Abstract
The effects of combined exposure to lead and cadmium on granulose cells were studied. Adult female rats were treated i.p. with either lead acetate (LA) or cadmium acetate (CA) both, alone, or in combination at a dose of 0.05 mg/kg body weight on a daily basis for 15 days. Both metals were accumulated in the ovary after metal exposure. Metal exposure caused a decrease in reduced glutathione content along with elevated lipid peroxidation in all groups. Granulose cells of both cadmium as well as combination group demonstrated a maximum increase in lipid peroxides and catalase activity, along with decreased glutathione status and superoxide dismutase activities. Combined treated animals exhibited an intermediate effect in antioxidant status. However, “in vitro” exposure showed no significant change in antioxidant enzymes in all metal exposed cells. Data from the present study indicates that lead and cadmium in isolation and in combination cause oxidative stress. Lead and cadmium in combination do not show additive or synergistic effect indicating the competition between them due to similarity in electronic affinities. Present study highlights the effects of toxic metals that disturb membrane integrity of cells via ROS and thereby classifying mechanism for altered receptor binding, steroidogenesis, and hormone production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Free radicals are generated continuously in aerobic cells (Clance et al. 1979). Cellular respiration involves the reduction of molecular oxygen to water in the electron transport chain. This reduction involves the production of reactive oxygen species (O2−, H2O2, OH•), which can damage cellular systems by attacking the lipid components of the membrane causing lipid peroxidation. Cells are protected by defense systems which remove these free radicals to prevent oxidative damage. Superoxide dismutase (SOD) scavenges the superoxide ions by rapid dismutation and forms hydrogen peroxide, which are again detoxified by catalase (CAT) to form water and oxygen. It has been suggested that cells may maintain a homeostatic level of free radicals and lipid peroxides to provide a suitable oxidative environment for controlling metabolic pathways throughout the ovarian cycle (Nelson et al. 1994). A sixfold rise in superoxide anion (O2−) was seen during proestrous stage along with decreased SOD activity (Duran Reyes et al. 1998) in rats. Whereas, the activities of anti-oxidative enzymes like SOD and CAT are known to increase during the process of ovulation, particularly in late proestrous to estrous stage (Sato et al. 1992; Miyazaki et al. 1991). To the best of our knowledge, there are no reports on the antioxidant status of granulose cells.
Both lead and cadmium belong to the class of redox inactive metals and the proposed mechanism for these metals for inducing toxicity is through their role in the generation of reactive oxygen species and their effect on the antioxidant system (Christie and Costa 1984; Adler et al. 1993). It is known that lead and cadmium, both have electron sharing affinities, which can result in the formation of covalent attachments (Bondy 1996) with sulfahydryl groups of proteins (Ouig 1998). This results in depletion of glutathione, cell’s major sulfahydryl reserve.
Cellular integrity is important for ovarian function as the gonadotropin receptors are present on the cell membrane of granulose cells of ovary. The gonadotropins (FSH and LH) bind to these receptors present on the membrane, to initiate the process of steroidogenesis, which controls ovulation and estrous cycle. It has been reported that exposure to either lead or cadmium causes decreased gonadotropin binding (Wiebe et al. 1988; Priya et al. 2004) leading to decreased steroid production (Paksy et al. 1997, 2001). However, the mechanism behind such a decrease is not understood. Moreover, there are several reports on the mechanism of lead and cadmium in relation to oxidative stress in other tissues, but little is known about mechanism behind the toxic effects of metal salts on the granulose cells of the ovary.
Most of the above reports are dealt with single metal exposure. In reality, population is continuously exposed to more than one heavy metal at a time. This kind of exposure could cause an additive, synergistic, or antagonistic effect. We have recently reported a decrease in gonadotropin binding, both in vivo and in vitro, in female rats exposed to lead, cadmium, and in combination, where the combined exposure resulted in intermediate effects compared with the effect of each of the metals individually (Nampoothiri and Gupta 2006; Priya et al. 2004). We have also demonstrated that this decrease is due to alteration in membrane structure (Nampoothiri and Gupta 2006). The aim of the present study was to understand further the mechanism responsible for decreased receptor binding and alteration in membrane integrity by lead and cadmium either alone or in combination on granulose cells of ovary.
Materials and methods
Animals
All experiments were performed in Charles foster female virgin rats (200–220 g of body weight). All the animals were maintained at a controlled room temperature and lighting (12 h light/12 h dark). All treatments and procedures were in accordance with the protocol of University’s Animal Care and Use Committee and Guidelines for the Care and Use of Experimental Animals.
“In vivo” study
There were four groups of animals in the study: control (sodium acetate), LA, CA, and LA + CA in combination. The animals were treated i.p at a dose of 0.05 mg/kg body weight on a daily basis for 15 days (Pillai et al. 2002). The LA + CA treated group received the same dose by taking half of the concentration of each metal, thereby a total of 0.05 mg/kg body weight. After 15 days of metal treatment, the animals in late diestrous stage of estrous cycle received 75 IU of hCG, to increase the yield of cells by super ovulation and they were killed after 24 h at proestrous stage of estrous cycle. Ovaries were removed and granulose cells were isolated (Campbell 1979).
“In vitro” study
Adult female virgin rats were treated with 75 IU hCG at late diestrous stage and were killed at proestrous stage and granulose cells were isolated from the ovaries and exposed to metals for 1 h, at a concentration of metals that reach the ovary when exposed to 0.05 mg/kg body weight/day for 15 days. These cells were then sonicated at 5 cycles/min and were then assayed for all the above parameters.
Chemicals
Ethylene glycol tetra acetic acid (EGTA), nitroso blue tetrazolium (NBT), phenyl methyl sulfonate (PMS), 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) were procured from Sisco Research Laboratories, Mumbai, India. All other chemicals were bought locally.
Granulose cell isolation
Ovaries were removed and granulose cells were isolated by modified method of Campbell (1979). In brief, ovaries were suspended in Hanks balance buffered saline (HBSS) and were treated with hypertonic sucrose and EGTA solutions. The ovaries were expressed with blunt spatula and resuspended in HBSS. Purity of granulose cells was evaluated by assessing 3β-hydroxy steroid dehydrogenase (3β-HSD) activity (pure preparation of cells should have undetectable 3β-HSD activity). The viability of cells was checked by trypan blue exclusion, which was about 75–80%. The live cells were only considered for the study.
Isolated granulose cells were sonicated at 5 cycles/min and these sonicated cells were used to assess oxidative parameters: reduced glutathione (Beutler and Gelbart 1985), lipid peroxides (Ohkawa et al. 1979), and antioxidant enzymes—catalase (Hugo 1987) and SOD (Kakkar et al. 1984).
Metal analysis
Metal analysis was performed in both ovarian samples. The ovaries were digested in a mixture of reagent grade nitric acid and per chloric acid in the ratio of 3:1 until the samples became colorless. Further samples were evaporated and reconstituted in hydrochloric acid. Then the acid mixture was evaporated and precipitate thus obtained was dissolved in a few drops of concentrated HCl. The sample was then diluted to 1 ml with distilled water and the readings were taken in GBC 902 double beam atomic absorption spectrophotometer. Sensitivities of the assays were 0.06 and 0.009 μg/ml for lead and cadmium, respectively. Detection limit using the instrument was 0.05 ng/ml for lead and 0.003 ng/ml for cadmium, respectively.
Estimation of reduced glutathione
Granulose cells (3 × 105) were taken in 100 μl and sonicated for 2 min with 5 cycles/min. Reduced glutathione was precipitated using precipitating agent and supernatant was treated with 5,5′-dithiobis-2-nitrobenzoic acid, which is readily reduced by sulfhydryl compounds forming a highly colored yellow anion, which can be read at 412 nm.
Estimation of lipid peroxides (TBARS)
Granulose cells (4 × 105) were taken in 100 μl of HBBS and washed with PBS twice, to remove HBSS. The cells are resuspended in 100 μl of PBS and sonicated for 2 min with 5 cycles/min. Sonicated cells which have lipid peroxides react with thiobarbituric acid (TBA) and give thiobarbituric reactive substance (TBARS) which gives a characteristic pink color that can be estimated at 532 nm.
Estimation of catalase activity
Catalase activity was estimated by the method of Hugo (1987). Catalase is a heme-containing enzyme which catalyzes dismutation of hydrogen peroxide into water and oxygen. Decomposition of hydrogen peroxide by catalase is measured spectrophotometrically at 240 nm, since hydrogen peroxide absorbs UV light maximally at this wavelength. Granulose cells (3 × 105) were taken in 200 μl of PBS (pH 7.4) and sonicated for 2 min at 5 cycles/min. Sonicated cells are used as an enzyme source. Ten microliters of ethanol was added to 1.0 ml supernatant and these tubes were incubated in ice water bath for 30 min. Just before the assay, 10 μl of Triton X-100 and 9 ml of phosphate buffer were added and estimated at 240 nm.
Estimation of superoxide dismutase activity
The activity was estimated according to the method of Kakkar et al. (1984). Granulose cells (4 × 105) were suspended in 100 μl of PBS and were sonicated for 2 min with 5 cycles/min. Sonicated cells were used as a source of enzyme. Mixtures of NADH and phenazine methosulfate (PMS) along with enzyme generate superoxide under nonacidic conditions via the univalent oxidation of reduced PMS, which is accepted by NBT and through its reduction into a stable, blue colored formazon product, which can be measured at 560 nm.
Statistical analysis
Results are expressed as mean + standard error mean. All data were analyzed employing one-way ANOVA, followed by Tukey’s test for multiple comparisons. Values of P < 0.05 were considered for statistical significance.
Results
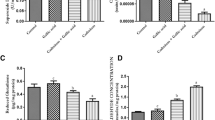
There were no significant differences in body weight in treated animals as compared to control animals. The concentrations of lead and cadmium in the ovary and granulosa cell count after 15 days of exposure are represented in Tables 1 and 2 respectively. Both Pb and Cd get accumulated in the ovaries after exposure. The animals receiving combined treatment exhibited intermediate values for both lead and cadmium metals compared to individual metal treated groups.
Figures 1, 2, 3, and 4 represent the effects of lead and cadmium either alone or in combination on reduced glutathione, TBARS, and antioxidant enzymes both “in vivo” and “in vitro”. Cells of cadmium treated animals showed a maximum decrease in reduced glutathione content and elevation in lipid peroxidation compared to cells of control animals. Cells of animals which were exposed to both LA + CA showed an intermediate change in both the parameters while cells of lead treated animals showed minimum change. Significant inhibition in the activity of SOD and marked elevation in catalase activity were seen in cells of cadmium exposed animals. The activity of SOD in combined treated group was inhibited to similar extent as the cells in cadmium exposed group while the activity of catalase was intermediate between individually exposed groups of lead and cadmium.
In the case of “in vitro” study, significant decrease in glutathione content has been demonstrated in cadmium and LA + CA treated group, whereas elevation in lipid peroxidation was seen only in cadmium treated groups.
Discussion
Toxic metals act as a catalyst in the generation of reactive oxygen species (El-Maraghy et al. 2001). The increase in free radicals can attack the lipid molecules causing lipid peroxidation and alterations in antioxidant status of the cells (Yiin and Lin 1995; Shafiq-ur-Rehman 1984; Stohs et al. 2001). Granulose cells exposed to either cadmium alone or in combination showed higher lipid peroxidation as compared to control. Several reports have accounted for such an elevation in lipid peroxidation upon cadmium exposure (Stohs et al. 2001; Wang et al. 2002). Antioxidant enzyme of the cell plays a role in maintaining the homeostasis of free oxygen radicals in the steroidogenic tissues (Qanungo et al. 1999). Superoxide dismutase requires Zn as a factor for its activity. Present study exhibits inhibition of SOD activity in both granulose cells suggesting displacement of zinc by lead and cadmium from its active site. Similar kind of inhibition was obtained by several other workers (Hussain et al. 1987; Kofod et al. 1991; Ariza et al. 1998). Our present study demonstrates a significant increase in catalase activity in all metal treated groups. This increase in activity could be due to the early displacement of the transition metals from the active site of SOD by the lead and cadmium, thereby causing no inhibition in catalase activity. Increase in catalase activity could be due to higher production of ROS by these heavy metals. Similar kind of increase in catalase activity has been reported in different tissues on metal exposure (Kostic et al. 1993; Mousa et al. 2002). A recent study by Strehlow et al. (2003) reported that estrogen upregulates SOD expression, without any change in CAT activity. It has been reported earlier that estrogen production is decreased on lead and cadmium exposure (Piasek et al. 2002; Paksy et al. 1992). Thereby, it could be plausible that decreased estrogen observed upon metal exposure could also contribute to decreased SOD expression.
An important protein involved in interaction of toxic elements is glutathione (Meister 1988) and the present study shows a decrease in GSH content in granulose cells. This could be attributed to the interaction of these divalent elements with sulfhydryl groups of GSH as suggested by several workers (Christie and Costa 1984; Stohs et al. 2001; Monterio et al. 1991a, b; Nigam et al. 1999). Various studies have indicated that increase in lipid peroxidation also causes an alteration in glutathione status, thus indicating direct correlation between the two components (Struzynska et al. 2002; Stohs et al. 2001). We have clearly demonstrated recently that similar exposure of lead and cadmium caused significant changes in lipid peroxidation and antioxidant status (Pillai and Gupta 2005) in liver tissue.
Free radicals produced by these heavy metals are known to cause change in membrane structure, which then can affect receptors, activity of enzymes, and membrane fluidity (Mishra et al. 1989; Nivsarkar et al. 1998). Gonadotropins mediate their action through binding with their receptors present on the membrane of granulose cells. Thereby, any alteration in the membrane structure would reflect a change in gonadotropin binding, leading to change in hormone action. This could be a possible mechanism behind the change in membrane components that led to decreased gonadotropin binding as reported earlier by our lab (Nampoothiri and Gupta 2006).
Present study did not demonstrate any change in antioxidant parameters upon “in vitro” exposure. Only cadmium treated cell membrane exhibited a slight elevation in lipid peroxidation and decrease in reduced glutathione level. This could be due to very low concentration of lead and cadmium to which cells are exposed. Also, cells were exposed to metals for 1 h only. There is a significant inhibition of enzyme by the metal exposure. However, percent inhibition obtained in “in vitro” study was less than that in the “in vivo” study, thus indicating both direct interaction of metal with enzyme as well as an altered membrane structure by the metals, responsible for the change in “in vivo” treated groups of animals. Metals when used in combination gave intermediate results, which could be due to competition between the two metals. This competition could be due to similarity in electronic affinities.
Present study indicates that both lead and cadmium alter antioxidant enzyme status, causing an increase in free radicals in granulose cells. This data clearly supports our earlier report (Nampoothiri and Gupta 2006) and determines the mechanism behind all observed effects on combined exposure to lead and cadmium. Thus, it is clear that increase in free radicals along with membrane damage is a major mechanism behind the decreased gonadotropin binding, which leads to reproductive dysfunction.
References
Adler AJ, Barth RH, Berlyne GM (1993) Effects of lead on oxygen free radical metabolism: inhibition of superoxide dismutase activity. Trace Elem Res 10:93–96
Ariza ME, Bijur GN, Williams MV (1998) Lead and mercury mutagenesis: role of H2O2, superoxide dismutase and xanthine oxidase. Environ Mol Mutagen 31:352–361
Beutler E, Gelbart J (1985) Plasma glutathione in health and in patients with malignant disease. J Lab Clin Med 105:581–584
Bondy SC (1996) Oxygen generation as a basis for neurotoxicity by metals. In: Chang LW (ed) Toxicology of metals.CRC Press, Baco Raton, pp 699–706
Campbell KL (1979) Ovarian granulosa cell isolated with EGTA and hypertonic sucrose: cellular integrity and function. Biol Rep 21:773–786
Christie NT, Costa M (1984) In vitro assessment of the toxicity of metal compounds. IV. Disposition of metals in cells: interaction with membranes, glutathione, metallothionein and DNA. Biol Trace Elem Res 6:139–158
Clance B, Sies H, Boveries A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605
Duran Reyes G, Gomez Melendez MR, Hicks Gomez JJ (1998) Importance of free radicals during the reproduction cycle. Ginecol Obstet Mex 66:371–376
El-Maraghy SA, Gad MZ, Fahim AT, Hamdy MA (2001) Effect of cadmium and aluminum intake on the antioxidant status and lipid peroxidation in rat tissues. J Biochem Mol Toxicol 15:207–214
Hugo EA (1987) Catalase. In: Bergmeyer HU, Bergmeyer J, Grabt M (eds) Methods of biochemical analysis, vol III. VCH Publishers, New York, pp 277–282
Hussain T, Shukla GS, Chandra SV (1987) Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: in vivo and in vitro studies. Pharmacol Toxicol 60:355–359
Kakkar P, Das B, Vishwanath PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Kofod P, Bauer R, Danielsen E, Larsen E, Bjerrum MJ (1991) 113 Cd-NMR investigation of a cadmium-substitution copper, zinc containing superoxide dismutase from yeast. Eur J Biochem 198:607–611
Kostic MM, Ognjanovic B, Dimitrijevic S, Zikic RV, Stajn A, Rosic GL, Zivkovic RV (1993) Cadmium-induced changes of antioxidant and metabolic status in red blood cells of rats: in vivo effects. Eur J Haematol 51:86–92
Meister A (1988) On the discovery of glutathione. Trends Biochem Sci 13:185–188
Mishra OP, Delivora-Papadopoulus M, Cahillane G, Wagerle DC (1989) Lipid peroxidation as the mechanism of modification of the affinity of the Na+ K+ ATPase active sites for ATP, K+, Na+ and strophanthidin in vitro. Neurochem Res 14:845–851
Miyazaki T, Sueoka K, Dharmarajan AM, Atlas SJ, Bulkley GB, Wallach EE (1991) Effect of inhibition of oxygen free radical on ovulation and progesterone production by the in-vitro perfused rabbit ovary. J Reprod Fertil 91:207–212
Monterio HP, Bechara EJH, Adballa DSP (1991a) Free radical involvement in neurological porphyries and lead poisoning. Mol Cell Biochem 103:73–83
Monterio HP, Bechara EJH, Adballa DSP (1991b) Free radical involvement in neurological porphyries and lead poisoning. Mol Cell Biochem 103:73–83
Mousa HM, Al-Qarawi AA, Ali BH, Abdel Rahman HA, El Mougy SA (2002) Effect of lead exposure on the erythrocytic antioxidant levels in goats. J Vet Med A Physiol Pathol Clin Med 49:531–553
Nampoothiri LP, Gupta S (2006) Simultaneous effect of lead and cadmium on granulosa cells: a cellular model for ovarian toxicity. Reprod Toxicol 21:179–185
Nelson SK, Bose SK, McCord JM (1994) The toxicity of high-dose superoxide dismutase suggests that superoxide can both initiate and terminate lipid peroxidation in the reperfused heart. Free Radic Biol Med 16:195–200
Nigam D, Shukla GS, Agarwal AK (1999) Glutathione depletion and oxidative damage in mitochondria following exposure to cadmium in rat liver and kidney. Toxicol Lett 106:151–157
Nivsarkar M, Cherian B, Patel S (1998) A regulatory role of sulfhydryl groups in modulation of sperm membrane confirmation by heavy metals:sulfhydryl groups as a marker for infertility assessment. Biochem Biophys Res Commun 2476:716–718
Ohkawa H, Ohishi N, Yagi K (1979). Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem 95:351–358
Ouig G (1998) Cysteine metabolism and metal toxicity. Altern Med Rev 3:262–270
Paksy K, Varga B, Lazar P (1992) Cadmium interferes with steroid biosynthesis in rat granulosa and luteal cells in vitro. Biometals 5:245–250
Paksy K, Rajczy K, Forgacs Z, Lazar P, Bernard A, Gati I, Kaali GS (1997) Effect of cadmium on morphology and steroidogenesis of cultures human granulose cells. Toxicology 17:321–327
Paksy K, Gati I, Naray M, Rajczy K (2001) Lead accumulation in human ovarian follicular fluid and in vitro effect of lead on progesterone production by cultured human granulosa cells. J Toxicol Environ Health, Part A 62:329–336
Piasek M, Laskey JW, Kostial K, Blanusa M (2002) Assessment of steroid disruption using cultures of whole ovary and/or placenta in rat and in human placental tissue. Int Arch Occup Environ Health 75:S36–S44
Pillai A, Gupta S (2005) Antioxidant enzyme activity and lipid peroxidation in liver of female rats co-exposed to lead and cadmium: effects of vitamin E and Mn2+. Free Radic Res 39:707–712
Pillai A, Laxmipriya, Rawal A, Gupta S (2002) Effect of low level exposure of lead and cadmium on hepatic estradiol metabolism in female rats. Indian J Exp Biol 40:807–811
Priya PN, Pillai A, Gupta S (2004). Effect of simultaneous exposure to lead and cadmium on gonadotropin binding and steroidogenesis on granulosa cells: an in vitro study. Indian J Exp Biol 42:143–148
Qanungo S, Sen A, Mukherjea M (1999) Antioxidant status and lipid peroxidation in human feto-placental unit. Clin Chim Acta 285:1–12
Sato EF, Kobuchi H, Edashige K, Takahashi M, Yoshioka T, Utsumi K, Inoue M (1992) Dynamic aspects of ovarian superoxide dismutase isozymes during the ovulatory process in the rat. FEBS Lett 303:121–125
Shafiq-ur-Rehman (1984) Lead induced regional lipid peroxidation in brain. Toxicol Lett 21:333–337
Stohs SJ, Bagchi D, Hassoun E, Bagchi M (2001). Oxidative mechanisms in the toxicity of chromium and cadmium ions. Environ Pathol Toxicol Oncol 20:77–88
Strehlow K, Rotter S, Wassmann S, Adam O, Grohe C, Laufs K, Bohm M, Nickenig G (2003) Modulation of antioxidant enzyme expression and function by estrogen. Circ Res 93:170–177
Struzynska L, Sulkowski G, Lenkiewicz A, Rafalowska U (2002) Lead stimulates the glutathione system in selective regions of rat brain. Folia Neuropathol 40:203–209
Wang X, Yin X, Bai X (2002) Combined effect of lead and cadmium on lipid peroxidation in renal tubular epithelial cells of rats. Wei Sheng Yan Jiu 31:232–234
Wiebe JP, Barr KJ, Buckingham KD (1988) Effect of prenatal & neonatal exposure to lead on gonadotropin receptors and steroidogenesis in rat ovaries. J Toxicol Environ Health 24:461–476
Yiin S, Lin TH (1995) Lead catalysed peroxidation of essential unsaturated fatty acid. Biol Trace Elem Res 50:167–172
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nampoothiri, L.P., Agarwal, A. & Gupta, S. Effect of co-exposure to lead and cadmium on antioxidant status in rat ovarian granulose cells. Arch Toxicol 81, 145–150 (2007). https://doi.org/10.1007/s00204-006-0133-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-006-0133-x