Abstract
Alveolar type II epithelial cells can regulate immune responses to sepsis-induced acute lung injury. Lipopolysaccharide (LPS), an outer membrane component of Gram-negative bacteria, can cause septic shock. This study was designed to evaluate the cytotoxic effects of LPS on human alveolar epithelial A549 cells and its possible molecular mechanisms. Exposure of A549 cells to LPS decreased cell viability in concentration- and time-dependent manners. In parallel, LPS concentration- and time-dependently induced apoptosis of A549 cells. Meanwhile, LPS only at a high concentration of 10 μg/ml caused mildly necrotic insults to A549 cells. In terms of the mechanism, exposure of A549 cells to LPS increased the levels of cellular nitric oxide and reactive oxygen species (ROS). Pretreatment with N-acetylcysteine (NAC), an antioxidant, significantly lowered LPS-caused enhancement of intracellular ROS in A549 cells and simultaneously attenuated the apoptotic insults. Sequentially, treatment of A549 cells with LPS caused significant decreases in the mitochondrial membrane potential and biosynthesis of adenosine triphosphate. In succession, LPS triggered the release of cytochrome c from the mitochondria to the cytoplasm. Activities of caspase-9 and caspase-6 were subsequently augmented following LPS administration. Consequently, exposure of A549 cells induced DNA fragmentation in a time-dependent manner. Pretreatment of A549 cells with NAC significantly ameliorated LPS-caused alterations in caspase-9 activation and DNA damage. Therefore, this study shows that LPS specifically induces apoptotic insults to human alveolar epithelial cells through ROS-mediated activation of the intrinsic mitochondrion–cytochrome c-caspase protease mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis induced by Gram-negative bacteria is a common complication of acute pulmonary infections and can lead to organ dysfunction or hypoperfusion abnormalities (Angus et al. 2001; Cazzola et al. 2004). Lipopolysaccharide (LPS), an outer membrane component of Gram-negative bacteria, is one of the major causes of septic shock (Raetz et al. 1991; Welbourn and Yong 1992). Pulmonary alveolar type II epithelial cells, located in the corners of the alveoli, play crucial roles in physiological and pathophysiological regulation of septic shock and acute lung injury by specifically synthesizing, secreting, and reutilizing surfactants (Mendelson 2000; Rooney 2001). Pulmonary surfactants have critical functions of reducing surface tension at the alveolar air–liquid interface, thereby preventing alveolar collapse upon expiration and allowing normal breathing (Clements and Avery 1998). In response to bacterial infection, levels of surfactant components in the lungs can be altered through a host’s defense mechanism (LeVine et al. 2000; Crouch and Wright 2001). Our previous study showed that LPS regulates surfactant protein-A biosynthesis in human alveolar epithelial A549 cells through a toll-like receptor-2-dependent pathway (Chuang et al. 2009). Vernooy et al. (2001) reported that intratracheal instillation of a high dose of LPS in mice directly caused the death of bronchial epithelial cells. Thus, LPS may have biphasic effects on affecting the activity and function of alveolar type II epithelial cells.
Apoptosis is an energy-dependent type of programmed cell death (Fiers et al. 1999). A variety of intrinsic and extrinsic factors are involved in regulating cell apoptosis (Goyal 2001; Chen et al. 2007; Ferrari et al. 2009). Reactive oxygen species (ROS) are apoptotic factors that can cause oxidative stress and subsequent cell apoptosis (Tai et al. 2007; Pellegrini and Baldari 2009). As an ROS, nitric oxide (NO) was implicated as an effector for death regulation (Wu et al. 2007; Cherng et al. 2008). Previous studies showed that exposure to LPS stimulated NO overproduction and eventually led to cell apoptosis (Waak et al. 2009; Lee et al. 2010). Mitochondria are energy-producing organelles. Maintenance of the mitochondrial membrane potential is critical to adenosine triphosphate (ATP) synthesis (Chang et al. 2009). Depolarization of the mitochondrial membrane potential increases the release of apoptotic factors such as cytochrome (Cyt) c from the mitochondria to the cytoplasm, which leads to apoptotic insults (Saikumar et al. 1998; Ho et al. 2009; Chen et al. 2010). After interacting with apoptotic protease-activating factor-1, Cyt c elicits cascade activation of caspase-9, caspase-3, and caspase-6, ultimately inducing DNA damage and cell apoptosis (Goyal 2001; Kagan et al. 2004; Lee et al. 2009). Alveolar type II epithelial cells can control septic shock-induced acute lung injury (Mendelson 2000; Rooney 2001). However, the cytotoxic effects of LPS on alveolar epithelial cells are still little known. Thus, in this study, we attempted to evaluate the toxic effects of LPS on human lung carcinoma type II epithelial-like A549 cells and the possible mechanisms.
Materials and methods
Cell culture and drug treatment
Human lung carcinoma type II epithelium-like A549 cells, purchased from the American Type Culture Collection (Rockville, MD, USA), were grown in Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 culture medium (Sigma Chemical, St. Louis, MO, USA) with 10% (v/v) heat-inactivated fetal calf serum, 100 U/ml penicillin, 2 mM l-glutamine and 100 μg/ml streptomycin in 75-cm2 culture flasks at 37°C in a humidified atmosphere with 5% CO2. LPS, purchased from Merck (Stanton, NJ, USA), was dissolved in dimethyl sulfoxide (DMSO) and sonicated to disperse large LPS aggregates as described before (Kitchens et al. 2001). The concentration of DMSO in the medium never exceeded 0.1% to avoid toxicity of this solvent to A549 cells. N-acetylcysteine (NAC), an antioxidant, was purchased from Sigma, dissolved in DMSO, and pretreated for 1 h before LPS administration. Control cells received DMSO only.
Assay of cell viability
Cell viability was determined using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described previously (Cherng et al. 2008). Briefly, A549 cells (1 × 104 cells per well) were seeded overnight in 96-well tissue culture plates. After drug treatment, A549 cells were cultured in new medium containing 0.5 mg/ml MTT for a further 3 h. The blue formazan products in A549 cells were dissolved in DMSO and spectrophotometrically measured at a wavelength of 550 nm.
Quantification of apoptotic cells
Apoptotic cells were determined by detecting cells which were arrested at the sub-G1 stage according to the method of Lee et al. (2009). After drug treatment, the harvested A549 cells were fixed with cold 80% ethanol, then incubated with 3.75 mM sodium citrate, 0.1% Triton X-100, and 30 μg/ml RNase A, and resuspended in 20 μg/ml propidium iodide. Stained nuclei were analyzed by flow cytometry (FACS Calibur, Becton–Dickinson, San Jose, CA, USA).
Assay of necrotic cells
Necrotic cells were quantified using a photometric immunoassay according to the standard protocol of the cell death detection kit (Roche Applied Sciences, Nonnenwald, Penzberg, Germany) as described previously (Chen et al. 2005a, b). Briefly, A549 cells (1 × 105 cells) were seeded in 96-well tissue culture plates overnight. After LPS administration, cell lysates and culture medium were collected, and necrotic cells were immunodetected using mouse monoclonal antibodies (mAbs) against histone. After the antibody reaction and washing, the colorimetric product was measured at 405 nm against the substrate solution as a blank.
Quantification of NO
Amounts of NO in A549 cells were determined according to the technical bulletin of the Bioxytech NO assay kit (OXIS International, Portland, OR, USA) as described previously (Lee et al. 2010). In this kit, nitrate reductase is provided to reduce nitrate to nitrite so total nitrite in the culture medium is detected. After exposure to LPS, the culture medium of A549 cells was centrifuged. The supernatant fractions were collected and reacted with nitrate reductase. Following a reaction of the supernatant with sulfanilamide and N-1-napthylethylenediamine, a colorimetric azo compound was formed and quantified using an Anthos 2010 microplate photometer (Anthos Labtec Instruments, Lagerhausstrasse, Wals/Salzburg, Austria). To avoid interruption of the detection of nitrite by acidity, pH values of the culture medium were monitored in our preliminary study.
Quantification of intracellular ROS
Levels of intracellular ROS were quantified to determine the oxidative stress to A549 cells in response to LPS stimulation according to a previously described method (Chang et al. 2010). Briefly, 5 × 105 A549 cells were cultured in 12-well tissue culture plates overnight, and then co-treated with LPS and 2′,7′-dichlorofluorescin diacetate, an ROS-sensitive dye. After drug treatment, A549 cells were harvested and suspended in 1× phosphate-buffered saline (PBS) (0.14 M NaCl, 2.6 mM KCl, 8 mM Na2HPO4, and 1.5 mM KH2PO4). Relative fluorescence intensities in A549 cells were quantified using a flow cytometer (Becton–Dickinson).
Quantification of the mitochondrial membrane potential
The mitochondrial membrane potential was determined following a previously described method (Chang et al. 2006). Briefly, A549 cells (5 × 105) were seeded in 12-well tissue culture plates overnight, and then treated with LPS. After drug administration, A549 cells were harvested and incubated with 3,3′-dihexyloxacarbocyanine (DiOC6), a positively charged dye, at 37°C for 30 min in a humidified atmosphere with 5% CO2. After washing and centrifugation, cell pellets were suspended in 1×PBS. Intracellular fluorescent intensities were analyzed using a flow cytometer (Becton–Dickinson).
Assay of cellular ATP levels
Levels of cellular ATP in A549 cells were determined by a bioluminescence assay, which was based on luciferase’s requirement for ATP in producing a light emission, according to the protocol of Molecular Probes’ ATP Determination kit (Molecular Probes, Eugene, OR, USA) as described previously (Chang et al. 2009). The luminent light (560 nm) emitted by the luciferase-mediated reaction of ATP and luciferin was detected by a WALLAC VICTOR 2TM 1420 multilabel counter (Welch Allyn, Turku, Finland).
Immunoblotting analyses of cytosolic Cyt c and β-actin
Protein analyses were carried out according to a previously described method (Chen et al. 2009). After drug treatment, cytosolic proteins were prepared in an ice-cold radioimmunoprecipitation assay (RIPA) buffer (25 mM Tris–HCl (pH 7.2), 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, 0.15 M NaCl, and 1 mM EDTA). To avoid degradation of the cytosolic proteins by proteinases, a mixture of 1 mM phenyl methyl sulfonyl fluoride, 1 mM sodium orthovanadate, and 5 μg/ml leupeptin was added to the RIPA buffer. Protein concentrations were quantified using a bicinchonic acid protein assay kit (Pierce, Rockford, IL, USA). Proteins (50 μg/well) were subjected to sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to nitrocellulose membranes. Immunodetection of Cyt c was carried out using a mouse mAb against human Cyt c (Transduction Laboratories, Lexington, KY, USA). Cellular β-actin protein was immunodetected using a mouse mAb against mouse β-actin (Sigma) as the internal standard. These protein bands were quantified using a digital imaging system (UVtec, Cambridge, UK).
Fluorogenic substrate assay for caspase activities
The activities of caspase-9 and caspase-6 were determined by a fluorogenic substrate assay (Wu et al. 2007). Briefly, cell extracts were prepared by lysing A549 cells in a buffer containing 1% Nonidet P-40, 200 mM NaCl, 20 mM Tris/HCl (pH 7.4), 10 μg/ml leupeptin, 0.27 U/ml aprotinin, and 100 μM PMSF. Caspase-9 and caspase-6 activities were determined by, respectively, incubating cell lysates (25 μg total protein) with 50 μM of the fluorogenic substrates, LEHD and VEID, in 200 μl of a cell-free system buffer consisting of 10 mM Hepes (pH 7.4), 220 mM mannitol, 68 mM sucrose, 2 mM NaCl, 2.5 mM KH2PO4, 0.5 mM EGTA, 2 mM·MgCl2, 5 mM pyruvate, 0.1 mM PMSF, and 1 mM dithiothreitol. Intensities of the fluorescent products in cells were measured with a spectrofluorometer.
Quantification of DNA fragmentation
DNA fragmentation in A549 cells was quantified to evaluate if LPS damaged nuclear DNA. BrdU-labeled histone-associated DNA fragments in the cytoplasm of cell lysates were detected according to the instructions of the cellular DNA fragmentation enzyme-linked immunosorbent assay (ELISA) kit (Boehringer Mannheim, Indianapolis, IN, USA) as described previously (Chang et al. 2006). Briefly, A549 cells (2 × 105) were subcultured in 24-well tissue culture plates and labeled with BrdU overnight. Cells were harvested and suspended in the culture medium. One hundred microliters of cell suspension was added to each well of 96-well tissue culture plates. A549 cells were cocultured with LPS or NAC for another 8 h at 37°C in a humidified atmosphere with 5% CO2. Amounts of BrdU-labeled DNA in the cytoplasm were quantified using an Anthos 2010 microplate photometer (Anthos Labtec Instruments, Lagerhausstrasse, Wals/Salzburg, Austria) at a wavelength of 450 nm.
Statistical analysis
Statistical differences were considered significant when the p value of Duncan’s multiple-range test was <0.05. Statistical analysis between groups over time was carried out by a two-way analysis of variance (ANOVA).
Results
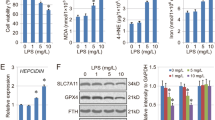
Exposure of A549 cells to 0.1 μg/ml LPS for 24 h did not affect cell viability (Fig. 1a). Meanwhile, when concentrations reached 1 and 10 μg/ml, LPS caused significant 49 and 74% decreases in the viability of A549 cells. Treatment of A549 cells with 1 μg/ml LPS for 12 h did not influence cell viability (Fig. 1b). After exposure to 1 μg/ml LPS for 24 and 48 h, viability of A549 cells was significantly reduced by 47 and 73%, respectively.
Concentration- and time-dependent effects of lipopolysaccharide (LPS) on the viability of A549 cells. A549 cells were exposed to 0.5, 1, and 10 μg/ml LPS for 24 h (a) or to 1 μg/ml LPS for 12, 24, and 48 h (b). Cell viability was assayed using a colorimetric method. Each value represents the mean ± SEM for n = 6. An asterisk indicates that the value significantly (p < 0.05) differs from the control group
Exposure of A549 cells to 0.1 μg/ml LPS for 24 h did not lead to cell apoptosis (Fig. 2a). When treatment concentrations reached 1 and 10 μg/ml, LPS induced apoptosis of A549 cells by 47 and 68%, respectively. Treatment of A549 cells with 1 μg/ml LPS for 12 h did not cause cell apoptosis (Fig. 2b). After exposure for 24 and 48 h, LPS, respectively, caused 53 and 72% of A549 cells to undergo apoptosis. A necrotic analysis showed that exposure of A549 cells to 0.5 and 1 μg/ml LPS for 24 h did not affect cell necrosis (Fig. 2c). However, LPS at a high concentration of 10 μg/ml moderately induced necrosis of A549 cells by 15%.
Concentration- and time-dependent effects of lipopolysaccharide (LPS) on apoptosis or necrosis of A549 cells. A549 cells were exposed to 0.5, 1, and 10 μg/ml LPS for 24 h (a) or to 1 μg/ml of LPS for 12, 24, and 48 h (b). After drug treatment, A549 cells were harvested, fixed, and stained with a propidium iodide dye. Apoptotic cells were quantified using a flow cytometer. A549 cells were treated with 0.1, 1, and 10 μg/ml LPS for 24 h (c). Necrotic cells were determined by a photometric immunoassay. Each value represents the mean ± SEM for n = 6. An asterisk indicates that the value significantly (p < 0.05) differs from the control group
Treatment of A549 cells with 1 μg/ml LPS for 12 h did not affect the biosynthesis of NO (Fig. 3a). Meanwhile, when the treatment time intervals reached 24 and 48 h, 1 μg/ml LPS caused significant 3.8- and 4.5-fold increases in the levels of cellular NO, respectively. In parallel, exposure to 1 μg/ml LPS for 12 h slightly increased the amount of intracellular ROS by 80% (Fig. 3b). After treatment for 24 and 48 h, LPS, respectively, led to 5.8- and 6.7-fold augmentations in the levels of intracellular ROS. Exposure of A549 cells to 1 μg/ml LPS for 24 h enhanced intracellular ROS levels by 6.1-fold (Fig. 3c). Pretreatment with NAC, an antioxidant, for 1 h slightly reduced basal levels of intracellular ROS. However, the LPS-caused promotion of intracellular ROS in A549 cells was significantly attenuated by 61% following pretreatment with NAC (Fig. 3c). In comparison, exposure to NAC alone did not influence apoptosis of A549 cells, but significantly reduced LPS-induced cell apoptosis by 63% (Fig. 3d).
Time-dependent effects of lipopolysaccharide (LPS) on the production of nitric oxide (NO) and intracellular reactive oxygen species (ROS). A549 cells were exposed to 1 μg/ml LPS for 12, 24, and 48 h. Levels of NO were analyzed by a Bioxytech NO assay kit (a). Amounts of intracellular ROS in A549 cells were quantified using flow cytometry (b). A549 cells were pretreated with 1 mM N-acetylcysteine (NAC), an antioxidant, for 1 h, and then exposed to 1 μg/ml LPS for another 24 h (c, d). Intracellular ROS (c) and apoptotic cells (d) were determined with the aid of a flow cytometer. Each value represents the mean ± SEM for n = 6. An asterisk and pound sign indicate that the values significantly (p < 0.05) differ from the control and LPS-treated groups, respectively
Exposure of A549 cells to 1 μg/ml LPS for 12 h did not alter the mitochondrial membrane potential (Fig. 4a). Meanwhile, after treatment with LPS for 24 and 48 h, the mitochondrial membrane potential had significantly decreased by 42 and 53%, respectively. Treatment of A549 cells with 1 μg/ml LPS for 12 h did not change the level of cellular ATP (Fig. 4b). Amounts of cellular ATP in A549 cells were considerably reduced by 36 and 46% following exposure to LPS for 24 and 48 h.
Time-dependent effects of lipopolysaccharide (LPS) on the mitochondrial membrane potential and adenosine triphosphate (ATP) synthesis. A549 cells were exposed to 1 μg/ml LPS for 12, 24, and 48 h. The mitochondrial membrane potential was determined using a flow cytometer (a). Levels of cellular ATP were measured by a bioluminescence assay (b). Each value represents the mean ± SEM for n = 6. An asterisk, indicates that the value significantly (p < 0.05) differs from the control group
Cyt c was detected in untreated A549 cells (Fig. 5a, top panel, lane 1). Exposure of A549 cells to 1 μg/ml LPS for 12 h did not affect the level of cytosolic Cyt c (lane 2). After treatment for 24 and 48 h, the amounts of Cyt c in the cytoplasm of A549 cells were obviously augmented (lanes 3 and 4). Levels of β-actin were immunodetected as the internal standard (Fig. 5a, bottom panel). These immunorelated protein bands were quantified and analyzed (Fig. 5b). Exposure of A549 cells to 1 μg/ml LPS for 24 and 48 h caused significant 2.1-fold and 94% increases in the levels of Cyt c, respectively.
Time-dependent effects of lipopolysaccharide (LPS) on the amounts of cytochrome c (Cyt C). A549 cells were exposed to 1 μg/ml LPS for 12, 24, and 48 h. Cytosolic proteins were prepared and electrophoretically separated. Levels of Cyt c were immunodetected by a mouse monoclonal antibody against human Cyt c (a, top panel). β-actin was determined as the internal control (bottom panel). These immunorelated protein bands were quantified and analyzed (b). Each value represents the mean ± SEM for n = 6. An asterisk indicates that the value significantly (p < 0.05) differs from the control group
Exposure of A549 cells to 1 μg/ml LPS for 12 h enhanced caspase-9 activity by 67% (Fig. 6a). Activities of caspase-9 were significantly increased by 2.4- and 2.5-fold following exposure to 1 μg/ml LPS for 24 and 48 h, respectively. Sequentially, treatment of A549 cells with 1 μg/ml LPS for 12 h did not affect caspase-6 activity (Fig. 6b). However, when the treatment time intervals reached 24 and 48 h, LPS caused significant 3.5- and 3.4-fold increases in caspase-6 activity. Consequently, exposure to LPS at 1 μg/ml for 12 h did not cause DNA fragmentation in A549 cells (Fig. 6c). After exposure for 24 and 48 h, LPS drastically induced DNA fragmentation of A549 cells by 2.4- and 3.2-fold, respectively.
Time-dependent effects of lipopolysaccharide (LPS) on the activities of caspases-9 and -3 and DNA fragmentation. A549 cells were exposed to 1 μg/ml LPS for 12, 24, and 48 h. Activities of caspase-9 and caspase-6 were measured using fluorogenic substrate assays (a, b). DNA fragmentation was quantified with a cellular DNA fragmentation enzyme-linked immunosorbent assay kit (c). Each value represents the mean ± SEM for n = 6. An asterisk and the pound sign indicate that values significantly (p < 0.05) differ from the control and LPS-treated groups, respectively
Exposure of A549 cells to 1 μg/ml LPS stimulated caspase-9 activation by 2.7-fold (Fig. 7a). Pretreatment with NAC alone did not affect caspase-9 activity, but caused a significant 45% reduction in this protease’s activity. In parallel, NAC did not cause DNA damage but significantly lessened LPS-induced DNA fragmentation by 41% (Fig. 7b).
Roles of reactive oxygen species (ROS) in lipopolysaccharide (LPS)-caused augmentations of caspase-9 activity and DNA fragmentation. A549 cells were pretreated with 1 mM N-acetylcysteine (NAC) for 1 h and then exposed to 1 μg/ml LPS for 24 h. Caspase-9 activity was assayed using a fluorogenic substrate method (a). DNA fragmentation was quantified with a cellular DNA fragmentation enzyme-linked immunosorbent assay kit (b). Each value represents the mean ± SEM for n = 6. An asterisk and the pound sign indicate that values significantly (p < 0.05) differ from the control and LPS-treated groups, respectively
Discussion
This study shows that LPS can induce insults to human lung carcinoma type II epithelium-like A549 cells via an apoptotic mechanism. Our previous study demonstrated the suppressive effects of LPS on regulating surfactant protein-A gene expression (Chuang et al. 2009). The present study further showed that LPS at a high concentration of 1 μg/ml can directly induce the death of A549 cells. In addition, our current results reveal that LPS augmented the fraction of A549 cells arrested at the sub-G1 phase. Cell arrest at the sub-G1 phase and shrunken morphologies are two typical characteristics exhibited by cells that are undergoing apoptosis (Fiers et al. 1999; Chen et al. 2005a, b). Furthermore, a necrotic analysis demonstrated that LPS only at a very high concentration of 10 μg/ml caused slight necrosis of A549 cells. Thus, 1 μg/ml LPS selectively caused apoptosis of A549 cells. Sauter and Wolfensberger (1980) reported that LPS at 1–2 μg could be lethal to the human. Furthermore, Bysani et al. (1990) showed that the plasma concentration of LPS in a patient with fatal Klebsiella pneumoniae sepsis was 25 ng/ml. Thus, the concentrations of LPS used in this study may be higher than its circulating levels in patients with acute lung injury or septic shock. LPS was reported to be one of the major causes of Gram-negative bacterium-caused septic shock and acute lung injury (Raetz et al. 1991; Welbourn and Yong 1992). Therefore, one of the possible mechanisms involved in LPS-induced acute lung injury during inflammation may occur through triggering apoptotic insults to alveolar type II epithelial cells.
ROS participate in LPS-induced apoptosis of A549 cells. Exposure of A549 cells to LPS time-dependently enhanced levels of intracellular ROS. Separately, the amounts of NO in A549 cells considerably increased following LPS administration. NO is an ROS (Wu et al. 2007; Cherng et al. 2008). Our previous studies showed that exposure of macrophages or osteoblasts to LPS with/without inflammatory cytokines caused NO overproduction and cellular oxidative stress (Chen et al. 2005a, b; Lee et al. 2010). Thus, augmentation of intracellular ROS in LPS-treated A549 cells is partially attributable to the production of NO. NAC is a well-known antioxidant (Chen et al. 2000). Pretreatment of A549 cells with NAC significantly suppressed LPS-enhanced intracellular ROS and concurrently protected against apoptotic insults. ROS are crucial apoptotic factors that can cause oxidative stress and subsequent cell apoptosis (Tai et al. 2007; Pellegrini and Baldari 2009). A previous study reported that NAC can lessen oxidative stress-induced allergic airway inflammation (Cho et al. 2008). As a result, augmentation of intracellular ROS may be one of the major factors contributing to LPS-induced apoptotic insults to alveolar type II epithelial A549 cells.
LPS induces mitochondrial dysfunction and cell apoptosis. Mitochondria are energy-producing organelles. This study showed that treatment of A549 cells with LPS decreased the mitochondrial membrane potential and autonomously reduced levels of cellular ATP in a time-dependent manner. Maintenance of the mitochondrial membrane potential is critical to the respiratory chain reaction and ATP synthesis (Chang et al. 2009). In consequence, LPS can disrupt the mitochondrial membrane potential and thus repress ATP synthesis in A549 cells. A previous study reported that Bax translocation to mitochondria from the cytoplasm can depolarize the mitochondrial membrane (Hsu et al. 1997). Thus, LPS decreases the mitochondrial membrane potential possibly by stimulating Bax translocation from the cytoplasm to mitochondria. In parallel with reducing the mitochondrial membrane potential, this study showed that LPS significantly increased intracellular ROS levels. ROS are mitochondrion-related apoptotic factors (Goyal 2001). A previous study stated that a decrease in cellular ATP synthesis can induce cell apoptosis (Blom et al. 2003). Therefore, LPS may cause mitochondrial dysfunction through suppression of the mitochondrial membrane potential in A549 cells and thus induce cell apoptosis.
Cyt c mediates LPS-induced apoptosis of A549 cells. Exposure of A549 cells to LPS time-dependently augmented cellular Cyt c levels. Cyt c is one of the key mitochondrion-related apoptotic factors (Saikumar et al. 1998). Depolarization of the mitochondrial membrane potential increases the release of apoptotic factors such as Cyt c from mitochondria to the cytoplasm, which leads to apoptotic insults (Saikumar et al. 1998; Ho et al. 2009; Chen et al. 2010). In this study, we showed that LPS decreased the mitochondrial membrane potential. Thus, LPS can enhance Cyt c release from mitochondria to the cytoplasm possibly through depolarization of the mitochondrial membrane. In an animal model of acute lung injury, Cyt c can mediate LPS-induced cell apoptosis (Koh et al. 2007). Furthermore, this study provides in vitro data to further demonstrate that LPS can specifically induce apoptotic insults to alveolar epithelial A549 cells in the course of upregulating the release of Cyt c from the mitochondria to the cytoplasm.
Cascade activation of caspase-9 and caspase-6 is involved in LPS-induced DNA fragmentation and cell apoptosis. Treatment of A549 cells with LPS increased caspase-9 activity in a time-dependent manner. After being released from mitochondria, Cyt c can interact with cytoplasmic apoptotic protease-activating factor-1 to form apoptosomes and mediate caspase-9 activation (Kagan et al. 2004). Thus, LPS-caused increases in Cyt c release can lead to caspase-9 activation. Consecutively, this study demonstrated the amplification of caspase-6 activity in LPS-treated A549 cells. After being triggered, caspase-9 has a cascade effect of promoting digestion of pro-caspase-6 into activated subunits (Goyal 2001). Hence, the LPS-caused activation of caspase-6 is due to the upstream commencement of caspase-9 triggered by this endotoxin. In parallel with sequential caspase activation, LPS induced DNA fragmentation of A549 cells. Additionally, our present results further demonstrate that pretreatment of A549 cells with NAC lowered LPS-stimulated caspase-9 activation. Consequently, LPS-induced apoptosis of A549 cells was significantly lessened by NAC. Caspase-9-mediated DNA fragmentation is a distinctive step that occurs in the intrinsic apoptotic pathway (Goyal 2001; Chen et al. 2007; Ferrari et al. 2009). Therefore, the LPS-induced cascade activation of caspase-9 and caspase-6 is involved in regulating apoptotic insults to alveolar type II epithelial A549 cells.
In conclusion, this study showed that LPS can directly damage alveolar type II epithelial A549 cells via an apoptotic mechanism. In parallel, exposure of A549 cells to LPS time-dependently raised the levels of cellular NO and intracellular ROS. Meanwhile, pretreatment of A549 cells with NAC, an antioxidant, appreciably alleviated LPS-caused increases in oxidative stress and cell apoptosis. Sequentially, LPS reduced the mitochondrial membrane potential and consequent ATP synthesis. After exposure to LPS, cytosolic Cyt c was significantly augmented in A549 cells. In succession, exposure of A549 cells to LPS caused cascade activation of caspase-9 and caspase-6. Concurrently, LPS time-dependently induced DNA fragmentation. However, pretreatment with NAC considerably attenuated LPS-induced caspase-9 activation and DNA damage. Therefore, according to the present data, we suggest that LPS induces apoptotic insults to alveolar type II epithelial A549 cells via an intrinsic mitochondrion-Cyt c-caspase protease pathway. There are certain limitations in the present study, including A549 cells are derived from human lung carcinoma. The mechanisms of LPS-induced oxidative stress and cell apoptosis in A549 cells may be different from normal alveolar epithelial cells. Thus, we performed translational study to evaluate the effects of LPS on alveolar epithelial cells of animals with acute lung injury.
References
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310
Blom WM, de Bont HJ, Nagelkerke JF (2003) Regional loss of the mitochondrial membrane potential in the hepatocyte is rapidly followed by externalization of phosphatidylserines at that specific site during apoptosis. J Biol Chem 278:12467–12474
Bysani GK, Shenep JL, Hildner WK, Stidham GL, Roberson PK (1990) Detoxification of plasma containing lipopolysaccharide by adsorption. Crit Care Med 18:67–71
Cazzola M, Page CP, Matera MG (2004) Alternative and/or integrative therapies for pneumonia under development. Curr Opin Pulmonary Med 10:204–210
Chang CC, Liao YS, Lin YL, Chen RM (2006) Nitric oxide protects osteoblasts from oxidative stress-induced apoptotic insults via a mitochondria-dependent mechanism. J Orthop Res 24:1917–1925
Chang HC, Chen TL, Chen RM (2009) Interruption of hepatocyte cytoskeletons by ketamine occurs through suppression of calcium mobilization and mitochondrial function. Drug Metab Dipos 37:24–31
Chang HC, Lin KH, Tai YT, Chen JT, Chen RM (2010) Lipoteichoic acid-induced TNF-α and IL-6 gene expressions and oxidative stress production in macrophages are suppressed by ketamine through downregulating toll-like receptor 2-mediated activation of ERK1/2 and NFκB. Shock 33:485–492
Chen RM, Chou MW, Ueng TH (2000) Induction of cytochrome P450 1A1 in human hepatoma HepG2 cells by 6-nitrochrysene. Toxicol Lett 117:69–77
Chen RM, Chen TL, Chiu WT, Chang CC (2005a) Molecular mechanism of nitric oxide-induced osteoblast apoptosis. J Orthop Res 23:462–468
Chen RM, Chen TL, Lin YL, Chen TG, Tai YT (2005b) Ketamine reduces nitric oxide biosynthesis in human umbilical vein endothelial cells through downregulating endothelial nitric oxide synthase expression and intracellular calcium levels. Crit Car Med 33:1044–1049
Chen TG, Chen TL, Chang HC, Tai YT, Cherng YG, Chang YT, Chen RM (2007) Oxidized low-density lipoprotein induces apoptotic insults to mouse cerebral endothelial cells via a Bax-mitochondria-caspase protease pathway. Toxicol Appl Pharmacol 219:42–53
Chen TL, Chang CC, Lin YL, Ueng YF, Chen RM (2009) Signal-transducing mechanisms of ketamine-caused inhibition of interleukin-1β gene expression in lipopolysaccharide-stimulated murine macrophage-like Raw 264.7 cells. Toxicol Appl Pharmacol 240:15–25
Chen TL, Wu GJ, Hsu CS, Fong TH, Chen RM (2010) Oxidative stress induces apoptotic insults to rat osteoblasts by suppressing phosphorylation of mitogen-associated protein kinases, activation of nuclear factor-kappaB and activator protein-1, and subsequent Bcl-XL expression. Chem Biol Interact 184:359–365
Cherng YG, Chang HC, Lin YL, Kuo ML, Chiu WT, Chen RM (2008) Apoptotic insults to human chondrocytes induced by nitric oxide are involved in sequential events, including cytoskeletal remodeling, phosphorylation of mitogen-activated protein kinase kinase kinase-1, and Bax-mitochondria-mediated caspase activation. J Orthop Res 26:1018–1026
Cho YS, Oh SY, Zhu Z (2008) Tyrosine phosphatase SHP-1 in oxidative stress and development of allergic airway inflammation. Am J Respir Cell Mol Biol 39:412–419
Chuang CY, Chen TL, Chen RM (2009) Molecular mechanisms of lipopolysaccharide-caused induction of surfactant protein-A gene expression in human alveolar epithelial A549 cells. Toxicol Lett 191:132–139
Clements JA, Avery ME (1998) Lung surfactant and neonatal respiratory distress syndrome. Am J Respir Crit Care Med 157:S59–S66
Crouch E, Wright JR (2001) Surfactant proteins A and D and pulmonary host defense. Ann Rev Physiol 63:521–554
Ferrari R, Ceconi C, Campo G, Cangiano E, Cavazza C, Secchiero P, Tavazzi L (2009) Mechanisms of remodelling: a question of life (stem cell production) and death (myocyte apoptosis). Circ J 73:1973–1982
Fiers W, Beyaert R, Declercq W, Vandenabeele P (1999) More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene 18:7719–7730
Goyal L (2001) Cell death inhibition: keeping caspases in check. Cell 104:805–808
Ho WP, Chan WP, Hsieh MS, Chen RM (2009) Runx2-mediated Bcl-2 gene expression contributes to nitric oxide protection against oxidative stress-induced osteoblast apoptosis. J Cell Biochem 108:1084–1093
Hsu YT, Wolter KG, Youle RJ (1997) Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proc Natl Acad Sci USA 94:3668–3672
Kagan VE, Borisenko GG, Tyurina YY, Tyurin VA, Jiang J, Potapovich AI, Kini V, Amoscato AA, Fujii Y (2004) Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Rad Biol Med 37:1963–1985
Kitchens RL, Thompson PA, Viriyakosol S, O’Keefe GE, Munford RS (2001) Plasma CD14 decreases monocyte responses to LPS by promoting the transfer of cell-bound LPS to plasma lipoproteins. J Clin Invest 108:485–493
Koh H, Tasaka S, Hasegawa N, Yamada W, Shimizu M, Nakamura M, Yonemaru M, Ikeda E, Adachi Y, Fujishima S, Yamaguchi K, Ishizaka A (2007) Protective role of vascular endothelial growth factor in endotoxin-induced acute lung injury in mice. Respir Res 8:60
Lee ST, Wu TT, Yu PY, Chen RM (2009) Apoptotic insults to human HepG2 cells induced by S-(+)-ketamine occurs through activation of a Bax-mitochondria-caspase protease pathway. Br J Anaesth 102:80–89
Lee CJ, Tai YT, Lin YL, Chen RM (2010) Molecular mechanisms of propofol-involved suppression of nitric oxide biosynthesis and inducible nitric oxide synthase gene expression in lipopolysaccharide-stimulated macrophage-like RAW 264.7 cells. Shock 33:93–100
LeVine AM, Whitsett JA, Gwozdz JA, Richardson TR, Fisher JH, Burhans MS, Korfhagen TR (2000) Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J Immunol 165:3934–3940
Mendelson CR (2000) Role of transcription factors in fetal lung development and surfactant protein gene expression. Ann Rev Physiol 62:875–915
Pellegrini M, Baldari CT (2009) Apoptosis and oxidative stress-related diseases: the p66Shc connection. Curr Mol Med 9:392–398
Raetz CR, Ulevitch RJ, Wright SD, Sibley CH, Ding A, Nathan CF (1991) Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J 5:2652–2660
Rooney SA (2001) Regulation of surfactant secretion. Comp Biochem Physiol 129:233–243
Saikumar P, Dong Z, Patel Y, Hall K, Hopfer U, Weinberg JM, Venkatachalam MA (1998) Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene 17:3401–3415
Sauter C, Wolfensberger C (1980) Interferon in human serum after injection of endotoxin. Lancet 18:852–853
Tai YT, Cherng YG, Chang CC, Hwang YP, Chen JT, Chen RM (2007) Pretreatment with low nitric oxide protects osteoblasts from high nitric oxide-induced apoptotic insults through regulation of c-Jun N-terminal kinase/c-Jun-mediated Bcl-2 gene expression and protein translocation. J Orthop Res 25:625–635
Vernooy JHJ, Mentener MA, van Suylen RJ, Buurman WA, Wouters EFM (2001) Intratracheal instillation of lipopolysaccharide in mice induces apoptosis in bronchial epithelial cells. Am J Respir Cell Mol Biol 24:569–576
Waak J, Weber SS, Waldenmaier A, Gorner K, Alunni-Fabbroni M, Schell H, Vogt-Weisenhorn D, Pham TT, Reumers V, Baekelandt V, Wurst W, Kahle PJ (2009) Regulation of astrocyte inflammatory responses by the Parkinson’s disease-associated gene DJ-1. FASEB J 23:2478–2489
Welbourn CRB, Yong Y (1992) Endotoxin, septic shock and acute lung injury: neutrophils, macrophages, and inflammatory mediators. Br J Surg 79:998–1003
Wu GJ, Chen TG, Chang HC, Chiu WT, Chang CC, Chen RM (2007) Nitric oxide from both exogenous and endogenous sources activates mitochondria-dependent events and induces insults to human chondrocytes. J Cell Biochem 101:1520–1531
Acknowledgments
This study was supported by the Department of Health, Taipei City Hospital (94002-62-005 and 95001-62-017), Taipei, Taiwan. The authors express their gratitude to Ms. Yi-Ling Lin for technical support and data collection during the experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chi-Yuan Chuang and Ta-Liang Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chuang, CY., Chen, TL., Cherng, YG. et al. Lipopolysaccharide induces apoptotic insults to human alveolar epithelial A549 cells through reactive oxygen species-mediated activation of an intrinsic mitochondrion-dependent pathway. Arch Toxicol 85, 209–218 (2011). https://doi.org/10.1007/s00204-010-0585-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-010-0585-x