Abstract
Magnolol, a compound extracted from the Chinese medicinal herb Magnolia officinalis, has several biological effects. However, its protective effects against endothelial injury remain unclear. In this study, we examined whether magnolol prevents oxidized low density lipoprotein (oxLDL)-induced vascular endothelial apoptosis. Incubation of oxLDL with magnolol (2.5–20 μM) inhibited copper-induced oxidative modification via diene formation, thiobarbituric acid reactive substances (TBARS) assay and electrophoretic mobility assay. Apoptotic cell death as characterized by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) stain. We measured the production of reactive oxygen species (ROS) by using the fluorescent probe 2′,7′-dichlorofluorescein acetoxymethyl ester (DCF-AM), and observed the activity of antioxidant enzymes. Furthermore, several apoptotic signaling pathways which showed NF-κB activation, increased cytosolic calcium, alteration of mitochondrial membrane potential, cytochrome c release and activation of caspase 3 were also investigated. We demonstrated that magnolol prevented the copper-induced oxidative modification of LDL. Magnolol attenuated the oxLDL-induced ROS generation and subsequent NF-κB activation. Furthermore, intracellular calcium accumulation and subsequent mitochondrial membrane potential collapse, cytochome c release and activation of caspase 3 caused by oxLDL were also inhibited by magnolol. Our results suggest that magnolol may have clinical implications in the prevention of atherosclerotic vascular disease through decreasing the oxLDL-induced ROS production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A high plasma level of cholesterol is a well-known risk factor for atherosclerotic disease. Oxidation of low density lipoprotein is believed to play an important role in the pathogenesis of atherosclerosis. In early atherosclerotic lesions, the amount of oxidized LDL (oxLDL) is usually low and exerts a mainly proinflammatory effect (Cushing et al. 1990). In more advanced atherosclerotic lesions, increased accumulation of oxLDL containing more peroxidation products may generate a cytotoxic effect contributing to plaque degeneration and rupture. OxLDL alters the intracellular redox status of the cells, in part through the generation of reactive oxygen species (ROS), and has been implicated as an important pro-oxidant signal in endothelial cells (Berliner et al. 1995). ROS regulation of signal transduction components include modifications of the activity of transcriptional factors such as NF-κB, a vital signaling factor for several vascular events, resulting in changes in gene expression and modifications in cellular responses, which may contribute to oxLDL-induced apoptosis (Harada-Shiba et al. 1998; Heermeier et al. 1999). Hsieh et al. (2001) demonstrated that ROS generation and apoptosis were tightly coupled in oxLDL-treated vascular cells. In addition, antioxidants that reduced ROS level inhibited apoptosis, while those that did not reduce ROS level were ineffective.
Magnolol, an active component isolated from the herb ‘Houpo’ (Magnolia officinalis), is widely used in the treatment of a number of diseases in traditional Chinese medicine due to its anti-allergic effects (Tohda et al. 1999), anxiolytic effects, anti-ulcer effects (Ikarashi et al. 2001) and inhibitory effects on skin tumors (Ikeda and Nagase 2002). It has been shown to possess antioxidant activity approximately 1,000 times greater than α-tocopherol (Lo et al. 1994). Therefore, it is expected that magnolol might play an important role in preventing the proatherogenic stimuli which leads to increased production of ROS within endothelial cells. Early studies have shown that magnolol protected rat heart mitochondria against lipid peroxidation (Lo et al. 1994), and free radical mediated cerebral ischaemic injury (Chang et al. 2003), reduced superoxide anion production and myeloperoxidase activity, an index of neutrophil infiltration in the ischemic myocardium (Lee et al. 2001). However, the molecular and cellular mechanisms of magnolol on oxLDL-induced endothelial apoptosis were not fully elucidated. To test the hypothesis that magnolol may have effects on the prevention of atherosclerosis, we undertook the current study to explore whether magnolol prevented oxidative modification of LDL and protected against oxLDL-induced cytotoxicity. Apoptotic signaling including ROS generation, NF-κB activation, intracellular calcium accumulation, mitochondrial destabilization and activation of caspase 3 were also investigated.
Materials and methods
Chemicals
Magnolol was prepared as previously described (Yang et al. 2003). Chemicals were obtained from the following companies: M199, fetal bovine serum, and trypsin-EDTA from Gibco (Grand Island, NY, USA); low serum growth supplement (Cascade, OR, USA), 4,6-diamidino-2-phenylindole (DAPI), ethylene diaminotetraacetic acid (EDTA), penicillin, and streptomycin from Sigma (St. Louis, MO, USA); lactate dehydrogenase (LDH) kits and TUNEL staining kit from Boehringer Mannheim (Mannheim, Germany); superoxide dismutase activity assay kit from Calbiochem (San Diego, CA, USA); Fura 2-AM, DCF-AM from Molecular Probes (Eugene, OR, USA); 5,58,6,68-tetraethylbenzimidazolcarbocyanine iodide (JC-1) and anti-active caspase 3 from BioVision (Palo Alto, CA, USA).
Cell cultures
Human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical cord with collagenase and used at passage 2–3 (Jaffe et al. 1973). After dissociation, the cells were collected and cultured on gelatin-coated culture dishes in medium 199 with low serum growth supplement, 100 IU/ml penicillin, and 0.1 mg/ml streptomycin. Subcultures were performed with trypsin-EDTA. Media were refreshed on every second day. The identity of umbilical vein endothelial cells was confirmed by their cobblestone morphology and strong positive immunoreactivity to von Willebrand factor.
Lipoprotein separation
Native LDL was isolated and oxidatively modified from fresh normolipidemic human serum by sequential ultracentrifugation as previously described (Havel et al. 1955). Immediately before oxidation tests LDL was separated from EDTA and from diffusible low molecular mass compounds by gel filtration on PD-10 Sephadex G-25 M gel (Pharmacia) in 0.01 mol/l phosphate-buffered saline (136.9 mmol/l NaCl, 2.68 mmol/l KCl, 4 mmol/l Na2HPO4, 1.76 mmol/l KH2PO4) pH 7.4. EDTA-free LDL was immediately used for the measurement of the kinetics of LDL oxidation by continuously monitoring the change in the absorbency of conjugated dienes at 232 nm at 37°C. Absorbance was measured with a Beckman DU740 spectrophotometer equipped with a six-position automated cuvette changer and a Peltier temperature controller (Beckman Instruments, Mississauga, ON, Canada), essentially as described by Esterbauer et al (1989). Briefly, 100 μg of LDL (protein) was diluted with 0.01 M phosphate-buffered saline, and oxidation was initiated by 10 μM CuSO4 (final concentration). Absorbance was recorded every 10 min, and the kinetics of oxidation were followed by measuring the increase in the levels of conjugated dienes at 37°C for up to 7 h. The lag time (lag phase, resistance time) was defined as the point at which the initial slope representing the initiation rate and the point at which the propagation slope intersected. This was calculated from the kinetic plot of the change of absorbance versus time as described previously (Wilson et al. 2002). In some experiments, LDL oxidation was also measured by thiobarbituric acid reactive substances (TBARS) as described previously (Buege and Aust 1978). Malondialdehyde was used as a standard. LDL oxidation was also checked by measuring the electrophoretic mobility of LDL on a 1% agarose gel in 0.1 M Tris–HCl pH 8.6. The gel was stained with Sudan Black as previously described (Terpstra et al. 1981). Depending on the degree of oxidation, the electrophoretic mobility of freshly oxidized LDL was approximately 1.4–1.7 times that of native LDL. The Cu++-modified LDL (1 mg protein/ml) was prepared by exposing LDL to 10 μM CuSO4 for 16 h at 37°C. Protein levels were measured by the methods of Bradford (Bradford 1976).
Determination of cytotoxicity and indices of apoptosis
To determine the effects of magnolol on the oxLDL-induced cytotoxicity, HUVECs were first incubated with magnolol (0.1–20 μM) for 2 h and then stimulated with oxidized LDL (200 μg/ml) for 16 h. At the end of stimulation, mitochondrial dehydrogenase activity was used as an index of cell viability and was assessed using MTT assay (Denizot and Lang 1986), and the lactate dehydrogenase (LDH) released was analyzed for the loss of plasma membrane integrity by using an LDH diagnostic kit according to the manufacturer’s instructions. Apoptotic cells were determined by a terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay under fluorescence microscopy or flow cytometer.
Measurement of ROS production
ROS, such as superoxide (O −2 ) and hydrogen peroxide (H2O2), are specific mediators of atherogenic stimuli that induce leukocyte adhesion to endothelial cells. The role of ROS in oxLDL-mediated cytotoxicity has been reported recently, in particular through the activation of the caspase cascade and apoptosis (Hsieh et al. 2001). The effect of magnolol on ROS production in HUVECs was determined by a fluorometric assay using 2′,7′-dichlorofluorescein acetoxymethyl ester (DCF-AM, Molecular Probes) as a probe for the presence of hydroxyl radicals. After preincubation for 2 h with the indicated concentrations of magnolol (2.5–20 μM), HUVECs were incubated with DCF-AM for 1 h and followed by incubation with 200 μg/ml oxLDL for indicated time periods. The fluorescence intensity was measured at 485-nm excitation and 538-nm emission using a fluorescence microplate reader (Labsystem, CA, USA).
Measurement of superoxide dismutase (SOD)
To determine the effects of magnolol on antioxidant enzymes after oxLDL treatment, SOD activity in the homogenate was determined by an enzymatic assay method using a commercial kit (Calbiochem) according to the manufacturer’s instructions. Enzyme activity was converted to U/mg protein.
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay was performed according to the method described by Aikawa et al. (2002) with minor modifications. HUVECs were pretreated for 2 h with or without indicated concentrations of magnolol followed by oxLDL (200 μg/ml) incubation for 1 h. Afterwards, HUVECs were gently harvested and washed twice with ice-cold PBS containing 0.3 mM PMSF, the nuclear fraction was extracted from the cell pellet of the cells using a NE-PER nuclear and cytoplasmic reagent. A double stranded oligonucleotide 5′-AGTTGAGGGGACTTTCCCAGGC-3′ used in our EMSA experiment contains the consensus NF-κB element in the κ light-chain enhancer, 5′-GGGACTTTCC-3′ (5′-GGGRNYYYCC-3′; R is an unspecified purine; Y is an unspecified pyrimidine; and N is any nucleotide) (Chen et al. 1998). Ten microgram of the nuclear fraction was incubated at 30°C for 30 min in the presence of 1 μg poly (dI-dC) with double-stranded NF-κB consensus oligonucleotides labeled at the 3′ terminal by biotin. The samples were separated on 6% PAGE and then transferred to a nitrocellulose membrane by electroblotting. After incubation with 1 μg/ml streptoavidin conjugated to horseradish peroxidase, peroxidase activity on the membrane was detected using an enhanced chemiluminescence substrate system.
Measurement of [Ca2+]i
To determine the effects of magnolol on the oxLDL-induced intracellular calcium rise, HUVECs were seeded onto 24-mm glass coverslips, pretreated with magnolol (2.5–20 μM) for 2 h and then stimulated with oxLDL (200 μg/ml) for 16 h. The cells on coverslips were loaded with 2 μM fura-2 AM (Molecular Probe) in M199 for 30 min at 37°C. After loading, the cells were washed with HEPES buffer (mM) (NaCl, 131; KCl, 5; CaCl2, 1.3; Mg2SO4, 1.3; KH2PO4, 0.4; HEPES, 20; glucose 25, pH 7.4) to remove excess fluorescence dye. Then, the fluorescence of the cells from each coverslip was measured and recorded using an inverted Olympus microscope IX-70. [Ca2+]i in endothelial cells were monitored by alternating excitation wavelengths between 340 and 380 nm and an emission wavelength of 510 nm with a Delta Scan System (Photon Technology International, Princeton, NJ, USA), and calculated using Grynkiewicz’s method (Grynkiewicz et al. 1985).
Measurement of mitochondria membrane potential
To explore the effects of magnolol on the mitochondria membrane potential (ΔΨm), the lipophilic cationic probe fluorochrome 5,58,6,68-tetraethylbenzimidazolcarbocyanine iodide (JC-1) was used. JC-1 exists either as a green fluorescent monomer at a depolarized membrane potential or as a red fluorescent J-aggregate at a hyperpolarized membrane potential. JC-1 exhibits potential-dependent accumulation in mitochondria, as indicated by the fluorescence emission shift from 530 to 590 nm. After a treatment of oxLDL (200 μg/ml) for 16 h in the presence or absence of 5 μM magnolol, cells (5 × 104 cells/24-well plates) were rinsed with M199, and JC-1 (5 μM) was loaded. After 20 min incubation at 37°C, cells were examined under a fluorescent microscope. Determination of the ΔΨm was also carried out using a FACScan flow cytometer (Bedner et al. 1999).
Isolation of cytosolic fractions for cytochrome c analysis
After a treatment of oxLDL in the presence and absence of magnolol (2.5–20 μM), the cells were collected and lysed with lysis buffer (20 mmol/l HEPES/pH 7.5, 250 mmol/l sucrose, 10 mmol/l KCl, 2 mmol/l MgCl2, 1 mmol/l EDTA, 1 mmol/l DTT, protease inhibitor cocktail) for 20 min on ice. The samples were homogenized via 10 passages through a 2-gauge needle. The homogenate was centrifuged at 12,000 rpm for 20 min at 4°C. Protein was measured by the method used by Bradford (1976). A volume of cell lysates containing 30 μg of protein was analyzed by Western blot analysis for cytochrome c (1:1,000) and β-actin (1:50,000).
Measurement of active caspase-3
To explore the effects of magnolol on the oxLDL-induced caspase-3 activation, HUVECs were pretreated with 5 μM magnolol for 2 h and then stimulated with oxLDL (200 μg/ml) for 16 h; and the contents of active caspase-3 were detected by flow cytometry, using a commercial fluorescein active caspase kit (Mountain View, CA, USA) (Telford et al. 2002).
Statistical analyses
Results are expressed as mean ± SEM, and data were analyzed for significant difference using Student’s t-test. Statistical significance was defined as P < 0.05.
Results
Effects of magnolol on the copper-induced oxidative modification of LDL
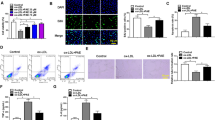
The copper-induced oxidation of LDL in the presence of various concentrations of magnolol was determined by the kinetics of conjugated diene formation, MDA formation and electrophoretic mobility. When LDL was oxidized by cupric ions, a very short lag-time (near 70 min) was observed. On the other hand, in the presence of various concentrations of magnolol (2.5–20 μM), no lag times were detected by 300 min, indicating that oxidation of LDL was delayed (Fig. 1a). The effective concentrations of magnolol inhibiting the copper ion-induced LDL oxidation were subsequently studied by measurement of TBARS. As shown in Fig. 1b, the presence of magnolol protected LDL from copper-induced oxidation, as indicated by the formation of nmol MDA per mg protein (−0.64 ± 0.2; 18.9 ± 2.0; 16.1 ± 2.9; 14.0 ± 2.2; 10.8 ± 1.8; 10.0 ± 1.0 for native LDL, copper-treated LDL and LDL with combined treatment of copper and magnolol at 2.5, 5, 10, and 20 μM, respectively; all P < 0.01). The relative mobility of oxidized LDL was approximately 1.7-fold faster than that of native LDL (Fig. 1c, lane 1 vs. lane 2 from left). A significant decrease in the mobility was observed in all four treatments with various concentrations of magnolol (2.5–20 μM). These results further indicate that magnolol is a potent antioxidant.
Effects of magnolol on copper-induced oxidation of LDL measured as an increase in lag time of diene formation (a), decrease in TBARS (b) and electrophoretic mobility (c) in the absence or presence of the indicated concentrations of magnolol. Data are shown as the mean ± SEM of three independent analyses. #P < 0.01 versus native LDL; *P < 0.01 versus oxLDL treatment
Magnolol inhibited the oxLDL-induced cytotoxicity on HUVECs in a concentration-dependent manner
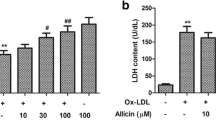
Phase-contrast microscopy was performed to examine the protective effects of magnolol on morphological features on the cultured HUVECs exposed to oxLDL. After a 16-h exposure to a cytotoxic concentration of oxLDL (200 μg of protein/ml of LDL), the number of shrinking cells or cells with blebbing membranes were significantly reduced by the presence of magnolol (Fig. 2a). The viability of cells incubated with oxLDL in the absence or presence of indicated concentrations of magnolol was carried out by MTT assay (32 ± 5% vs. 32 ± 4% (P = NS), 33 ± 5% (P < 0.01), 54 ± 7% (P < 0.01), 82 ± 8% (P < 0.01), 104 ± 10% (P < 0.01), 115 ± 8% (P < 0.01), 115 ± 5% (P < 0.01) for oxLDL, oxLDL with 0.1, 0.5, 1, 2.5, 5, 10, 20 μM magnolol compared to control, respectively, Fig. 2b), and the membrane permeability was assayed by LDH release [245 ± 7% vs. 247 ± 6% (P = NS), 239 ± 5.0% (P = NS), 246 ± 10% (P = NS), 179 ± 11% (P < 0.01), 139 ± 25% (P < 0.01), 111 ± 16% (P < 0.01), 72 ± 2% (P < 0.01) for oxLDL, oxLDL with 0.1, 0.5, 1, 2.5, 5, 10, 20 μM magnolol compared to control, respectively, Fig. 2C]. The 50% effective concentration (ED50) was calculated to be 1.7 μM for cell viability and 3.1 μM for cytotoxicity. Therefore, 2.5–20 μM were finally selected for the following experiments. To further ascertain the induction of apoptosis in HUVECs, oxLDL-treated cells were biochemically analyzed via TUNEL and DAPI staining assay. As shown in Fig. 3, cells incubated with oxLDL for 16 h showed typical apoptosis, including the formation of condensed and fragmented nuclei, which was not observed in the 5 μM magnolol pretreated-HUVECs. The results of cell viability assay together with phenotypic observation of apoptosis under microscopy and flow cytometry suggest that magnolol is a potent inhibitor of the oxidized LDL-induced cytotoxicity in cultured HUVECs.
Effects of magnolol on oxDL-induced endothelial cell death. HUVECs were incubated with oxLDL (200 μg protein/ml) in the absence (middle) and presence (right) of 5 μM of magnolol for 16 h. Photomicrographs from phase-contrast microscopy (a). Viability was determined via MTT assay (b) and LDH release (c). The values represent means ± SEM from three separate experiments. #P < 0.01 versus control; *P < 0.01 versus oxLDL treatment
Effects of magnolol on oxidized LDL-induced endothelial cell apoptosis. HUVECs were incubated with oxLDL (200 μg protein/ml) in the absence (middle) and presence (right) of 5 μM magnolol for 16 h. Top Cells stained with DAPI. Middle Cells stained using TUNEL assay. Bottom Flow cytometric analysis. (control white, oxLDL black, oxLDL with magnolol gray)
Magnolol decreased the suppression of superoxide dismutase which led to a reduction in oxLDL-induced ROS generation
We next turned our attention to ROS as a potential factor related to the oxLDL-induced endothelial cell injury and assayed the activity of the endogenous antioxidant. Pretreatment with magnolol (2.5–20 μM) of HUVECs for 2 h before exposure to 200 μg/ml of oxLDL significantly decreased ROS generation in a dose-dependent and time-dependent manner (all P < 0.01) as shown in Fig. 4a. In accordance with these findings, magnolol also reduced the suppression of SOD activity as shown in Fig. 4b (0.60 ± 0.03, 0.31 ± 0.03, 0.44 ± 0.03, 0.44 ± 0.03, 0.49 ± 0.03 and 0.58 ± 0.04% U/mg protein for control, oxidized LDL and oxidized LDL with 2.5, 5, 10, 20 μM magnolol pretreatment, respectively; all P < 0.01).
Time course of inhibitory effects of magnolol on oxLDL-induced ROS production in HUVECs. After preincubation for 2 h with the indicated concentrations of magnolol (2.5–20 μM), HUVECs were incubated with DCF-AM for 1 h followed by incubation with 200 μg/ml oxLDL. a Fluorescence distribution of DCF-AM oxidation was expressed as a percentage of increased intensity. b The activities of SOD in HUVECs stimulated with oxLDL for 2 h in the absence or presence of magnolol were determined. Data show the mean ± SEM of three independent analyses. #P < 0.01 versus control; *P < 0.01 versus oxLDL treatment
Magnolol inhibited the oxLDL-induced NF-κB activation of HUVECs
NF-κB, a potent transcription factor, is thought to be important in oxidative stress and implicated in atherosclerosis since activated NF-κB is present in human atherosclerotic lesions (Brand et al. 1996). We therefore examined the effects of magnolol on NF-κB activation. As shown in Fig. 5, electrophoretic mobility shift assay detected an increase band upwards and an unbound band in a mixture of biotin-labeled NF-κB consensus oligonucleotides with the nuclear fraction of HUVECs treated with oxLDL, and magnolol prevented NF-κB activation at all concentrations.
Effects of magnolol on NF-κB activation stimulated by oxLDL. HUVECs were pretreated for 2 h with or without indicated concentrations of magnolol followed by 200 μg/ml oxLDL incubation for 1 h. Nuclear extracts from these cells were analyzed by EMSA with a labeled probe. A representative autoradiograph from three experiments is shown
Magnolol reduced oxLDL-induced intracellular calcium accumulation
Unlike oxLDL-induced ROS generation and NF-κB activation that happened within 2 h, cytotoxicity caused by oxLDL needs a longer incubation period. Thus, HUVECs were treated with oxLDL for 16 h in the following experiments. To investigate the effects of exposure of endothelial cells to oxLDL on intracellular calcium, we incubated HUVECs with a cytotoxic concentration (200 μg/ml) of oxLDL in either the absence or presence of magnolol. As shown in Fig. 6, the basal [Ca2+]i increased in oxLDL-treated cells after 16 h, whereas magnolol significantly inhibited the oxLDL-enhanced intracellular calcium rise (49 ± 1, 301 ± 20, 104 ± 3, 85 ± 3, 90 ± 2, 86 ± 3 nM for control, oxidized LDL and oxidized LDL combined with 2.5, 5, 10, 20 μM magnolol, respectively; all P < 0.01).
Effects of magnolol on cytoplasmic Ca2+ increase stimulated by oxLDL in fura 2-loaded HUVECs. Images were processed as indicated in Materials and methods. Calcium changes are color coded (color bar) such that warm colors indicate high calcium. Upper left control; upper middle oxLDL; upper right oxLDL plus magnolol 2.5 μM; lower left oxLDL plus magnolol 5 μM; lower middle oxLDL plus magnolol 10 μM; lower right oxLDL plus magnolol 20 μM. The values represent means ± SEM of more than 250 individual cells from three separate experiments. *P < 0.01 versus oxLDL treatment
Effects of magnolol on mitochondrial transmembrane permeability transition
To examine whether inhibition of mitochondrial disruption may account for the anti-apoptotic effects of magnolol, we tested the effects of oxLDL on mitochondrial permeability. When HUVECs were exposed to oxLDL (200 μg/ml), the ΔΨm was depolarized, as shown by the increase in green fluorescence (Fig. 7a, middle panel). Pretreatment with magnolol reduced the change in ΔΨm as indicated by repression of green fluorescence and restoration of red fluorescence (Fig. 7a, right panel). These findings were supported using flow cytometry (Fig. 7b). OxLDL caused a marked increase in JC1 green fluorescence (middle) compared to control (left). Pretreatment with magnolol caused a marked inhibition of oxLDL-induced apoptosis (right). Indeed, disruption of mitochondrial membrane function resulted in the specific release of the mitochondrial enzyme cytochrome c into the cytosol. Cytosolic proteins were separated and detected by Western blot. As shown in Fig. 7c, incubation of HUVECs with oxLDL for 16 h induced the release of cytochrome c into the cytosolic fraction ∼3 folds compared with the cytochrome c amount determined in the cytosolic fraction of control cells. Notably, magnolol significantly prevented oxLDL-induced cytochrome c release.
Effects of magnolol on mitochondrial transmembrane permeability transition. a Δψm was assessed via a signal from monomeric and J-aggregate JC-1 fluorescence, as described in Materials and methods. Left No treatment; middle oxLDL; and right oxLDL+ 5 μM magnolol. b JC-1 fluorescence was confirmed by using flow cytometry. c cytochrome c was detected by Western blotting as described in Materials and methods. Lane 1 control; lane 2 oxLDL; lane 3, 4, 5, 6, oxLDL + 2.5, 5, 10, 20 μM magnolol, respectively
Magnolol reduced oxLDL-induced caspase-3 activation
It is well known that oxLDL-induced cytochrome c release may lead to activation of caspase-3, an effector of apoptosis. We subsequently determined the active form of caspase-3 using flow cytometry. As shown in Fig. 8, active caspase-3 was significantly increased in cells treated with oxLDL for 16 h. In contrast, the activation of caspase-3 by oxLDL was suppressed by approximately 50% in cells pretreated with 5 μM of magnolol.
Discussion
OxLDL exhibit a wide range of biological effects on cultured arterial cells including apoptosis. Evidence has been presented that oxidized lipids from oxLDL induce modification of cell protein structure, elicit ROS generation and lipid peroxidation of cellular lipids, and alter the regulation of various signaling pathways and gene expression (Napoli 2003). In the present study, we demonstrated that magnolol, an active component isolated from the herb ‘Houpo’ (Magnolia officinalis), exerted potent inhibitory effects on oxLDL-induced apoptosis through inhibition of ROS generation. Our findings indicate that the anti-oxidative actions of magnolol inhibited the copper-induced LDL oxidation (Fig. 1). Magnolol reduced apoptosis and cell death are especially relevant since increased endothelial cell apoptosis may initiate atherosclerotic lesions, which are prone to rupture at vulnerable plaque (Littlewood and Bennett 2003). Results from our experiments indicated that cell death of HUVECs exposed to a toxic concentration of oxLDL (200 μg protein/ml) could be reversed by magnolol; evidenced by DAPI stain and by TUNEL assay (Fig. 2). OxLDL induced time-dependent increase in ROS production during 2 h as well as decreased SOD activities were reversed in the presence of magnolol. In addition, several apoptotic signaling pathways including intracellular calcium accumulation and subsequent mitochondrial membrane potential collapse, cytochome c release and activation of caspase 3 caused by oxLDL were also inhibited by magnolol. All these findings strongly indicate the anti-oxidative effects of magnolol in response to oxLDL treatment in HUVECs.
High levels of oxLDL in endothelial membrane can foster the formation of a necrotic lipid core by apoptosis in atherosclerotic lesions (Heermeier et al. 1999). There is a plethora of signal transduction and nuclear events that are involved in the phenomenon, including TNFR1 and TNFR2 activation, the complex cascade of kinases, regulatory events of the Bcl-2 family of genes, activation of death-effector caspases and sphingomyelinase, and regulatory action of the transcription factors (Escargueil-Blanc et al. 1998; Harada-Shiba et al. 1998; Sata and Walsh 1998). NF-κB is often viewed as a global regulator of cytokines and mitogenic products that may influence vascular smooth muscle proliferation as well as endothelial apoptosis and contribute to atherogenesis (Selzman et al. 1999). Several lines of evidence suggest a role for ROS as a common and critical intermediate for various NF-κB activating signals. This conclusion is based largely on the inhibition of NF-κB activation by a variety of antioxidants (Schreck et al. 1992) and by overexpression of antioxidant enzymes. It was also noted that NF-κB expression was considerably increased in endothelial cells following oxLDL treatment (Cominacini et al. 2000) and the presence of oxLDL is associated with activation of the NF-κB in the endothelium of an animal model (Calara et al. 1998). Activation of NF-κB coordinates the expression of vascular cell adhesion molecules (VCAM-1) (Neish et al. 1992), E-selectin (Collins et al. 1993) and monocyte chemoattractant protein-1 (MCP-1) gene (Lindner 1998) may lead to an adherence of endothelial cells and leukocytes which is associated with initial events in the development of atherosclerotic lesion. Therefore, suppression of NF-κB can be expected to result in prevention of atherogenesis. This was supported by our finding that the activation of NF-κB by oxLDL was significantly inhibited by pretreatment of magnolol (Fig. 5). Whether magnolol reduced oxLDL-induced NF-κB activation is involved in the release of inflammatory cytokines requires further study.
There is an increasing interest in the application of antioxidants for the prevention and treatment of cardiovascular disease since oxygen-derived free radicals and alteration of intracellular Ca2+ ion homeostasis are now considered major contributing factors to atherosclerotic coronary artery disease. An intense, delayed, and sustained calcium signal is a trigger of cell death but the initial targets and the subsequent sequence of events leading to cell death are only partly understood (Vindis et al. 2005). Previous studies demonstrated that calcium channel blockers inhibite experimental atherosclerosis in cholesterol-fed animals (Cristofori et al. 2000), improve endothelial cell functions by upregulating the nitric oxide system (Ding and Vaziri 2000), and downregulating the endothelial receptor for oxidized LDL (LOX-1) as well as inhibiting the CPP32-like protease activity (Sugano et al. 2002). In addition, magnolol block receptor-operated cation channel, voltage dependent calcium channel and inhibit calcium release from the sarcolemmal membrane through blocking InsP3-sensitive and ryanodine-sensitive pathways on distal colon of guinea pig in vitro (Bian et al. 2006), inhibit calcium influx through voltage-operated calcium channels in porcine trachea (Zhai et al. 2003). This may explain, at least partially, the mechanisms underlying the protective effects of magnolol against oxLDL-induced intracellular calcium accumulation.
The oxidant stress imposed by oxLDL appears to activate the signaling pathways leading to apoptosis of endothelial cells, since ROS generation is the earliest apoptotic signal and it usually happens within 5 min of oxLDL addition. ROS generation may inhibit Ca2+-ATPases, and lead to a sustained elevation of [Ca2+]i, which is associated with mitochondrial dysfunction mediated by loss of membrane potential and release of cytochrome c. Accordingly, the anti-apoptotic effects of magnolol are hypothesized to be resulted from its inhibitory effect on ROS generation that, in turn, represses the release of endothelial [Ca2+]i and stabilizes the mitochondrial membrane, thereby preventing the release of mitochondrial protein cytochrome c, a molecule required for the activation of caspase-3 that executes the cell death program (Littlewood and Bennett 2003). Pathways to caspase-3 activation have been identified that are either dependent on or independent of mitochondrial cytochrome c release and caspase-9 function. In some instance, apoptotic cell death does not always involve the cytochrome c-dependent activation of caspase-3 (Indrajit et al. 2006). Our data demonstrated that pretreatment HUVECs with 5 μM magnolol almost complete abolished the oxLDL-induced transmembrane permeability transition of mitochondria (Fig. 7) but only suppressed the activation of caspase-3 by approximately 50% (Fig. 8). We assumed that magnolol at dose of 5 μM was sensitive enough to reverse the cytochrome c-dependent but not cytochrome c-independent activation of caspase 3.
In accordance with our observations, several previous studies have demonstrated that magnolol has the beneficial functions of anti-oxidant and anti-inflammatory activities, which stem from the inhibition of neutrophil infiltration and reactive oxygen species production (Liou et al. 2003a, b; Park et al. 2004) and protection of mitochondrial respiratory chain enzyme activity against NADPH-induced peroxidative stress (Haraguchi et al. 1997). With regard to the underlying mechanisms of anti-inflammation, magnolol suppresses IL-6-induced signal transducer and activator of transcription protein 3 (STAT3) activation and gene expression in endothelial cells (Chen et al. 2006) and magnolol acts as a potent inhibitor of NF-κB activation, which is a key transcription factor in the expression of inflammatory cytokines (Lee et al. 2005). In addition to these reported beneficial effects, earlier studies have shown that magnolol relaxes smooth muscle due to the blockade of receptor-operated cation channels and voltage-operated Ca2+ channels (Ko et al. 2003; Lu et al. 2003). Taken together, all these findings suggest that magnolol might be a plausible lead candidate for further development in the prevention of cardiovascular diseases.
In summary, results from our experiments indicate that magnolol, a natural aromatic product from plants, could prevent atherogenesis, probably via its anti-oxidative effects. In addition, magnolol inhibited oxLDL-induced apoptosis in HUVECs. Our findings on the intracellular signaling pathways modulating the oxidative insults induced by oxLDL in endothelial cultures shed some light on the potential implications of magnolol in the prevention of the atherosclerotic process.
References
Aikawa Y, Yamamoto M, Yamamoto T, Morimoto K, Tanaka K (2002) An anti-rheumatic agent T-614 inhibits NF-kappaB activation in LPS- and TNF-alpha-stimulated THP-1 cells without interfering with IkappaBalpha degradation. Inflamm Res 51:188–194
Bedner E, Li X, Gorczyca W, Melamed MR, Darzynkiewicz Z (1999) Analysis of apoptosis by laser scanning cytometry. Cytometry 35:181–195
Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ (1995) Atherosclerosis: basic mechanisms : oxidation, inflammation, and genetics. Circulation 91:2488–2496
Bian ZX, Zhang G.S, Wong KL, Hu XG, Liu L, Yang Z, Li M (2006) Inhibitory effects of magnolol on distal colon of guinea pig in vitro. Biol Pharm Bull 29:790–795
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA, Neumeier D (1996) Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest 97:1715–1722
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Calara F, Dimayuga P, Niemann A, Thyberg J, Diczfalusy U, Witztum JL, Palinski W, Shah PK, Cercek B, Nilsson J, Regnstrom J (1998) An animal model to study local oxidation of LDL and its biological effects in the arterial wall. Arterioscler Thromb Vasc Biol 18:884–893
Chang CP, Hsu YC, Lin MT (2003) Magnolol protects against cerebral ischaemic injury of rat heatstroke. Clin Exp Pharmacol Physiol 30:387–392
Chen FE, Huang DB, Chen YQ, Ghosh G. (1998) Crystal structure of p50/p65 heterodimer of transcription factor NF-[kappa]B bound to DNA. Nature 391:410–413
Chen SC, Chang YL, Wang DL, Cheng JJ (2006) Herbal remedy magnolol suppresses IL-6-induced STAT3 activation and gene expression in endothelial cells. Br J Pharmacol 148:226–232
Collins T, Palmer HJ, Whitley MZ, Neish AS, Williams AJ (1993) A common theme in endothelial activation: insights from the structural analysis of the genes for E-selectin and VCAM-1. Trends Cardiovasc Med 3:92–97
Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, Rigoni A, Pastorino AM, Lo Cascio V, Sawamura T (2000) Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL Receptor-1 in endothelial cells induces the activation of NF-kappa B through an increased production of intracellular reactive oxygen species. J Biol Chem 275:12633–12638
Cristofori P, Lanzoni A, Gaviraghi G, Turton J, Sbarbati A (2000) Anti-atherosclerotic activity of the calcium antagonist lacidipine in cholesterol-fed hamsters. Biomed Pharmacother 54:93–99
Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM (1990) Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci USA 87:5134–5138
Denizot F, Lang R (1986) Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89:271–277
Ding Y, Vaziri ND (2000) Nifedipine and Diltiazem but Not Verapamil up-regulate endothelial nitric-oxide synthase expression. J Pharmacol Exp Ther 292:606–609
Escargueil-Blanc I, Andrieu-Abadie N, Caspar-Bauguil S, Brossmer R, Levade T, Negre-Salvayre A, Salvayre R (1998) Apoptosis and activation of the sphingomyelin-ceramide pathway induced by oxidized low density lipoproteins are not causally related in ECV-304 endothelial cells. J Biol Chem 273:27389–27395
Esterbauer H, Striegl G, Puhl H, Rotheneder M (1989) Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun 6:67–75
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Harada-Shiba M, Kinoshita M, Kamido H, Shimokado K (1998) Oxidized low density lipoprotein induces Apoptosis in cultured human umbilical vein endothelial cells by common and unique mechanisms. J Biol Chem 273:9681–9687
Haraguchi H, Ishikawa H, Shirataki N, Fukuda A (1997) Antiperoxidative activity of neolignans from Magnolia obovata. J Pharm Pharmacol 49:209–212
Havel RJ, Eder HA, Bragdon JH (1955) The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest 34:1345–1353
Heermeier K, Schneider R, Heinloth A, Wanner C, Dimmeler S, Galle J (1999) Oxidative stress mediates apoptosis induced by oxidized low-density lipoprotein and oxidized lipoprotein(a). Kidney Int 56:1310–1312
Hsieh CC, Yen MH, Yen CH, Lau YT (2001) Oxidized low density lipoprotein induces apoptosis via generation of reactive oxygen species in vascular smooth muscle cells. Cardiovasc Res 49:135–145
Ikarashi Y, Yuzurihara M, Sakakibara I, Nakai Y, Hattori N, Maruyama Y (2001) Effects of the extract of the bark of Magnolia obovata and its biphenolic constituents magnolol and honokiol on histamine release from peritoneal mast cells in rats. Planta Med 67:709–713
Ikeda K, Nagase H (2002) Magnolol has the ability to induce apoptosis in tumor cells. Biol Pharm Bull 25:1546–1549
Indrajit C, Binu T, Ganapathy KB (2006) Current concepts in apoptosis: the physiological suicide program revisited. Cell Mol Biol Lett V11:506–525
Jaffe EA, Nachman RL, Becker CG, Minick CR (1973) Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52:2745–2756
Ko CH, Chen HH, Lin YR, Chan MH (2003) Inhibition of smooth muscle contraction by magnolol and honokiol in porcine trachea. Planta Med 69:532–536
Lee J, Jung E, Park J, Jung K, Lee S, Hong S, Park J, Park E, Kim J, Park S, Park D (2005) Anti-inflammatory effects of magnolol and honokiol are mediated through inhibition of the downstream pathway of MEKK-1 in NF-kappaB activation signaling. Planta Med 71:338–343
Lee YM, Hsiao G, Chen HR, Chen YC, Sheu JR, Yen MH (2001) Magnolol reduces myocardial ischemia/reperfusion injury via neutrophil inhibition in rats. Eur J Pharmacol 422:159–167
Lindner V (1998). The NF-kappaB and IkappaB system in injured arteries. Pathobiology 66:311–320
Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK (2003a) The anti-inflammatory effect of honokiol on neutrophils: mechanisms in the inhibition of reactive oxygen species production. Eur J Pharmacol 475:19–27
Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK (2003b) Honokiol protects rat brain from focal cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res 992:159–166
Littlewood TD, Bennett MR (2003) Apoptotic cell death in atherosclerosis. Curr Opin Lipidol 14:469–475
Lo YC, Teng CM, Chen CF, Chen CC, Hong CY (1994) Magnolol and honokiol isolated from Magnolia officinalis protect rat heart mitochondria against lipid peroxidation. Biochem Pharmacol 47:549–553
Lu YC, Chen HH, Ko CH, Lin YR, Chan MH (2003) The mechanism of honokiol-induced and magnolol-induced inhibition on muscle contraction and Ca2+ mobilization in rat uterus. Naunyn Schmiedebergs Arch Pharmacol 368:262–269
Napoli C (2003) Oxidation of LDL, atherogenesis, and apoptosis. Ann N Y Acad Sci 1010:698–709
Neish AS, Williams AJ, Palmer HJ, Whitley MZ, Collins T (1992) Functional analysis of the human vascular cell adhesion molecule 1 promoter. J Exp Med 176:1583–1593
Park J, Lee J, Jung E, Park Y, Kim K, Park B, Jung K, Park E, Kim J, Park D (2004) In vitro antibacterial and anti-inflammatory effects of honokiol and magnolol against Propionibacterium sp. Eur J Pharmacol 496:189–195
Sata M, Walsh K (1998) Oxidized LDL activates Fas-mediated endothelial cell apoptosis. J Clin Invest 102:1682–1689
Schreck R, Albermann K, Baeuerle PA (1992) Nuclear factor kappa B: an oxidative stress–responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun 17:221–237
Selzman CH, Shames BD, Reznikov LL, Miller SA, Meng X, Barton HA, Werman A, Harken AH, Dinarello CA, Banerjee A (1999) Liposomal delivery of purified inhibitory-{kappa}B{alpha} inhibits tumor necrosis factor-{alpha}induced human vascular smooth muscle proliferation. Circ Res 84:867–875
Sugano M, Tsuchida K, Makino N (2002) Nifedipine prevents apoptosis of endothelial cells induced by oxidized low-density lipoproteins. J Cardiovasc Pharmacol 40:146–152
Telford WG, Komoriya A, Packard BZ (2002) Detection of localized caspase activity in early apoptotic cells by laser scanning cytometry. Cytometry 47:81–88
Terpstra AH, Woodward CJ, Sanchez-Muniz FJ (1981) Improved techniques for the separation of serum lipoproteins by density gradient ultracentrifugation: visualization by prestaining and rapid separation of serum lipoproteins from small volumes of serum. Anal Biochem 111:149–157
Tohda Y, Haraguchi R, Kubo H, Muraki M, Fukuoka M, Nakajima S (1999) Effects of saiboku-to on dual-phase bronchoconstriction in asthmatic guinea pigs. Methods Find Exp Clin Pharmacol 21:449–452
Vindis C, Elbaz M, Escargueil-Blanc I, Auge N, Heniquez A, Thiers JC, Negre-Salvayre A, Salvayre R (2005) Two distinct calcium-dependent mitochondrial pathways are involved in oxidized LDL-Induced apoptosis. Arterioscler Thromb Vasc Biol 25:639–645
Wilson T, March H, Ban WJ, Hou Y, Adler S, Meyers CY, Winters TA, Maher MA (2002) Antioxidant effects of phyto-and synthetic-estrogens on cupric ion-induced oxidation of human low-density lipoproteins in vitro. Life Sci 70:2287–2297
Yang SE, Hsieh MT, Tsai TH, Hsu SL (2003) Effector mechanism of magnolol-induced apoptosis in human lung squamous carcinoma CH27 cells. Br J Pharmacol 138:193–201
Zhai H, Nakade K, Mitsumoto Y, Fukuyama Y (2003) Honokiol and magnolol induce Ca2+ mobilization in rat cortical neurons and human neuroblastoma SH-SY5Y cells. Eur J Pharmacol 474:199–204
Acknowledgments
This study was supported by grants from the National Science Council (NSC 94-2314-B-075A-012) and Taichung Veterans General Hospital (TCVGH-953505C, TCVGH-TTMHH958502), Taiwan, Republic of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ou, HC., Chou, FP., Sheu, W.HH. et al. Protective effects of magnolol against oxidized LDL-induced apoptosis in endothelial cells. Arch Toxicol 81, 421–432 (2007). https://doi.org/10.1007/s00204-006-0172-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-006-0172-3