Abstract
To investigate the effects of silver nanoparticles on the histological structure and properties of the mucosubstances in the intestinal mucosa, Sprague–Dawley rats were divided into four groups (10 rats in each group): vehicle control, low-dose group (30 mg/kg), middle-dose group (300 mg/kg), and high-dose group (1,000 mg/kg), and administered silver nanoparticles (60 nm) for 28 days, following OECD test guideline 407 and using GLP. The control sections contained no silver nanoparticles; however, the treated samples showed luminal and surface particles and the tissue also contained silver nanoparticles. A dose-dependent increased accumulation of silver nanoparticles was observed in the lamina propria in both the small and large intestine, and also in the tip of the upper villi in the ileum and protruding surface of the fold in the colon. The silver nanoparticle-treated rats exhibited higher numbers of goblet cells that had released their mucus granules than the controls, resulting in more mucus materials in the crypt lumen and ileal lumen. Moreover, cell shedding at the tip of the villi was frequent. Lower amounts of neutral and acidic mucins were found in the goblet cells in the silver nanoparticle-treated rats, plus the amount of sialomucins was increased, while the amount of sulfomucins was decreased. In particular, in the colon of the silver nanoparticle-treated rats, sialyated mucins were detected in the lamina propria, the connective tissue under the epithelia. Therefore, the present results suggest that silver nanoparticles induce the discharge of mucus granules and an abnormal mucus composition in the goblet cells in the intestines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various studies have already established the strong antibacterial property of silver nanoparticles (Feng et al. 1999; Sondi and Salopek-Sondi 2004; Morones et al. 2005; Li et al. 2005; Percival et al. 2005; Li et al. 2006; Kim et al. 2007). The extremely small size of nanoparticles means their surface area is large relative to their volume and their properties are enhanced when compared to the bulk material. In the case of silver nanoparticles, this allows them to interact easily with other particles and increases their antibacterial efficiency. Silver nanoparticles kill all types of fungal infections, bacteria, and viruses, including antibiotic-resistant strains. Meanwhile, the strong toxicity exhibited by various chemical forms of silver toward a wide range of microorganisms is also widely known (Liau et al. 1997; Gupta and Silver 1998; Nomiya et al. 2004).
The U.S. Agency for Toxic Substances and Disease Registry (ATSDR) previously published a review of the toxicology of silver nitrate, silver chloride, silver sulfide, and silver oxide, in which they stated that the health effects resulting from short- and long-term exposure to air containing specific levels of silver are not known (ATSDR 2005). Nonetheless, a previous study by the current authors on the 28-day oral toxicity of silver nanoparticles (60 nm) at doses of 30 mg/kg, 300 mg/kg, and 1,000 mg/kg, following OECD test guideline 407 and good laboratory practices (GLP), indicated that exposure to over 300 mg of silver nanoparticles could result in slight liver damage, plus a gender-related difference was noted in the accumulation of silver in the kidneys, with a twofold higher accumulation in the female kidneys when compared with the male kidneys (Kim et al. 2008).
It has already been shown that silver nanoparticles can enter the human body through several ports, and there have been several excellent reviews on the intestinal uptake of particles (Florence and Hussain 2001; Hussain et al. 2001). In this context, mucins are large glycoproteins secreted by mucous cells in the intestinal epithelium that act as a medium for lubrication, transport between the luminal contents and the epithelial cells, and protection against mechanical damage and penetration by microorganisms and bacterial toxins (Allen et al. 1976; Neutra and Forstner 1987; Forstner et al. 1995; Belly et al. 1999). Mucins also serve as a protective barrier against acid and pepsin digestion (Kemper and Specian 1991).
Accordingly, this study investigated the changes in the histological structures and properties of mucins to assess the effects of oral exposure to silver nanoparticles on the intestinal mucosa in rats.
Materials and methods
Silver nanoparticles
The silver nanoparticles (52.7–70.9 nm, average; 60 nm) were purchased from Namatech Co., Ltd. (Korea) and at least 99.98% pure (Kim et al. 2008). The vehicle, 0.5% aqueous Carboxy methylcellulose (CMC), was obtained from Sigma (USA).
Animals and conditions
Four-week-old male and female, specific pathogen-free (SPF) Sprague–Dawley rats were purchased from OrientBio (Korea) and acclimated for 2 weeks before starting the experiments. During the acclimation and experimental periods, the rats were housed in polycarbonate cages (maximum of 3 rats per cage) in a room with controlled temperature (23 ± 2°C) and humidity (55 ± 7%), and a 12-h light/dark cycle. The rats were fed rodent chow (Harlan Teklab, Plaster International Co., Korea) and filtered water ad libitum. At 5 weeks, the rats were divided into four groups (10 rats in each group): vehicle control (0.5% carboxy methyl cellulose), low-dose group (30 mg/kg per day), middle-dose group (300 mg/kg per day), and high-dose group (1,000 mg/kg per day). At 6 weeks, the rats were exposed to silver nanoparticles following OECD test guideline 407 (OECD 1995), based on 28 days of repeated oral administration (dosing volumes were 10 ml/kg) using GLP.
Histological analysis
At the conclusion of the 4-week experiment, the rats were 10 weeks old. Before the necropsy, food was withheld for 24 h and the rats anesthetized with ether. After collecting their blood, the rats were sacrificed by cervical dislocation. The ileum, colon, and rectum were all carefully removed, then weighed and fixed in a 10%-formalin solution containing neutral phosphate buffered saline. Thereafter, the organs were embedded in paraffin, stained with hematoxylin and eosin (H–E) and a Periodic acid Schiff (PAS) reaction, and examined under a light microscope.
Histochemistry of mucins
To determine the general histochemistry of the mucins, the following staining procedures were used: a PAS reaction to detect neutral mucins, alcian blue (AB) staining at pH 2.5 to detect acidic mucins, AB staining at pH 2.5 and a PAS reaction to characterize the neutral mucins and acidic mucins, and high iron diamine (HID) and AB staining at pH 2.5 to distinguish between sulfated and nonsulfated (sialyated) mucins.
Results
Histological findings
The experimental samples at the high and middle dose revealed luminal and surface particles, plus silver nanoparticles were found within the tissue. Higher numbers of silver nanoparticles were also found in the lamina propria (connective tissue under the epithelia) in both the small and large intestine, and in the tip of the upper villi in the ileum and protruding surface of the fold in the colon (figures not shown).
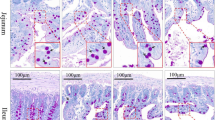
In the control rat ileum, the goblet cells in the villi and crypt epithelium were revealed as densely stained mucus granules that filled the apical cytoplasm (Fig. 1a). Meanwhile, in the silver nanoparticle-administered rats, the ileal mucosa exhibited two different regions, where the first showed no change in the architecture of the epithelia compared to the control rats, while the other showed disordered goblet cells. In the silver nanoparticle-treated rats, the numbers of goblet cells that had released their mucus granules were higher than those in the controls, there were also large amounts of mucus material in the crypt lumen and ileal lumen (Fig. 1b), and cell shedding at the tip of the villi was frequent.
AB pH 2.5-PAS staining of ileum mucosa from control and silver nanoparticle-administered rats. In the silver nanoparticle-administered rats (b), higher numbers of goblet cells were found to have released their mucus granules, resulting in more mucus materials in the crypt lumen and ileal lumen, plus the staining intensity of the goblet cells was lower when compared with that for the control rats (a). Magnification ×400
In rats, the proximal colon differs from the distal colon with respect to the surface structure, the form of the crypt, and the depth of the crypt. However, this study was limited to the proximal region of the colon, and no significant changes in the goblet cells from the proximal colon were observed for the silver nanoparticles-treated rats. In contrast, the goblet cells in the rectum of the silver nanoparticle-treated rats were found to have released their mucus granules and were occasionally destroyed (Fig. 2b).
AB pH 2.5-PAS staining of rectum mucosa from control and silver nanoparticle-administered rats. In the control rectum mucosa (a), goblet cells with densely stained granules were observed along the length of the crypt. Meanwhile, in the silver nanoparticle-treated rectum mucosa (b), the number of stained mucus cells was decreased, the crypt lumen was expanded, and mucus was released from the goblet cells. Magnification ×400
Histochemistry of mucins
The histochemical results for the mucins in the ileum, colon, and rectum mucosa using PAS, AB pH 2.5, AB pH 2.5-PAS, and HID-AB pH 2.5 staining are given in Tables 1, 2, and 3. The numerical values from 1 to 4 correspond to an increasing intensity of staining. However, the results represent a composite of subjective evaluations covering a range of values, thus the findings may have differed for any one individual. A numerical evaluation of the staining of the brush border of the epithelial cells was not attempted.
Neutral and acidic mucosubstances were both demonstrated in goblet cells from the villi and crypts of the ileum mucosa in the control rats, and sulfated mucins were more prominent than nonsulfated (sialyated) mucins. Furthermore, while neutral mucosubstances were found in epithelial goblet cells throughout the large intestine, acid mucosubstances were generally predominant, with sulfated mucins being particularly prominent in the distal colon. Meanwhile, sialyated mucins, with variations in the individual amounts present, were most prominent in the lower regions of the crypts, yet mixed with sulfomucins in the upper regions. Finally, in the distal colon, sulfated mucins were predominant throughout, with occasional cells showing some sialyated mucins.
As shown in Tables 1, 2, and 3, goblet cells from the ileum, colon, and rectum mucosa of the silver nanoparticle-administered rats showed decreased amounts of neutral and acidic mucins (Figs. 1b, 2b), and this was even more marked in the regions shedding goblet cells. In particular, in the ileum of the silver nanoparticle-treated rats, the mucin distribution differed greatly between the normal and abnormal regions. A significant decrease of both neutral and acidic mucins was observed in the ileum mucosa (Fig. 1b). Plus, in the silver nanoparticle-treated rats, the proportion of sulfated mucins decreased, while the proportion of nonsulfated (sialyated) mucins increased, however, the total amount of acidic mucins decreased (Figs. 3b, 4b). In particular, an HID-positive reaction was detected in the lamina propria (connective tissue under the epithelia) of the colon, indicating the presence of sialyated mucins, which was not found in the control rats (Fig. 5).
Discussion
The antibacterial activity of silver nanoparticles has resulted in their widespread use in bedding, washers, water purification, toothpaste, shampoo and rinse, nipples and nursing bottles, fabrics, deodorants, filters, kitchen utensils, toys, and humidifiers (Maynard 2006; KISTI 2006). Silver nanoparticles have also recently been shown to be a promising antimicrobial material (Sondi and Salopek-Sondi 2004). However, the toxicity of silver nanoparticles in biological systems remains a controversial research area, due to the limited availability of information.
In a previous inhalation study, the present authors found no significant toxicity in Sprague–Dawley rats that had been repeatedly exposed to silver nanoparticles via inhalation, based on 6 h each day, 5 days a week, for a total of 4 weeks. Plus, no significant changes were found in the male and female body weights or hematology and blood biochemical values relative to various concentrations of silver nanoparticles during the 28-day experiment (Ji et al. 2007). Meanwhile, in another oral toxicity study using male and female Sprague–Dawley rats, the present authors found that oral exposure to silver nanoparticles (60 nm) at concentrations of 30, 300, and 1,000 mg/kg for 28 days had no affect on micronucleated polychromatic erythrocytes or bone marrow cells. However, the repeated oral doses of silver nanoparticles did induce liver toxicity and had a coagulation effect on peripheral blood. The histopathologically evaluated liver toxicity included dilatation of the central vein, bile duct, and hyperplasia (Kim et al. 2008).
In this study, the experimental samples revealed luminal and surface particles and the tissue also contained silver nanoparticles. An increased accumulation of silver nanoparticles was observed in the lamina propria (connective tissue under the epithelia) in both the small and large intestine.
The mucus secreted into the lumen from intestinal goblet cells forms a gel layer that covers the mucosal surface of the intestinal tract, acting as a semipermeable barrier between the lumen and epithelium. The stability of this mucus layer is then essential in preserving the integrity of the intestinal epithelium, and any breakdown of this protection can lead to mucosal injury. Therefore, this study investigated whether silver nanoparticles would modulate the secretion of intestinal mucus, a major component of physiological defense mechanisms. The oral administration of silver nanoparticles was found to induce the discharge of mucus from goblet cells, mostly in the ileum and rectum, yet not in the proximal colon. Thus, since goblet cells releasing mucus were frequently found in the silver nanoparticle-administered rats, this indicates that the silver nanoparticles did not allow the cells to be replenished by mucin synthesis. As the gel layer formed by the mucus on the surface of the intestinal epithelium has a barrier function, the present data may be relevant physiologically to the defense mechanisms of the gastrointestinal tract. Goblet cells secrete mucins via an unregulated constitutive pathway that is dependent on the continuous movement of mucin granules from the Golgi apparatus to the apex of the cell and by a regulated process dependent on the sudden release of mucins from the granules. This regulatory process is controlled by a wide variety of stimuli, including nerve activation and inflammatory mediators (Neutra et al. 1982; Branka et al. 1997; Plaisancie et al. 1998; Verburg et al. 2002; Smirnova et al. 2003; Blanchard et al. 2004). In this study, silver nanoparticles were demonstrated to be one of these stimuli.
This study also found that the intestinal goblet cells from the silver nanoparticle-administered rats showed a decreased amount of neutral and acidic mucins. Meanwhile, the proportion of sulfated mucins was decreased, while the proportion of sialyated mucins was increased, although the total amount of acidic mucins was decreased. The increase in sialyated mucins observed in this study was similar to the intestinal mucin change in the case of active ulcerative colitis (Filipe and Dawson 1970), small intestine carcinoma (Filipe and Fenger 1979), and the administration of organophosphorus pesticides to rats (Lee 1979; Jo and Kim 1987), although the toxicants were different. Filipe and Branfoot (1974) reported that the transitional mucosa, the normal mucosa adjacent to carcinomas, in the large intestine is characterized by an increase of sialyated mucins, accompanied usually by a decrease or absence of sulfated mucins, which are predominant in the normal mucosa of the large intestine. In addition, higher quantities of neuraminidase-sensitive sialic acids in the mucosa around a tumor have also been reported (Filipe 1971; Filipe and Cooke 1974; Dawson et al. 1978). Thus, evidence of sialyated mucins in the mucosa around a tumor may be an index of the tumor’s aggressiveness. As such, the presence of sialyated mucins in the lamina propria (connective tissue under the epithelia) of the colon in this study, which was not found in the control rats, would seem to indicate that repeated silver nanoparticle-exposure may produce pathologic regions.
In conclusion, this study suggests that silver nanoparticles are a powerful intestinal secretagogue and induce an abnormal mucin composition in the intestinal mucosa. Thus, further studies are needed to determine the relevance of these findings to the pathophysiology of the gastrointestinal tract.
References
Allen A, Pain RH, Robson TR (1976) Model for the structure of the gastric mucous gel. Nature 264:88–89
ATSDR (Agency for Toxic Substances and Disease Registry) (2005) Toxprofiles 2005, US Department of Health & Human Services, Atlanta, GA
Belly A, Keller K, Gottke M, Chadee K, Goettke M (1999) Intestinal mucins in colonization and host defense against pathogens. Am Trop Med Hyg 60:10–15
Blanchard C, Durual S, Estienne M, Bouzakri K, Heim MH, Blin N, Cuber JC (2004) IL-4 and IL-13 up-regulate intestinal trefoil factor expression: requirement for STAT6 and de novo protein synthesis. J Immunol 172:3775–3783
Branka JE, Vallette G, Jarry A, Laboisse CL (1997) Stimulation of mucin exocytosis from human epithelial cells by nitric oxide: evidence for a cGMP-dependent and a cGMP-independent pathway. Biochem J 323:521–524
Dawson PA, Patel I, Filipe MI (1978) Variation in sialomucins in the mucosa of the large intestine in malignancy: a quantimet and statistical analysis. Histochem J 10:559–572
Feng QL, Cui FZ, Kim TN, Kim JW (1999) Ag-substituted hydroxyapatite coatings with both antimicrobial effects and biocompatibility. J Mater Sci Lett 18:559–561
Filipe MT (1971) The mucous membrane of the normal human large intestine and the changes which occur in it immediately adjacent to proven carcinoma—a histochemical, autoradiographic and chemical study. PhD thesis, University of London
Filipe MI, Branfoot AC (1974) Abnormal patterns of mucous secretion in apparently normal mucosa of large intestine with carcinoma. Cancer 34:282–290
Filipe MI, Cooke BK (1974) Changes in mucin composition in the mucosa adjacent to carcinoma of the colon as compared with the normal—a biochemical investigation. J Clin Pathol 27:315–318
Filipe MI, Dawson I (1970) The diagnostic value of mucosubstances in rectal biopsies from patients with ulcerative colitis and Crohn’s disease. Gut 11(3):229–234
Filipe MI, Fenger C (1979) Histochemical characteristics of mucins in the small intestine. A comparative study of normal mucosa, benign epithelial tumours and carcinoma. Histochem J 11(3):277–287
Florence AT, Hussain N (2001) Transcytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Adv Drug Deliv Rev 50:S69–S89
Forstner J, Oliver M, Sylvester F (1995) Production, structure and biologic relevance of gastrointestinal mucins. In: Blaser M, Smith P, Ravdin J, Greenberg H, Guerrant R (eds) Infections of the gastrointestinal tract. Raven Press, New York, pp 71–88
Gupta A, Silver S (1998) Silver as biocide: will resistance become a problem? Nat Biotechnol 16:888
Hussain N, Jaitley V, Florence AT (2001) Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Deliv Rev 50:107–142
Ji JH, Jung JH, Kim SS, Yoon JU, Park JD, Choi BS, Chung YH, Kwon IH, Jeong J, Han BS, Shin JH, Sung JH, Song KS, Yu IJ (2007) A twenty-eight-days inhalation toxicity study of silver nanoparticles in Sprague–Dawley rats. Inhalat Toxicol 19(10):857–871
Jo UB, Kim BS (1987) Histochemical study on the effect of the pyridine herbicide, gramoxone, on the mucosubstances of the goblet cells in the rat small intestine. Korean J Anat 20(2):299–317 (in Korean)
Kemper AC, Specian RD (1991) Rat small intestinal mucins: a quantitative analysis. Anat Rec 229:219–226
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH (2007) Antimicrobial effects of silver nanoparticles. Nanomedicine 3:95–101
Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, Choi BS, Lim R, Chang HK, Chung YH, Kwon IH, Jeong J, Han BS, Yu IJ (2008) Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague–Dawley Rats. Inhalat Toxicol 20(6):575–583
KISTI (Korea Institute of Science and Technology Information) (2006) The present status of Korean nanotechnology industrialization. Nano Weekly, No. 210. KISTI, Seoul, Korea
Lee MK (1979) Histochemical studies on the effect of organophosphorus pesticides on the mucosubstances in the duodenal glands and goblet cells of the duodenal mucosa in the rat. Korean J Anat 12:111–126 (in Korean)
Li P, Li J, Wu C, Wu Q, Li J (2005) Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology 16:1912–1917
Li Y, Leung P, Yao L, Song QW, Newton E (2006) Antimicrobial effect of surgical masks coated with nanoparticles. J Hosp Infect 62:58–63
Liau SY, Read DC, Pugh WJ, Furr JR, Russell AD (1997) Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterial action of silver ions. Lett Appl Microbiol 25:279–283
Maynard AD (2006) Nanotechnology: a research strategy for addressing risk. Woodrow Wilson International Center for Scholars, Washington, DC
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353
Neutra MR, Forstner JF (1987) Gastrointestinal mucus: synthesis, secretion and function. In: Johnson LR (ed) Physiology of the gastrointestinal tract. Raven Press, New York, pp 975–1009
Neutra MR, O’Malley LJ, Specian RD (1982) Regulation of intestinal goblet cell secretion. A survey of potential secretagogues. Am J Physiol Gastrointest Liver Physiol 242:G380–G387
Nomiya K, Yoshizawa A, Tsukagoshi K, Kasuga NC, Hirakawa S, Watanabe J (2004) Synthesis and structural characterization of silver (I), aluminium (III) and cobalt (II) complexes with 4-isopropyltropolone (hinokitiol) showing noteworthy biological activities. Action of silver (I)-oxygen bonding complexes on the antimicrobial activities. J Inorg Biochem 98:46–60
OECD (1995) OECD guidelines for the testing of chemicals, Test guideline 407. Repeated dose 28-day oral toxicity study in rodent, Paris
Percival SL, Bowler PG, Russell D (2005) Bacterial resistance to silver in wound care. J Hosp Infect 60:1–7
Plaisancie P, Barcelo A, Moro F, Claustre J, Chayvialle JA, Cuber JC (1998) Effects of neurotransmitters, gut hormones, and inflammatory mediators on mucus discharge in rat colon. Am J Physiol Gastrointest Liver Physiol 275:G1073–G1084
Smirnova MG, Guo L, Birchall JP, Pearson JP (2003) LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol 221:42–49
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interf Sci 275:177–182
Verburg M, Renes IB, Van Nispen DJ, Ferdinandusse S, Jorritsma M, Buller HA, Einerhand AW, Dekker J (2002) Specific responses in rat small intestinal epithelial mRNA expression and protein levels during chemotherapeutic damage and regeneration. J Histochem Cytochem 50:1525–1536
Acknowledgments
This research was supported by Nano R&D program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2009-0082677).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeong, G.N., Jo, U.B., Ryu, H.Y. et al. Histochemical study of intestinal mucins after administration of silver nanoparticles in Sprague–Dawley rats. Arch Toxicol 84, 63–69 (2010). https://doi.org/10.1007/s00204-009-0469-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-009-0469-0