuring 32 urinary mycotoxin biomarkers to assess human mycotoxin exposure (Huybrechts et al. 2014). In contrast to DON, OTA, citrinin and their metabolites, which were frequently detected in urine samples collected from 29 Belgian adult volunteers, no ZEN or any of its metabolites were found to be present at detectable levels (Huybrechts et al. 2014). These findings were essentially confirmed in a larger study cohort of 239 adults and 155 children from Belgium (Heyndrickx et al. 2015). No ZEN or its metabolites were detected in children urines, and only one and two out of 239 samples collected from Belgium adults tested positive for α-ZEN and β-ZEL-14-GlcA, respectively. Noteworthy, their α-ZEN urine concentration was about twofold higher than the maximum concentration (5.0 ng/ml vs. 2.49 ng/ml) reported for Haiti (Huybrechts et al. 2014; Gerding et al. 2015). Based on the assumptions and exposure estimates made by Gerding et al. (2015), the ZEN exposure of this Belgian individual would be expected to be above the TDI.
In urines of 220 infants (age 1.5–4.5 years) from regions of Cameroon with high mycotoxin contamination levels of staple food (i.e., maize, peanuts, cassava), ZEN and its metabolites were found in only 3.6 % (ZEN), 4 % (α-ZEL) and 8 % (β-ZEL) of all samples with relatively high maximum concentrations using a LC–MS/MS method with prior liquid–liquid extraction and SAX SPE cleanup (Njumbe Ediage et al. 2013). Although ZEN-14-GlcA seems to be the main urinary excretion product of ZEN, it was not detected in any of the samples probably due to the high value of LOD (3.65 ng/ml) of the method used. There was a co-occurrence of ZEN and β-ZOL in only one sample and no co-occurrence of α-and β-ZEL in any of the positive samples. It was suggested that interindividual variability in ZEN metabolism may account for these unexpected results (Njumbe Ediage et al. 2013). The occurrence rate of ZEN and its metabolites was reported to be highest in children from the northwest region, where maize represents the most common main dietary staple (Njumbe Ediage et al. 2013).
In Cameroonian adults, 5 % of urine samples obtained from HIV-positive individuals (n = 145) were reported to contain ZEN, ZEN-14-GlcA or α-ZEL. However, neither ZEN nor its metabolites were detected in urine of HIV sero-negative individuals (n = 30) (Abia et al. 2013). Warth et al. (2014) utilized a “dilute and shoot” LC–MS/MS-based urinary multi-biomarker approach to monitor exposure to major mycotoxins (fumonisins, trichothecenes, AFB1, OTA, and ZEN) in the Bangkok metropolitan area and surrounding provinces (Warth et al. 2014). Based on the absence of ZEN, ZEN-14-GlcA, and α- and β-ZEL in urine of 60 healthy volunteers, the authors concluded that exposure to ZEN is unlikely to present a significant risk in this region (Warth et al. 2014).
Biomonitoring studies using indirect approaches with enzymatic hydrolysis for analysis of urinary ZEN, α- and β-ZEL
Results of human biomonitoring studies utilizing enzymatic cleavage of phase-II metabolites by β-glucuronidase/arylsulphatase treatment and subsequent sample cleanup with enrichment of analytes prior to analysis of urinary ZEN and α-and β-ZEL are summarized in Table 2.
To investigate possible additional workplace exposure to mycotoxins, Föllmann et al. (2016) employed a targeted LC–MS/MS method to determine urinary concentrations of biomarkers including ZEN and α- and β-ZEL in a small German cohort consisting of 17 mill workers and 13 aged-matched controls with dietary exposure only (Föllmann et al. 2016). ZEN was detected in all hydrolyzed urine samples at levels ranging from 0.007–0.09 ng/ml in the control group and 0.004–0.1 ng/ml in the mill workers. No significant differences in urinary ZEN levels between both cohorts were observed. The metabolites α- and β-ZEL were present in 46 and 23 % of urines from the control, respectively, and less frequently in the mill worker urines, and in both groups at lower concentrations than ZEN.
In a study designed to assess multi-mycotoxin exposure in 52 male and female residents of Southern Italy based on urinary biomarkers, ZEN was detected in all hydrolyzed samples at mean levels of 0.057 ng/ml (max. 0.12 ng/ml). The ZEN metabolites α- and β-ZEL were found to be present in most urines (100, 98 %) at mean levels of 0.077 ng/ml (max. 0.176 ng/mL) and 0.09 ng/ml (max. 0.135 ng/ml), respectively (Solfrizzo et al. 2014). No gender-related differences in the urinary concentrations of ZEN and α- and β-ZEL and their ratios were evident (Fig. 2). Using a mean 24-h urinary excretion rate of 36.8 % for total ZEN in pigs (Gambacorta et al. 2013), a mean human body weight of 60 kg and mean 24-h human urine volume of 1.5 L, exposure to ZEN was estimated for each volunteer based on the urinary concentration of total ZEN (as the sum of ZEN + α-ZEL + β-ZEL). Based on these calculations, the mean and maximum probable daily intakes (PDI) were 0.015 and 0.029 µg/kg b.w., respectively, and thus well below the current TDI of 0.25 µg/kg b.w. (Solfrizzo et al. 2014). By using a mean excretion rate of 9.4 % for total ZEN (Warth et al. 2013a), mean and maximum PDI would be about three times higher, i.e., 0.059 and 0.113 µg/kg b.w., respectively.
The same analytical method was used to determine ZEN, α- and β-ZEL in urine samples from 53 female farmers in the former Transkei, South Africa (Shephard et al. 2013), and from 252 adults participating in the Swedish national dietary survey (Wallin et al. 2015). The occurrence rate and mean ZEN biomarker levels in the Swedish cohort were found to be somewhat lower than in volunteers from Southern Italy (Wallin et al. 2015). In contrast to the Italian cohort, however, concentrations of urinary biomarkers of ZEN exposure were reported to be significantly higher (p < 0.001) in women compared to men (Wallin et al. 2015). In urines obtained from female Transkei farmers (n = 53), ZEN, α-ZEL and β-ZEL were detected in 100, 92 and 75 % of the samples, and at mean levels significantly higher than in Europe (Shephard et al. 2013). Maximum levels for ZEN, α- and β-ZEL detected in urines from this region were 3.15, 3.72 and 5.94 ng/ml, respectively, and thus exceeded maximum concentrations in the European cohorts by more than an order of magnitude (Shephard et al. 2013) (Fig. 3). Based on the assumptions made by Solfrizzo et al. (2014), it is expected that probable daily intakes of individuals from this study cohort may exceed the TDI of 0.25 µg/kg b.w.
Conclusions and recommendations
At present, biomonitoring for ZEN is still in its infancy. Multi-mycotoxin direct approaches (dilute and shoot) designed to monitor human exposure to a wide range of mycotoxins are generally less sensitive for ZEN and its metabolites than indirect analytical methods using enzymatic hydrolysis and immunoaffinity cleanup. Consequently, “direct” methods without hydrolysis and enrichment of analytes typically detect only high urinary ZEN concentrations corresponding to exposure levels at or above the TDI, but may not be suitable for estimating typical exposures. Biomonitoring of ZEN in different European cohorts using indirect analytical approaches indicates some (small) geographical differences in the mean levels of urinary ZEN and its major urinary metabolites, with somewhat higher concentrations in Southern Italy as compared to Sweden and Germany. These findings are consistent with slightly lower levels of exposure in Finland, Sweden and Germany as compared to Italy based on ZEN occurrence in certain food commodities and food consumption data (EFSA 2011). Compared to European study cohorts, urinary ZEN biomarker concentrations in South Africa, where maize is a major staple food, were significantly higher, reflecting the higher dietary intake of ZEN. Thus, urinary excretion of ZEN and its metabolites appears to be suitable indicators of human exposure to ZEN. Very preliminary approaches to translate urinary ZEN concentrations into probable daily intakes suggest exposure levels below the TDI of 0.25 µg/kg b.w. in European study cohorts, whereas exposure of some individuals from Haiti and in African countries may exceed the TDI. However, it needs to be emphasized that exposure estimates based on urinary ZEN are still hampered by a number of uncertainties, most importantly the absence of reliable human data on urinary excretion rates of ZEN and its metabolites, including dose–response and impact of age and gender. Although data from an Italian study cohort indicate no gender-related differences in the levels and ratios of ZEN relative to α- and β-ZEL (Fig. 2), it remains to be established if differences between individuals reflect differences in dietary intake of ZEN or interindividual variation in 3α-hydroxysteroid dehydrogenase-dependent biotransformation of ZEN. Based on the presently available data, it appears that relying on a single biomarker (e.g. ZEN only) may not accurately reflect human exposure to ZEN. Biomonitoring approaches should therefore determine both the parent compound ZEN and α- and β-ZEL after enzymatic hydrolysis of their respective phase-II conjugates. This seems to be particularly important as α- and β-ZEL-derived mycotoxins commonly co-occur with ZEN in food and contribute to human exposure (EFSA 2014b). Thus, measuring ZEN and α- and β-ZEL would provide a more comprehensive picture as it also covers exposure to modified ZEN mycotoxins. Bearing in mind that both α- and β-ZEL are endocrine-active compounds, consideration should be given to expressing exposure to ZEN and its modified forms as estrogen equivalents to further refine risk assessment of ZEN.
In conclusion, data on urinary excretion in (one) human and findings in pigs receiving different doses of ZEN support biomonitoring as a viable approach to assess human exposure to ZEN. However, further validation studies are needed to be able to translate urinary biomarker concentrations into exposure estimates.
Abbreviations
- AFB1 :
-
Aflatoxin B1
- DON:
-
Deoxynivalenol
- FB1 :
-
Fumonisin B1
- HBM:
-
Human biomonitoring
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantitation
- OTA:
-
Ochratoxin A
- PDI:
-
Probable daily intake
- TDI:
-
Tolerable daily intake
- ZEN:
-
Zearalenol
- ZEL:
-
Zearalenol
References
Abia WA, Warth B, Sulyok M, Krska R, Tchana A, Njobeh PB, Turner PC, Kouanfack C, Eyongetah M, Dutton M, Moundipa PF (2013) Bio-monitoring of mycotoxin exposure in Cameroon using a urinary multi-biomarker approach. Food Chem Toxicol 62:927–934
Biehl ML, Prelusky DB, Koritz GD, Hartin KE, Buck WB, Trenholm HL (1993) Biliary excretion and enterohepatic cycling of zearalenone in immature pigs. Toxicol Appl Pharmacol 121:152–159
Choi J, Mørck TA, Polcher A, Knudsen LE, Joas A, (2014) Review of the state of the art of human biomonitoring for chemical substances and its application to human exposure assessment for food safety. In: EFSA (ed) EFSA supporting publication 2015: EN-724, p 321
Danicke S, Brussow KP, Valenta H, Ueberschar KH, Tiemann U, Schollenberger M (2005) On the effects of graded levels of Fusarium toxin contaminated wheat in diets for gilts on feed intake, growth performance and metabolism of deoxynivalenol and zearalenone. Mol Nutr Food Res 49:932–943
De Boevre M, Jacxsens L, Lachat C, Eeckhout M, Di Mavungu JD, Audenaert K, Maene P, Haesaert G, Kolsteren P, De Meulenaer B, De Saeger S (2013) Human exposure to mycotoxins and their masked forms through cereal-based foods in Belgium. Toxicol Lett 218:281–292
Devreese M, Antonissen G, Broekaert N, De Baere S, Vanhaecke L, De Backer P, Croubels S (2015) Comparative toxicokinetics, absolute oral bioavailability, and biotransformation of zearalenone in different poultry species. J Agric Food Chem 63:5092–5098
EFSA (2011) Scientific opinion on the risks for public health related to the presence of zearalenone in food. EFSA J 9:2197
EFSA (2014a) Evaluation of the increase of risk for public health related to a possible temporary derogation from the maximum level of deoxynivalenol, zearalenone and fumonisins for maize and maize products. EFSA J 12:3699
EFSA (2014b) Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J 12:3916
Fitzpatrick DW, Arbuckle LD, Hassen AM (1988) Zearalenone metabolism and excretion in the rat: effect of different doses. J Environ Sci Health B 23:343–354
Föllmann W, Ali N, Blaszkewicz M, Degen G, (2016) Biomonitoring of mycotoxins in urine: pilot study in mill workers. J Toxicol Environ Health, Part A in press
Frizzell C, Ndossi D, Verhaegen S, Dahl E, Eriksen G, Sorlie M, Ropstad E, Muller M, Elliott CT, Connolly L (2011) Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol Lett 206:210–217
Gambacorta I, Solfrizzo M, Visconti A, Powers S, Cossalter A, Pinton P, Oswald IP (2013) Validation study on urinary biomarkers of exposure for aflatoxin B1, ochratoxin A, fumonisin B1, deoxynivalenol and zearalenone in piglets. World Mycotoxin J 6:299–308
Gerding J, Cramer B, Humpf HU (2014) Determination of mycotoxin exposure in Germany using an LC-MS/MS multibiomarker approach. Mol Nutr Food Res 58:2358–2368
Gerding J, Ali N, Schwartzbord J, Cramer B, Brown DL, Degen GH, Humpf HU (2015) A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany, and Haiti using a rapid and sensitive LC–MS/MS approach. Mycotoxin Res 31:127–136
Gutzwiller A, Gafner JL, Silacci P (2014) Urinary zearalenone measured with ELISA as a biomarker of zearalenone exposure in pigs. Mycotoxin Res 30:187–190
Heyndrickx E, Sioen I, Huybrechts B, Callebaut A, De Henauw S, De Saeger S (2015) Human biomonitoring of multiple mycotoxins in the Belgian population: results of the BIOMYCO study. Environ Int 84:82–89
Huybrechts B, Martins JC, Debongnie P, Uhlig S, Callebaut A (2014) Fast and sensitive LC–MS/MS method measuring human mycotoxin exposure using biomarkers in urine. Arch Toxicol 89:1993–2005
Metzler M, Pfeiffer E, Hildebrand A (2010) Zearalenone and its metabolites as endocrine disrupting chemicals (EDC). World Mycotoxin J 3:385–401
Mirocha CJ, Pathre SV, Robison TS (1981) Comparative metabolism of zearalenone and transmission into bovine milk. Food Cosmet Toxicol 19:25–30
Molina-Molina JM, Real M, Jimenez-Diaz I, Belhassen H, Hedhili A, Torne P, Fernandez MF, Olea N (2014) Assessment of estrogenic and anti-androgenic activities of the mycotoxin zearalenone and its metabolites using in vitro receptor-specific bioassays. Food Chem Toxicol 74:233–239
Njumbe Ediage E, Diana Di Mavungu J, Song S, Sioen I, De Saeger S (2013) Multimycotoxin analysis in urines to assess infant exposure: a case study in Cameroon. Environ Int 57–58:50–59
Olsen ME, Pettersson HI, Sandholm KA, Kiessling KH (1985) Quantitative liquid chromatographic method using fluorescence detection for determining zearalenone and its metabolites in blood plasma and urine. J Assoc Off Anal Chem 68:632–635
Rychlik M, Humpf HU, Marko D, Danicke S, Mally A, Berthiller F, Klaffke H, Lorenz N (2014) Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res 30:197–205
SCF (2000) Opinion of the scientific committee on food on fusarium toxins part 2: zearalenone (ZEA) (expressed on 22 June, 2000). http://ec.europa.eu/food/safety/docs/cs_contaminants_catalogue_out65_en.pdf
Shephard GS, Burger HM, Gambacorta L, Gong YY, Krska R, Rheeder JP, Solfrizzo M, Srey C, Sulyok M, Visconti A, Warth B, van der Westhuizen L (2013) Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food Chem Toxicol 62:217–225
Shin BS, Hong SH, Bulitta JB, Hwang SW, Kim HJ, Lee JB, Yang SD, Kim JE, Yoon HS, Kim DJ, Yoo SD (2009) Disposition, oral bioavailability, and tissue distribution of zearalenone in rats at various dose levels. J Toxicol Environ Health A 72:1406–1411
Solfrizzo M, Gambacorta L, Lattanzio VM, Powers S, Visconti A (2011) Simultaneous LC–MS/MS determination of aflatoxin M1, ochratoxin A, deoxynivalenol, de-epoxydeoxynivalenol, alpha and beta-zearalenols and fumonisin B1 in urine as a multi-biomarker method to assess exposure to mycotoxins. Anal Bioanal Chem 401:2831–2841
Solfrizzo M, Gambacorta L, Visconti A (2014) Assessment of multi-mycotoxin exposure in southern Italy by urinary multi-biomarker determination. Toxins (Basel) 6:523–538
Wallin S, Gambacorta L, Kotova N, Lemming EW, Nalsen C, Solfrizzo M, Olsen M (2015) Biomonitoring of concurrent mycotoxin exposure among adults in Sweden through urinary multi-biomarker analysis. Food Chem Toxicol 83:133–139
Warth B, Sulyok M, Berthiller F, Schuhmacher R, Krska R (2013a) New insights into the human metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone. Toxicol Lett 220:88–94
Warth B, Sulyok M, Krska R (2013b) LC–MS/MS-based multibiomarker approaches for the assessment of human exposure to mycotoxins. Anal Bioanal Chem 405:5687–5695
Warth B, Petchkongkaew A, Sulyok M, Krska R (2014) Utilising an LC–MS/MS–based multi-biomarker approach to assess mycotoxin exposure in the Bangkok metropolitan area and surrounding provinces. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 31:2040–2046
Zollner P, Jodlbauer J, Kleinova M, Kahlbacher H, Kuhn T, Hochsteiner W, Lindner W (2002) Concentration levels of zearalenone and its metabolites in urine, muscle tissue, and liver samples of pigs fed with mycotoxin-contaminated oats. J Agric Food Chem 50:2494–2501
Acknowledgments
The authors would like to thank Lucia Gambacorta for analyses with indirect method as well as Nicole Lorenz and all members of the Mycotoxin Panel of the Committee of Contaminants and other Undesirable Substances in the Food Chain of the Federal Institute for Risk Assessment (BfR) for constructive discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Mally, A., Solfrizzo, M. & Degen, G.H. Biomonitoring of the mycotoxin Zearalenone: current state-of-the art and application to human exposure assessment. Arch Toxicol 90, 1281–1292 (2016). https://doi.org/10.1007/s00204-016-1704-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1704-0