Abstract
Lung cancers with an epidermal growth factor receptor (EGFR) gene mutation account for ~40 % of adenocarcinomas in East Asians and ~15 % of those in Caucasians and African Americans, which makes them one of the most common molecularly defined lung cancer subsets. The discriminative clinical and pathological features of lung cancers with EGFR mutations have been intensively studied, and the predictive role of an EGFR mutation for treatment with EGFR tyrosine kinase inhibitors (EGFR-TKIs) is well established. However, controversial issues remain regarding the clinical and therapeutic implications of EGFR mutations in lung cancers. These include the prognostic impact of the EGFR mutation, its predictive implication for successful treatment with anticancer agents other than EGFR-TKIs, appropriate cytotoxic agents for lung cancers with this mutation, and the chemosensitivity of EGFR-mutation-positive lung cancers after acquisition of resistance to EGFR-TKIs. In this review, we discuss these unanswered but important questions, referring to in vitro studies, basic research, retrospective analyses, and the results of phase III clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ten years have passed since three research groups in the USA reported the presence of epidermal growth factor receptor (EGFR) gene mutations in lung cancers (Lynch et al. 2004; Paez et al. 2004; Pao et al. 2004). The EGFR mutation is of both clinical and research interests because its presence strongly predicts the efficacy of the EGFR tyrosine kinase inhibitors (TKIs) that was applied in clinic a few years earlier. The role of EGFR mutations as a strong predictive biomarker of the response to EGFR-TKI treatment was examined in retrospective analyses (as summarized in Mitsudomi and Yatabe 2007) and finally confirmed by the biomarker analyses of the Iressa Pan-Asian Study (IPASS) trial (Fukuoka et al. 2011; Mok et al. 2009). This dramatic treatment effect by EGFR-TKIs reflects the fact that the proliferation and survival of lung cancers with the EGFR mutation solely depend on the aberrant signaling originating from this mutation. This reliance on a single gene is referred to as “oncogene addiction,” and it forms the basis for the clinically impressive results of EGFR-TKI therapy for lung cancers (Weinstein 2002). Moreover, researchers and clinicians have identified many other aberrations in proto-oncogenes leading to oncogene addiction in lung cancers. These include translocations in anaplastic lymphoma kinase (ALK), ROS1, and RET; mutations in ERBB2, BRAF, and mitogen-activated protein kinase kinase 1 (MEK 1); the amplification of MET in lung adenocarcinomas; mutations in discoidin domain receptor tyrosine kinase 2 (DDR2), phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA), and EGFR vIII; and aberrations in fibroblast growth factor receptor (FGFR) family members in lung squamous cell carcinomas (as summarized in Suda and Mitsudomi 2014). However, lung cancers with EGFR mutations are still the largest subset of molecularly defined lung cancers, accounting for ~40 % of lung adenocarcinomas in East Asians and ~15 % of those in Caucasians and African Americans (Suda and Mitsudomi 2015).
During the past 10 years, clinicians and researchers have identified many of the characteristics and features of lung cancers with EGFR mutations. These are summarized in Table 1. However, two fundamental questions remain regarding these tumors, and they are discussed controversially among clinicians and researchers: (1) What is the prognostic impact of the EGFR mutation? (2) What is the predictive role of the EGFR mutation regarding tumor response to cytotoxic agents? In this review, we examine the answers that have emerged thus far and summarize the information from the accumulated studies on lung cancers with EGFR mutations.
EGFR and its activating mutations: background
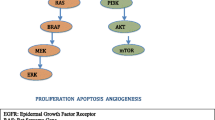
EGFR is a transmembrane receptor tyrosine kinase. Upon binding to its ligands (EGF, TGF-α, amphiregulin, etc.), EGFR forms either homodimers or heterodimers with the other ERBB family members (ERBB2, ERBB3, or ERBB4) (Hynes and Lane 2005), which stimulates intrinsic receptor tyrosine kinase activity and triggers the autophosphorylation of specific tyrosine residues within the cytoplasmic regulatory domains of these enzymes. The phosphorylated tyrosine residues activate several downstream signaling pathways, including mitogen-activated protein kinase (MAPK) pathway, phosphatidylinositol 3-kinase (PI3K)⁄AKT pathway, and the signal transducer and activator of transcription (STAT) pathway (Fig. 1). These pathways promote cell proliferation, migration and metastasis, evasion from apoptosis, and angiogenesis, all of which are associated with cancer phenotypes.
Structure and mechanism of activation of the EGFR. The EGFR protein consists of extracellular, transmembrane, tyrosine kinase, and regulatory domains. Conformational changes in the EGFR occur when a specific ligand binds to the extracellular domain, resulting in the formation of either homodimers or heterodimers with other ERBB family members (ERBB2, ERBB3, or ERBB4). During this process, the respective kinase domains dimerize asymmetrically, in a tail-to-head orientation, which stimulates the intrinsic tyrosine kinase activity of the receptors and triggers autophosphorylation of specific tyrosine residues within the cytoplasmic regulatory domains. These phosphorylated tyrosine residues serve as specific binding sites for several adaptor proteins, inducing proliferative or antiapoptotic signaling pathways, such as those of MAPK, phosphatidylinositol 3-kinase (PI3K)⁄AKT, and STAT
EGFR mutations in lung cancers usually occur in the first four exons of the tyrosine kinase domain (exons 18–21) and induce the ligand-independent activation of EGFR. The most common EGFR mutations are exon 19 deletion mutations and exon 21 L858R point mutations, accounting for >90 % of all EGFR mutations. Lung cancers with EGFR mutations are highly responsive to EGFR-TKIs, and the type of mutation correlates with the sensitivity of the lung tumors to the various types of these inhibitors (Table 1). Accordingly, patients whose lung cancers have EGFR mutations have a significantly prolonged overall survival (OS) after treatment with EGFR-TKIs compared with patients whose lung tumors are negative for EGFR mutations (Takano et al. 2008).
Prognostic and predictive biomarkers
Prior to the discussion of the prognostic impact of EGFR mutations and the predictive role of these mutations for responsiveness to cytotoxic agents, the terms “prognostic” and “predictive” should be appropriately defined. Stated simply, a predictive biomarker identifies patients who will or will not respond effectively to a certain drug, while a prognostic biomarker identifies patients who have a favorable or poor prognosis irrespective of treatment. It is often difficult to determine whether a biomarker is predictive or prognostic. For example, if a subgroup of patients with biomarker A lives longer than a control group after treatment with drug B, it is not clear whether biomarker A is a predictive biomarker for drug B or if biomarker A merely defines those patients with a favorable prognosis.
The interrelationship between EGFR mutations and clinicopathological prognostic/predictive factors
An additional difficulty in precisely discussing the prognostic or predictive roles of EGFR mutations is the confounding relationship between these mutations and other prognostic/predictive factors (or candidates thereof). An example is smoking status. Smoking status is a well-known poor prognostic factor and probably a poor predictive factor in the treatment for lung cancers (Cuyun Carter et al. 2014). Because the incidence of EGFR mutations in lung cancers is inversely correlated with smoking status (Table 1), the influence of smoking must be excluded in analyses of the prognostic or predictive roles of EGFR mutations. Similarly, sex, which is closely related to smoking status, especially in Asian countries, is another example of a clinical prognostic and predictive factor (Cuyun Carter et al. 2014) that correlates with the presence of EGFR mutations.
Histological subtypes, as defined by the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification, also influence analyses of the prognostic role of EGFR mutations. Several retrospective studies found that the prognosis of patients with surgically resected lung adenocarcinomas with solid or micropapillary subtypes is poorer than that of patients with lepidic, papillary, or acinar tumor subtypes (as discussed in Suda et al. 2014). The relationship between the presence of EGFR mutations and histological subtypes has been intensively investigated by several groups (Chen et al. 2014; Hu et al. 2014; Jie et al. 2014; Nakamura et al. 2014; Shim et al. 2011; Song et al. 2013; Sun et al. 2012, 2014; Yanagawa et al. 2014; Yoshizawa et al. 2013). Their findings are summarized in Fig. 2, which shows that the percentages of histological subtypes of lung cancers with EGFR mutations are different from those of lung cancers without these mutations.
Correlation between EGFR mutation status and histological subtypes according to the IASLC/ATS/ERS lung adenocarcinoma classification (East Asian population). Histological subtypes of lung adenocarcinomas with EGFR mutations mostly include those with a better prognosis ones, such as lepidic-, papillary-, and acinar-predominant adenocarcinomas, and fewer predominantly solid adenocarcinomas and invasive mucinous adenocarcinomas. Data are from (Chen et al. 2014; Hu et al. 2014; Jie et al. 2014; Nakamura et al. 2014; Shim et al. 2011; Song et al. 2013; Sun et al. 2012, 2014; Yanagawa et al. 2014; Yoshizawa et al. 2013)
Thus, in interpreting results regarding the prognostic or predictive role of EGFR mutation status, the influence of these and possibly other closely related factors must be kept in mind.
Prognostic role of EGFR mutations
Many studies have assessed the prognostic role of EGFR mutations in lung cancers, with conflicting results (D’Angelo et al. 2012; Izar et al. 2013; Janjigian et al. 2011; Kim et al. 2013; Kobayashi et al. 2008; Kosaka et al. 2009; Lim et al. 2007; Lin et al. 2014; Liu et al. 2010, 2014; Marks et al. 2008; Sonobe et al. 2007). Most have focused on OS, and some included patients treated with EGFR-TKIs. However, in studies that evaluated OS, the true prognostic significance of the EGFR mutation could not be determined because, as described above, it is virtually impossible to distinguish whether EGFR mutation status is an inherent prognostic factor or a predictive factor of treatment.
The true prognostic implication of a biomarker can be evaluated through a comparison of patient groups, classified by the biomarker, without any treatment (i.e., following the so-called natural history of the disease). Since this type of analysis is ethically unacceptable in cases of malignant disease, the most reasonable method to evaluate the prognostic implication of a certain biomarker is to compare the recurrence-free survival (RFS) rates of patients who have undergone complete tumor resection, preferably without postsurgical adjuvant chemotherapy (Suda et al. 2012b).
One such study was recently carried out in Taiwan. Lin et al. analyzed the RFS rates of 163 patients with pathological stage I, surgically resected lung adenocarcinoma. The tumor size was <2 cm in its maximal dimension, and none of the patients had received adjuvant chemotherapy. The results showed that the presence of an EGFR mutation was not associated with RFS (p = 0.286), while elevated preoperative serum carcinoembryonic antigen levels, the presence of visceral pleural surface invasion, histological differentiation, and a TP53 mutation were significant risk factors for relapse (Lin et al. 2014). Similar results have been reported from Japan (Kobayashi et al. 2008), Korea (Kim et al. 2013), and China (Liu et al. 2014), although some of the included patients had received adjuvant chemotherapy. These studies suggested that the EGFR mutation is frequently associated with other positive prognostic factors, non- or mild-smoking status, female sex, earlier disease stage, and a lepidic tumor pattern (indicative of less invasiveness) and is not an independent prognostic factor.
Conflicting results were obtained by Izar et al. (2013) in their retrospective analysis of 307 patients with completely resected stage I non-small-cell lung cancers (NSCLCs) not treated with adjuvant therapy. In that cohort, Caucasian patients accounted for >90 % of the cases. The authors found that the median RFS in the group negative for EGFR mutations was 7.0 years compared with 8.83 years in the EGFR-mutant-positive group (p = 0.0085). In a multivariate analysis, the presence of the EGFR mutation [hazard ratio (HR) 0.326, p = 0.026] and tumor size (HR 1.37, p = 0.04) was a significant prognostic factor. The discordance between this study and those described above can be explained by the fact that Izar et al. included patients with non-adenocarcinoma NSCLCs in their analyses. However, it may also possible that the prognostic implication of the EGFR mutation differs between different ethnic and racial groups.
Predictive molecular biomarkers for cytotoxic agents in lung cancers
Before discussing the predictive role of the EGFR mutation for tumor responsiveness to cytotoxic agents, we summarize what is known about well-known predictive molecular biomarkers or their candidates (Table 2). Although none of these biomarkers has been generally accepted, some are being evaluated in clinical trials. The rationale for studying molecular biomarkers is that they may be involved in resistance or, conversely, confer sensitivity to certain cytotoxic agents, as described below.
Because many cytotoxic agents kill cancer cells via DNA damage, DNA repair genes and specifically their expression levels are candidate predictive biomarkers for the efficacy of cytotoxic agents. The high-level expression of DNA repair genes such as the excision repair cross-complementation group 1 (ERCC1), breast cancer 1 (BRCA1), and the MutS homologue 2 (MSH2) has been suggested to protect cancer cells from cytotoxic agents that induce DNA damage, such as those that are platinum-based, resulting in resistance to these drugs. [Note that the currently available anti-ERCC1 antibodies did not specifically detect the unique functional ERCC1 isoform (Friboulet et al. 2013)]. The expression levels of the target genes of cytotoxic agents are a second group of candidate predictive biomarkers. Higher expression of a target gene implies the need for higher concentrations of cytotoxic agents, which may lead to drug resistance. The third group of candidate biomarkers are drug-degrading enzymes (again, specifically their expression levels), such as dihydropyrimidine dehydrogenase (DPD), which reduces the effect of 5-fluorouracil (5-FU). Membrane transporters or drug efflux pumps can increase the sensitivity or resistance, respectively, to cytotoxic agents that are substrates of these molecules. For example, folate receptor alpha (FRA) transports folates and antifolates into cells; therefore, its higher expression may induce the greater efficacy of pemetrexed (antifolates). In the case of drug efflux pumps such as ABC transporters, their high-level expression leads to lower intracellular concentrations of their substrate drugs and, accordingly, resistance to them. The predictive biomarkers commonly used to assess the efficacy of cytotoxic agents in lung cancer treatment are summarized in Table 2 (Ceppi et al. 2006a; Christoph et al. 2013; Filipits and Pirker 2011; Giovannetti et al. 2005; Mizuuchi et al. 2015; Mochinaga et al. 2014; Olaussen et al. 2006; Postel-Vinay et al. 2012; Scagliotti et al. 2008; Vilmar and Sorensen 2011).
Predictive roles of EGFR mutations in response to cytotoxic agents
EGFR mutation is a strong predictive biomarker for the response to treatment with EGFR-TKIs; however, can it also predict sensitivity to cytotoxic chemotherapy? The EGFR mutation does not fall into any of the categories of the molecular predictive biomarkers described above, such as DNA repair genes, target genes of cytotoxic agents, and drug efflux pumps. However, correlations between the presence of the EGFR mutation and molecular predictive biomarkers for the response to cytotoxic agents have been determined through analyses of clinical specimens. As summarized in Table 3, although in some cases the results are controversial, a negative correlation between ERCC1 expression and the presence of the EGFR mutation has been repeatedly reported. [Again, note that the currently available anti-ERCC1 antibodies did not specifically detect the unique functional ERCC1 isoform.] While the mechanism that links the one to the other is unclear, lung cancers with EGFR mutations may be more responsive to platinum-doublet chemotherapy which is currently the gold standard chemotherapeutic regimen for the treatment for NSCLC.
The predictive role of the EGFR mutation has also been evaluated retrospectively, using the data of lung cancer patients who were treated with frontline cytotoxic chemotherapies (Dong et al. 2013; Fang et al. 2014; Hotta et al. 2007; Kalikaki et al. 2010). Most of these studies reported that among lung cancer patients with EGFR mutations who were treated with platinum-doublet chemotherapies, progression-free survival (PFS) was better for patients with EGFR-mutation-positive tumors than for those with tumors negative for the mutation. In multivariate analyses adjusting for other predictive factors, such as disease stage, smoking status, sex, and tumor histology, the EGFR mutation was an independent predictive biomarker for responsiveness to platinum-doublet chemotherapy. Some of these studies were relatively large and included >200 patients (Dong et al. 2013; Fang et al. 2014).
Nonetheless, there are limitations in evaluating the predictive role of EGFR mutations retrospectively. For example, the platinum-doublet chemotherapy regimens differed, and some patients were treated with a reduced dose. In addition, there were differences in the follow-up schedule; more frequent follow-ups would be better able to detect disease progression, which would be reflected in shorter PFS. There may also have been publication bias.
The most reliable data to analyze the predictive implication of EGFR mutations for responsiveness to cytotoxic agents are the subgroup analyses of two randomized phase III trials: the above-mentioned IPASS (Mok et al. 2009) and First-SIGNAL (first-line single-agent iressa versus gemcitabine and cisplatin trial in never smokers with adenocarcinoma of the lung) (Han et al. 2012). Both enrolled patients in East Asia with previously untreated lung adenocarcinoma who had never smoked or were former light smokers (IPASS) or never smokers (First-SIGNAL); these criteria increased the number of lung cancer patients with EGFR mutations. Patients were randomly assigned to gefitinib or platinum-doublet chemotherapy (carboplatin–paclitaxel in IPASS and cisplatin–gemcitabine in First-SIGNAL). EGFR mutations were analyzed in tissue samples, and subgroup analyses were performed based on the EGFR mutation status. In the IPASS trial, 214 patients were assigned to the chemotherapy arm and had data on EGFR mutation status compared to 43 in the First-SIGNAL trial. In the two trials, the clinical backgrounds of the patient with/without EGFR mutation were similar, due to the inclusion criteria. The objective response rates (ORRs) in the IPASS trial of patients with and without EGFR mutations who were treated with carboplatin–paclitaxel were 47 and 24 %, respectively. The disease control rates (DCRs) were 88 and 84 %, and PFS was 6.3 and 5.5 months, respectively (Table 4). The difference in PFS was not statistically significant [HR 0.78; 95 % confidence interval (CI) 0.57–1.06; p = 0.11]. In the First-SIGNAL trial, similar ORRs were reported in patients with and without EGFR mutations who were treated with cisplatin–gemcitabine (38 vs. 52 %, respectively; p = 0.36). The PFS of the two subgroups was the same (6.3 vs. 6.4 months, respectively; HR 0.679; 95 % CI 0.34–1.35; p = 0.27).
Based on these reports, it can be concluded that lung cancers with EGFR mutation respond better to frontline platinum-doublet chemotherapy. However, the predictive impact of the EGFR mutation is quite small if clinical and pathological factors are strictly aligned.
What is the appropriate cytotoxic agent for lung cancers with EGFR mutations?
Is there a specific cytotoxic agent with higher or lower efficacy in the treatment for lung cancers with EGFR mutations? For the treatment for NSCLCs, platinum (cisplatin or carboplatin) combined with so-called third-generation cytotoxic agents (such as paclitaxel, docetaxel, gemcitabine, and vinorelbine) was the standard treatment before the era of pemetrexed, because the treatment effects of these regimes were similar (Kelly et al. 2001; Ohe et al. 2007; Scagliotti et al. 2002; Schiller et al. 2002). However, in a phase III study that evaluated the efficacy of pemetrexed, a multitargeted antifolate, a preplanned subset analysis showed that cisplatin plus pemetrexed was better than cisplatin plus gemcitabine in the OS of patients with tumors of non-squamous histology (Scagliotti et al. 2008). (For patients with squamous cell carcinoma, cisplatin plus gemcitabine resulted in a longer OS than cisplatin plus pemetrexed.) The molecular basis of this result has been attributed to the lower expression levels of thymidylate synthase (TS), the main target of pemetrexed, in tumors with a non-squamous histology than in squamous cell carcinoma (Ceppi et al. 2006b).
Because almost all lung cancers with EGFR mutations are adenocarcinomas, pemetrexed-based chemotherapy seems to be the most effective regimen. Further support for the use of pemetrexed in this setting comes from the finding of lower TS mRNA expression in lung adenocarcinomas positive for EGFR mutations or with an ALK translocation than in tumors that are negative for either mutation (Table 3). In addition, in a retrospective analysis, patients with lung cancers expressing an EGFR mutation had a better pemetrexed response rate (p = 0.016) and longer PFS (p = 0.030) than those whose tumors expressed the wild-type EGFR (Wu et al. 2011). Prospective evidence was provided by a phase II study (n = 51) that analyzed the efficacy of frontline carboplatin plus pemetrexed in patients with non-squamous NSCLCs. Median PFS was longer in patients with EGFR-mutation-positive disease (7.9 months) than in those whose tumors lacked EGFR mutations (6.3 months), although this difference was not statistically significant (p = 0.09) (Kim et al. 2012).
Table 4 summarizes the results of prospective phase III studies that compared EGFR-TKIs with platinum-doublet chemotherapies. The chemotherapy regimens included carboplatin–paclitaxel, cisplatin–gemcitabine, cisplatin–docetaxel, carboplatin–gemcitabine, and cisplatin–pemetrexed. PFS was 4.6–6.9 months. Although PFS rates determined in different chemotherapy trials cannot be compared directly, the combination of cisplatin and pemetrexed resulted in the numerically longest PFSs. In addition, in the preplanned integrated analysis of the Lux-Lung 3 and Lux-Lung 6 studies, cisplatin plus pemetrexed yielded longer PFS than cisplatin plus gemcitabine in patients with EGFR-mutation-positive lung cancers, whereas the PFS achieved with afatinib was roughly the same between these two trials (Yang et al. 2015). These results support the choice of regimens containing pemetrexed in the treatment for lung cancers with EGFR mutations.
A retrospective analysis reported that among patients with lung adenocarcinoma who underwent curative pulmonary resection followed by uracil–tegafur adjuvant chemotherapy, those whose tumors were negative for EGFR mutations had a significantly prolonged survival, whereas this was not the case in patients with EGFR-mutation-positive tumors (Suehisa et al. 2007). Although the findings of basic research also support this observation (Mochinaga et al. 2014; Suehisa et al. 2007), there is not enough evidence supporting the avoidance of adjuvant 5-FU-based agents in the treatment for lung cancers with EGFR mutations in clinical practices.
Treatment strategy for lung cancers with EGFR mutations
In clinical practice, however, fewer patients with EGFR-mutation-positive lung cancers receive platinum-doublet chemotherapy as a frontline therapy, because in all seven phase III trials, the PFS of patients who received EGFR-TKI treatment (gefitinib, erlotinib, or afatinib) was superior to that of platinum-doublet chemotherapy (Table 4) (Maemondo et al. 2010; Mitsudomi et al. 2010; Rosell et al. 2012; Wu et al. 2013; Wu et al. 2014; Yang et al. 2012; Zhou et al. 2011). However, the emergence of acquired resistance to frontline EGFR-TKI is almost inevitable, such that the median PFS is only in the range of 8.4–13.7 months (Table 4). The disease progression patterns include involvement of the central nervous system (CNS)-only progression, oligo-progression, and systemic progression (Fig. 3). Treatment strategies are usually based on the disease progression pattern, but cytotoxic agents play important roles in all three after the development of resistance to frontline EGFR-TKI. For example, in the NEJ002 trial that compared gefitinib with carboplatin/paclitaxel in the first-line setting, 68 patients (88 %) of the 77 patients who had stopped receiving frontline gefitinib received cytotoxic chemotherapy (Inoue et al. 2013). [However, in a population-based study, only 46 % of patients received cytotoxic chemotherapy after EGFR-TKI treatment failure (Mariano et al. 2014)]. In the next section, we consider whether the chemosensitivity of lung cancers with EGFR mutation is altered after they acquire resistance to EGFR-TKIs.
Patterns of disease progression after a good response to EGFR-TKIs. Disease progression can be classified into three patterns, CNS-only progression, oligo-progression, and systemic progression. Representative treatment strategies, including a role for cytotoxic agents, are also described. (Modified from slides presented by Gandara et al. at the American Society of Clinical Oncology meeting of 2013)
Mechanisms of acquired resistance to EGFR-TKIs
Before discussing chemosensitivity after the acquisition of resistance to EGFR-TKIs, it is necessary to briefly review the resistance mechanisms to EGFR-TKIs (Fig. 4) (Suda et al. 2012a). Acquisition of the T790M gatekeeper mutation of the EGFR (as a secondary mutation), which substitutes a methionine for a threonine at amino acid position 790 (Kobayashi et al. 2005; Pao et al. 2005), is the most common mechanism of acquired resistance to the reversible EGFR-TKIs (gefitinib and erlotinib), accounting for 68–83 % according to the high-sensitivity method of detection (Fig. 4b) (Arcila et al. 2011; Su et al. 2012). Initially, the larger methionine residue was thought to sterically block the binding of gefitinib or erlotinib; however, a later study demonstrated the increased ATP affinity of EGFR with a T790M mutation as the mechanism of resistance (reviewed in Suda et al. 2009). Afatinib is an irreversible EGFR-TKI that was expected to overcome acquired resistance by T790M secondary mutations, based on their ability to bind the EGFR irreversibly (Engelman et al. 2007a; Li et al. 2008). However, this drug is also active against the wild-type EGFR (Engelman et al. 2007a; Li et al. 2008), leading to dose limitation and thus a lower clinically achievable concentration. Therefore, a T790M secondary mutation is also considered as an acquired resistance mechanism to afatinib.
Summary of the mechanisms of acquired resistance to EGFR-TKIs in lung cancers with EGFR mutations. Lung cancers with EGFR mutation are highly responsive to EGFR-TKIs (a). However, acquired resistance occurs almost inevitably by: a secondary mutation in the receptor that interferes with the binding of its inhibitor (b), activation of bypass signaling (c), activation of EGFR downstream signaling (d), or other mechanisms (e). Resistance mechanisms thought to induce an altered chemosensitivity are highlighted in red
The activation of bypass signaling is another mechanism by which resistance to EGFR-TKIs is acquired. Despite the effective inhibition of EGFR by EGFR-TKI, cancer cells survive due to bypass signaling, that is, by activating other receptor tyrosine kinases. The molecular basis is either MET gene amplification (Bean et al. 2007; Engelman et al. 2007b) or the high-level expression of the ligand (HGF) (Yano et al. 2008), ERBB2 amplification, AXL amplification, and insulin-like growth factor 1 receptor activation (Fig. 4c). The third method of acquired resistance is the activation of downstream molecules of EGFR (Fig. 4d). The candidate molecules are PTEN (down-regulation), CRKL (amplification), ERK (reactivation of signaling by amplification or down-regulation of negative regulators of ERK signaling), and BRAF (mutation). Other acquired resistance mechanisms (Fig. 4e) thus far include epithelial-to-mesenchymal transition (EMT), stem-cell-like changes, small-cell lung cancer (SCLC) transformation, and loss of the mutant EGFR allele (Ercan et al. 2012; Ohashi et al. 2012; Sequist et al. 2011; Shien et al. 2013; Suda et al. 2012a; Tabara et al. 2012; Takezawa et al. 2012; Zhang et al. 2012). Analyses of clinical specimens suggest that these molecular mechanisms of resistance are usually mutually exclusive (Sequist et al. 2011; Suda et al. 2010; Yu et al. 2013).
The altered chemosensitivity of lung cancers with EGFR mutations after acquired resistance to EGFR-TKIs
Convincing data regarding the chemosensitivity of lung cancers with EGFR mutations before and after the acquisition of resistance to EGFR-TKIs have come from in vitro models of acquired resistance. These have been established by chronically exposing sensitive cultured cells to EGFR-TKIs, which gradually induces inhibitor resistance (Engelman et al. 2007b; Suda et al. 2011; Turke et al. 2010). These in vitro isogenic models have been used to analyze the molecular mechanism underlying the chemosensitivity of cells with acquired resistance. A summary of the results of these analyses, which include our own, is that cells with acquired resistance show the same chemosensitivity as the EGFR-TKI-sensitive parental cells if resistance was acquired by specific mechanisms that involve a single proto-oncogene, such as an EGFR T790M mutation, MET amplification, or IGF-1R activation. The exception appears to be PTEN down-regulation, which causes acquired resistance to both EGFR-TKIs and cisplatin (Suda and Mitsudomi 2013) and therefore would result in insensitivity to platinum-doublet chemotherapy. Other in vitro isogenic models of acquired resistance to EGFR-TKI, with EMT or stem-cell-like features, show altered chemosensitivity toward antimicrotubule agents (taxanes and vinorelbine). The overexpression of ABCB1, which encodes a drug efflux pump, is the apparent molecular basis of this cross-resistance (Mizuuchi et al. 2015; Shien et al. 2013). Thus, because “specific resistance mechanisms” are the main cause of acquired resistance to EGFR-TKIs (Yu et al. 2013), the chemosensitivity to cytotoxic agents does not usually change after the acquisition of resistance to these inhibitors in lung cancers with EGFR mutations. Clinical evidence supports this hypothesis. In the above-mentioned NEJ002 study, the protocol recommended a crossover regimen as second-line treatment, although any treatment was permitted. Among the 77 patients who stopped receiving gefitinib, 52 (67.5 %) received carboplatin–paclitaxel as the second-line treatment. The ORR of these patients was 28.8 %, comparable to the 30.7 % determined for patients in the frontline carboplatin–paclitaxel arm (Maemondo et al. 2010). Tseng et al. retrospectively analyzed the data of patients with lung adenocarcinomas with EGFR mutations who had been treated with pemetrexed plus platinum as the frontline therapy and as second-line therapy after EGFR-TKI treatment failure. The ORRs of patients without versus with prior EGFR-TKI were similar (38.6 vs. 24.6 %), as was the DCR (65.9 vs. 62.3 %) and PFS (6.1 vs. 6.1 months) (Tseng et al. 2014).
SCLC transformation is a rare mechanism of acquired resistance to EGFR-TKIs. Clinical cases in which the lung cancer responded to cytotoxic chemotherapy for classical SCLCs after the acquisition of resistance to EGFR-TKI have been reported (Sequist et al. 2011).
Conclusions
This review provided a summary of the clinical and therapeutic features of lung cancers with EGFR mutations, focusing on the prognostic roles and predictive implications for cytotoxic chemotherapies. Lung cancers with EGFR mutations define a subset of those with a better prognosis, probably due to the accompanying clinical characteristics. EGFR-TKIs are the treatment of choice for lung cancers with EGFR mutations, with cytotoxic chemotherapy serving as the second-line treatment. In vitro and clinical evidence suggest that in many cases, the chemosensitivity of lung cancers with EGFR mutations does not change after the acquisition of resistance to EGFR-TKIs.
References
Arcila ME, Oxnard GR, Nafa K et al (2011) Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res 17(5):1169–1180
Bean J, Brennan C, Shih JY et al (2007) MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA 104(52):20932–20937
Ceppi P, Volante M, Novello S et al (2006a) ERCC1 and RRM1 gene expressions but not EGFR are predictive of shorter survival in advanced non-small-cell lung cancer treated with cisplatin and gemcitabine. Ann Oncol 17(12):1818–1825
Ceppi P, Volante M, Saviozzi S et al (2006b) Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 107(7):1589–1596
Chen Z, Liu X, Zhao J, Yang H, Teng X (2014) Correlation of EGFR mutation and histological subtype according to the IASLC/ATS/ERS classification of lung adenocarcinoma. Int J Clin Exp Pathol 7(11):8039–8045
Christoph DC, Asuncion BR, Hassan B et al (2013) Significance of folate receptor alpha and thymidylate synthase protein expression in patients with non-small-cell lung cancer treated with pemetrexed. J Thorac Oncol 8(1):19–30
Cuyun Carter G, Barrett AM, Kaye JA, Liepa AM, Winfree KB, John WJ (2014) A comprehensive review of nongenetic prognostic and predictive factors influencing the heterogeneity of outcomes in advanced non-small-cell lung cancer. Cancer Manag Res 6:437–449
D’Angelo SP, Janjigian YY, Ahye N et al (2012) Distinct clinical course of EGFR-mutant resected lung cancers: results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J Thorac Oncol 7(12):1815–1822
Dong X, Zhao X, Hao Y, Wei Y, Yin Q, Du J (2013) Response to first-line chemotherapy in patients with non-small-cell lung cancer according to epidermal growth factor receptor and K-RAS mutation status. Clin Lung Cancer 14(6):680–687
Engelman JA, Zejnullahu K, Gale CM et al (2007a) PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 67(24):11924–11932
Engelman JA, Zejnullahu K, Mitsudomi T et al (2007b) MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316(5827):1039–1043
Ercan D, Xu C, Yanagita M et al (2012) Reactivation of ERK signaling causes resistance to EGFR kinase inhibitors. Cancer Discov 2(10):934–947
Fang S, Wang Z, Guo J et al (2014) Correlation between EGFR mutation status and response to first-line platinum-based chemotherapy in patients with advanced non-small cell lung cancer. OncoTargets Ther 7:1185–1193
Filipits M, Pirker R (2011) Predictive markers in the adjuvant therapy of non-small cell lung cancer. Lung cancer. 74(3):355–363
Friboulet L, Olaussen KA, Pignon JP et al (2013) ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med 368(12):1101–1110
Fukuoka M, Wu YL, Thongprasert S et al (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 29(21):2866–2874
Gandara DR, Grimminger P, Mack PC et al (2010) Association of epidermal growth factor receptor activating mutations with low ERCC1 gene expression in non-small cell lung cancer. J Thorac Oncol 5(12):1933–1938
Giovannetti E, Mey V, Nannizzi S et al (2005) Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cells. Mol Pharmacol 68(1):110–118
Han JY, Park K, Kim SW et al (2012) First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 30(10):1122–1128
Hotta K, Kiura K, Toyooka S et al (2007) Clinical significance of epidermal growth factor receptor gene mutations on treatment outcome after first-line cytotoxic chemotherapy in Japanese patients with non-small cell lung cancer. J Thorac Oncol 2(7):632–637
Hu H, Pan Y, Li Y et al (2014) Oncogenic mutations are associated with histological subtypes but do not have an independent prognostic value in lung adenocarcinoma. OncoTargets Ther 7:1423–1437
Hynes NE, Lane HA (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5(5):341–354
Inoue A, Kobayashi K, Maemondo M et al (2013) Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 24(1):54–59
Izar B, Sequist L, Lee M et al (2013) The impact of EGFR mutation status on outcomes in patients with resected stage I non-small cell lung cancers. Ann Thorac Surg 96(3):962–968
Janjigian YY, Park BJ, Zakowski MF et al (2011) Impact on disease-free survival of adjuvant erlotinib or gefitinib in patients with resected lung adenocarcinomas that harbor EGFR mutations. J Thorac Oncol 6(3):569–575
Jie L, Li XY, Zhao YQ et al (2014) Genotype-phenotype correlation in Chinese patients with pulmonary mixed type adenocarcinoma: relationship between histologic subtypes, TITF-1/SP-a expressions and EGFR mutations. Pathol Res Pract 210(3):176–181
Kalikaki A, Koutsopoulos A, Hatzidaki D et al (2010) Clinical outcome of patients with non-small cell lung cancer receiving front-line chemotherapy according to EGFR and K-RAS mutation status. Lung Cancer 69(1):110–115
Kelly K, Crowley J, Bunn PA Jr et al (2001) Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non–small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol 19(13):3210–3218
Kim YH, Hirabayashi M, Togashi Y et al (2012) Phase II study of carboplatin and pemetrexed in advanced non-squamous, non-small-cell lung cancer: Kyoto Thoracic Oncology Research Group Trial 0902. Cancer Chemother Pharmacol 70(2):271–276
Kim YT, Seong YW, Jung YJ et al (2013) The presence of mutations in epidermal growth factor receptor gene is not a prognostic factor for long-term outcome after surgical resection of non-small-cell lung cancer. J Thorac Oncol 8(2):171–178
Kobayashi S, Boggon TJ, Dayaram T et al (2005) EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 352(8):786–792
Kobayashi N, Toyooka S, Ichimura K et al (2008) Non-BAC component but not epidermal growth factor receptor gene mutation is associated with poor outcomes in small adenocarcinoma of the lung. J Thorac Oncol 3(7):704–710
Kosaka T, Yatabe Y, Onozato R, Kuwano H, Mitsudomi T (2009) Prognostic implication of EGFR, KRAS, and TP53 gene mutations in a large cohort of Japanese patients with surgically treated lung adenocarcinoma. J Thorac Oncol 4(1):22–29
Li D, Ambrogio L, Shimamura T et al (2008) BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 27(34):4702–4711
Li H, Xie L, Lai RS (2013) Association of EGFR mutations with low BRCA1 gene expression in non-small cell lung cancer. Mol Clin Oncol 1(1):195–199
Lim KH, Huang MJ, Liu HC, Kuo HT, Tzen CY, Hsieh RK (2007) Lack of prognostic value of EGFR mutations in primary resected non-small cell lung cancer. Med Oncol 24(4):388–393
Lin MW, Wu CT, Shih JY, Chang YL, Yang PC (2014) Clinicopathologic characteristics and prognostic significance of EGFR and p53 mutations in surgically resected lung adenocarcinomas </=2 cm in maximal dimension. J Surg Oncol 110(2):99–106
Liu HP, Isaac Wu HD, Chang JW et al (2010) Prognostic implications of epidermal growth factor receptor and KRAS gene mutations and epidermal growth factor receptor gene copy numbers in patients with surgically resectable non-small cell lung cancer in Taiwan. J Thorac Oncol 5(8):1175–1184
Liu WS, Zhao LJ, Pang QS, Yuan ZY, Li B, Wang P (2014) Prognostic value of epidermal growth factor receptor mutations in resected lung adenocarcinomas. Med Oncol 31(1):771
Lynch TJ, Bell DW, Sordella R et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350(21):2129–2139
Maemondo M, Inoue A, Kobayashi K et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362(25):2380–2388
Mariano C, Bosdet I, Karsan A et al (2014) A population-based review of the feasibility of platinum-based combination chemotherapy after tyrosine kinase inhibition in EGFR mutation positive non-small cell lung cancer patients with advanced disease. Lung Cancer 83(1):73–77
Marks JL, Broderick S, Zhou Q et al (2008) Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J Thorac Oncol 3(2):111–116
Mitsudomi T, Yatabe Y (2007) Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci 98(12):1817–1824
Mitsudomi T, Morita S, Yatabe Y et al (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11(2):121–128
Mizuuchi H, Suda K, Sato K, et al (2015) Collateral chemoresistance to anti-microtubule agents in a lung cancer cell line with acquired resistance to erlotinib. PloS One 10(4):e0123901
Mochinaga K, Tsuchiya T, Nagasaki T et al (2014) High expression of dihydropyrimidine dehydrogenase in lung adenocarcinoma is associated with mutations in epidermal growth factor receptor: implications for the treatment of non–small-cell lung cancer using 5-fluorouracil. Clinical Lung Cancer 15(2):136–144
Mok TS, Wu YL, Thongprasert S et al (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361(10):947–957
Nakamura, H, Saji, H, Shinmyo, T, et al. (2014) Association of IASLC/ATS/ERS histologic subtypes of lung adenocarcinoma with epidermal growth factor receptor mutations in 320 resected cases. Clin Lung Cancer 16(3):209–15
Ohashi K, Sequist LV, Arcila ME et al (2012) Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci USA 109(31):E2127–E2133
Ohe Y, Ohashi Y, Kubota K et al (2007) Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: four-Arm Cooperative Study in Japan. Ann Oncol 18(2):317–323
Okuda K, Sasaki H, Dumontet C et al (2008) Expression of excision repair cross-complementation group 1 and class III beta-tubulin predict survival after chemotherapy for completely resected non-small cell lung cancer. Lung Cancer 62(1):105–112
Olaussen KA, Dunant A, Fouret P et al (2006) DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 355(10):983–991
Paez JG, Janne PA, Lee JC et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304(5676):1497–1500
Pao W, Miller V, Zakowski M et al (2004) EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA 101(36):13306–13311
Pao W, Miller VA, Politi KA et al (2005) Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2(3):e73
Postel-Vinay S, Vanhecke E, Olaussen KA, Lord CJ, Ashworth A, Soria JC (2012) The potential of exploiting DNA-repair defects for optimizing lung cancer treatment. Nat Rev 9(3):144–155
Ren S, Chen X, Kuang P et al (2012) Association of EGFR mutation or ALK rearrangement with expression of DNA repair and synthesis genes in never-smoker women with pulmonary adenocarcinoma. Cancer 118(22):5588–5594
Rosell R, Carcereny E, Gervais R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13(3):239–246
Scagliotti GV, De Marinis F, Rinaldi M et al (2002) Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 20(21):4285–4291
Scagliotti GV, Parikh P, von Pawel J et al (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26(21):3543–3551
Schiller JH, Harrington D, Belani CP et al (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346(2):92–98
Schmid-Bindert G, Wang Y, Jiang H et al (2013) EBUS-TBNA provides highest RNA yield for multiple biomarker testing from routinely obtained small biopsies in non-small cell lung cancer patients—a comparative study of three different minimal invasive sampling methods. PLoS One 8(10):e77948
Sequist LV, Waltman BA, Dias-Santagata D et al (2011) Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Science translational medicine. 3(75):75ra26
Sequist LV, Yang JC, Yamamoto N et al (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31(27):3327–3334
Shien K, Toyooka S, Yamamoto H et al (2013) Acquired resistance to EGFR inhibitors is associated with a manifestation of stem cell-like properties in cancer cells. Cancer Res 73(10):3051–3061
Shim HS, Lee da H, Park EJ, Kim SH (2011) Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med 135(10):1329–1334
Song Z, Zhu H, Guo Z, Wu W, Sun W, Zhang Y (2013) Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Medical oncology. 30(3):645
Sonobe M, Nakagawa M, Takenaka K et al (2007) Influence of epidermal growth factor receptor (EGFR) gene mutations on the expression of EGFR, phosphoryl-Akt, and phosphoryl-MAPK, and on the prognosis of patients with non-small cell lung cancer. J Surg Oncol 95(1):63–69
Su KY, Chen HY, Li KC et al (2012) Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol 30(4):433–440
Suda K, Mitsudomi T (2013) PTEN—a molecule that cancers abuse to overcome their achilles heels. In: Ke Xu (ed) PTEN structure, mechanisms-of-action, role in cell signaling and regulation, 1st edn. Nova Science Publishers, New York, p 17
Suda K, Mitsudomi T (2014) Successes and limitations of targeted cancer therapy in lung cancer. Prog Tumor Res 41:62–77
Suda K, Mitsudomi T (2015) Racial differences in lung cancer genetics. J Thorac Oncol 10(2):230–231
Suda K, Onozato R, Yatabe Y, Mitsudomi T (2009) EGFR T790M mutation: a double role in lung cancer cell survival? J Thorac Oncol 4(1):1–4
Suda K, Murakami I, Katayama T et al (2010) Reciprocal and complementary role of MET amplification and EGFR T790M mutation in acquired resistance to kinase inhibitors in lung cancer. Clin Cancer Res 16(22):5489–5498
Suda K, Tomizawa K, Fujii M et al (2011) Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol 6(7):1152–1161
Suda K, Mizuuchi H, Maehara Y, Mitsudomi T (2012a) Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation-diversity, ductility, and destiny. Cancer Metastasis Rev 31(3–4):807–814
Suda K, Tomizawa K, Mizuuchi H et al (2012b) Genetic and prognostic differences of non-small cell lung cancer between elderly patients and younger counterparts. Aging Dis 3(6):438–443
Suda K, Sato K, Shimizu S et al (2014) Prognostic implication of predominant histologic subtypes of lymph node metastases in surgically resected lung adenocarcinoma. BioMed Res Int 2014:645681
Suehisa H, Toyooka S, Hotta K et al (2007) Epidermal growth factor receptor mutation status and adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. J Clin Oncol 25(25):3952–3957
Sun PL, Seol H, Lee HJ et al (2012) High incidence of EGFR mutations in Korean men smokers with no intratumoral heterogeneity of lung adenocarcinomas: correlation with histologic subtypes, EGFR/TTF-1 expressions, and clinical features. J Thorac Oncol 7(2):323–330
Sun Y, Yu X, Shi X, Hong W, Zhao J, Shi L (2014) Correlation of survival and EGFR mutation with predominant histologic subtype according to the new lung adenocarcinoma classification in stage IB patients. World J Surg Oncol 12:148
Tabara K, Kanda R, Sonoda K et al (2012) Loss of activating EGFR mutant gene contributes to acquired resistance to EGFR tyrosine kinase inhibitors in lung cancer cells. PLoS One 7(7):e41017
Takano T, Fukui T, Ohe Y et al (2008) EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: a historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol 26(34):5589–5595
Takezawa K, Pirazzoli V, Arcila ME et al (2012) HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site egfrt790M mutation. Cancer Discov 2(10):922–933
Tseng JS, Yang TY, Chen KC et al (2014) Prior EGFR tyrosine-kinase inhibitor therapy did not influence the efficacy of subsequent pemetrexed plus platinum in advanced chemonaive patients with EGFR-mutant lung adenocarcinoma. OncoTargets Ther 7:799–805
Turke AB, Zejnullahu K, Wu YL et al (2010) Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell 17(1):77–88
Vilmar AC, Sorensen JB (2011) Customising chemotherapy in advanced nonsmall cell lung cancer: daily practice and perspectives. Eur Respir Rev 20(119):45–52
Weinstein IB (2002) Cancer. Addiction to oncogenes–the Achilles heal of cancer. Science. 297(5578):63–64
Wu SG, Yang CH, Yu CJ et al (2011) Good response to pemetrexed in patients of lung adenocarcinoma with epidermal growth factor receptor (EGFR) mutations. Lung Cancer 72(3):333–339
Wu YL, Liam CK, Zhou C, Wu G (2013) First-line erlotinib versus cisplatin/gemcitabine (GP) in patients with advanced EGFR mutation-positive non-small-cell lung cancer (NSCLC): Interim analyses from the phase i, open-label, ENSURE study. J Thorac Oncol 8(Suppl. 2):s603 WCLC abstracts
Wu YL, Zhou C, Hu CP et al (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15(2):213–222
Xu CW, Wang G, Wang WL et al (2015) Association between epidermal growth factor receptor mutations and the expression of excision repair cross-complementing protein 1 and ribonucleotide reductase subunit M1 mRNA in patients with non-small cell lung cancer. Exp Ther Med 9(3):880–884
Yamashita F, Azuma K, Yoshida T et al (2013) Prognostic value of EGFR mutation and ERCC1 in patients with non-small cell lung cancer undergoing platinum-based chemotherapy. PLoS One 8(8):e71356
Yanagawa N, Shiono S, Abiko M, Ogata SY, Sato T, Tamura G (2014) The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac Surg 98(2):453–458
Yang JC-H, Schuler MH, Yamamoto N et al (2012) LUX-Lung 3: A randomized, open-label, phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations. J Clin Oncol 30(suppl):7500 abstr LBA7500
Yang JC, Wu YL, Schuler M et al (2015) Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 16(2):141–151
Yano S, Wang W, Li Q et al (2008) Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res 68(22):9479–9487
Yoshizawa A, Sumiyoshi S, Sonobe M et al (2013) Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 8(1):52–61
Yu HA, Arcila ME, Rekhtman N et al (2013) Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 19(8):2240–2247
Zhang Z, Lee JC, Lin L et al (2012) Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 44(8):852–860
Zhou C, Wu YL, Chen G et al (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12(8):735–742
Acknowledgments
Current research in my laboratory is supported by Grants-in-Aid of Cancer Research from the Ministry of Education, Science, Sports, and Culture of Japan (K. Suda, 15K18450), grants from the Japan Surgical Society (JSS) Young Researcher Award 2014 (K. Suda), the Kaibara Morikazu Medical Science Promotion Foundation (K. Suda), the Uehara Memorial Foundation (T. Mitsudomi), and the Takeda Science Foundation (T. Mitsudomi).
Conflict of interest
T. Mitsudomi has received honoraria from AstraZeneca, Chugai, Boehlinger-Ingelheim, Pfizer, Roche, Novartis, and Taiho and has played an advisory role for AstraZeneca, Chugai, Boehlinger-Ingelheim, Pfizer, Roche, and Clovis Oncology. K. Suda declares no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suda, K., Mitsudomi, T. Role of EGFR mutations in lung cancers: prognosis and tumor chemosensitivity. Arch Toxicol 89, 1227–1240 (2015). https://doi.org/10.1007/s00204-015-1524-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1524-7