Abstract
Although several studies have reported that diesel exhaust particles (DEP) affect cardiorespiratory health in animals and humans, the effect of DEP on animal models with spontaneous allergic disorders has been far less intensively studied. The Nc/Nga mouse is known to be a typical animal model for human atopic dermatitis (AD). In the present study, we investigated the effects of repeated pulmonary exposure to DEP on airway inflammation and cytokine expression in NC/Nga mice. The animals were randomized into two experimental groups that received vehicle or DEP by intratracheal instillation weekly for six weeks. Cellular profiles of bronchoalveolar lavage (BAL) fluid and expressions of cytokines and chemokines in both the BAL fluid and lung tissues were evaluated 24 h after the last instillation. The DEP challenge produced an increase in the numbers of total cells, neutrophils, and mononuclear cells in BAL fluid as compared to the vehicle challenge (P<0.01). DEP exposure significantly induced the lung expressions of interleukin (IL)-4, keratinocyte chemoattractant (KC), and macrophage inflammatory protein (MIP)-1α when compared to the vehicle challenge. These results indicate that intratracheal exposure to DEP induces the recruitment of inflammatory cells, at least partially, through the local expression of IL-4 and chemokines in NC/Nga mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary exposure to particulate matter (PM) can have important acute and/or long-term health effects (Dockery and Pope 1994). These effects include aggravation of respiratory allergies to common allergens such as pollen (van Zijverden et al 2001) and house dust mites (Ichinose et al 2004). In urban areas, diesel engines are considered to be the major source of PM. The immunomodulatory effects of DEP, including adjuvant activity, have been demonstrated in several allergic models in vivo (Fujimaki et al 1997; Takafuji et al 1987; Takano et al 1997), as well as in experimental human studies (Diaz-Sanchez et al 1999). However, the effect of DEP on animal models with spontaneous allergic disorders without antigen challenge has never been investigated.
Recent epidemiological studies have shown that the prevalence of allergic diseases is rising in Western and developing countries (Eichenfield et al 2003; Laughter et al 2000; Woolcock and Peat 1997). Atopic dermatitis (AD) is a chronic skin allergy associated with cutaneous hyperreactivity to environmental triggers (Leung and Bieber 2003). The AD patients are affected by a Th1/Th2 inbalance that includes enhanced Th2 type cytokine expression in the skin (Hamid et al 1994). On the other hand, previous observations suggest that AD is the cutaneous manifestation of a systemic allergic response that also gives rise to bronchial asthma, food allergy, and allergic rhinitis (Leung and Bieber 2003; Spergel and Paller 2003; Eichenfield et al 2003).
The Nc/Nga mouse is an established animal model for human AD (Matsuda et al 1997; Matsumoto et al 1999). This strain of mouse spontaneously develops dermatitis associated with IgE overproduction under conventional conditions. Dermatitis is proposed to result from a combination of genetic propensity and environmental factors (Tsudzuki et al 1997). However, the effects of environmental particles or/and chemicals—both important ambient environmental factors—on this strain of mice have been studied far less (Fujimaki et al 2002). Furthermore, there has been no study of the effects of pulmonary exposure to PM on the airways in NC/Nga mice.
The aim of this study was to elucidate whether pulmonary exposure to DEP induces airway inflammation and local expression of cytokines and chemokines in NC/Nga mice.
Materials and methods
Animals and study protocol
Male NC/Nga mice (ten weeks old) were purchased from Charles River (Kanazawa, Japan). Food and water were given ad libitum. Animals were housed at temperature between 23 and 25 °C and 55–70% humidity with a 12-h/12-h light/dark cycle until the end of the experiment. This study adhered to the National Institute of Health guidelines for the use of experimental animals and was approved by the National Institute for Environmental Studies Animal Care and Use Committee.
Preparation of DEP
A 4JB1-type, light-duty, four-cylinder, 2.74 l, Isuzu diesel engine (Isuzu Automobile Co., Tokyo, Japan), under computer control, was connected to a dynamometer (Meiden-sya, Tokyo, Japan). A detailed description of the condition of engine and the collection of DEP has been published previously (Sagai et al 1996).
Study protocol
The mice were divided into two groups. The vehicle group received phosphate-buffered saline (PBS) at pH 7.4 (Nissui Pharmaceutical Co., Tokyo, Japan), containing 0.05% Tween 80 (Nacalai Tesque, Kyoto, Japan), every week for six weeks. The DEP group received 100 μg of suspended DEP in the same vehicle every week for six weeks. In each group, vehicle or DEP was dissolved in 0.1 ml aliquots, and inoculated by intratracheally through a polyethylene tube under deep anesthesia from 4% halothane (Hoechst, Japan; Tokyo, Japan), as described previously (Takano et al 1997). The cellular profiles in bronchoalveolar lavage (BAL) fluid, protein levels of cytokines and chemokines in both the BAL and total lung tissue supernatants of the animals were studied 24 h after the last intratracheal administration.
Bronchoalveolar lavage (BAL)
The trachea was cannulated after exsanguination. The lungs were lavaged with 1.2 ml of sterile saline at 37 °C, instilled bilaterally by syringe. The lavaged fluid was harvested by gentle aspiration. This procedure was conducted two more times. The average volume retrieved was 90% of the 3.6 ml that was instilled; the amounts did not differ by treatment. The fluid collections were combined and cooled to 4 °C. The lavage fluid was centrifuged at 300×g for 10 min, and the total cell count was determined on a fresh fluid specimen using a hemocytometer. Slides were prepared using Autosmear (Sakura Seiki Co., Tokyo, Japan) and were stained with Diff-Quik (International Reagents Co., Kobe, Japan). A total of 500 cells were counted under oil immersion microscopy (n=14 in each group). Aliquots of the supernatants were stored without further treatment at −80 °C until analyzed for cytokines and chemokines. After the BAL procedure, the lungs were removed, snap-frozen in liquid nitrogen, and stored at −80 °C.
Analyses of cytokine and chemokine protein levels in BAL supernatants, enzyme-linked immunosorbent assays (ELISA) for IL-4 (Amersham, Buckinghamshire, UK), IL-5 (Endogen, Cambridge, MA, USA), keratinocyte chemoattractant (KC), and macrophage inflammatory protein (MIP)-1α (R&D systems, Minneapolis, MN, USA) were conducted using matching antibody pairs according to the manufactor’s instruction. The second antibodies were conjugated to horseradish peroxidase. The readings at 550 nm were substracted from the readings at 450 nm and then converted to pg/ml using values obtained from standard curves generated for limits of detection of 5, 5, 2 and 1.5 pg/ml for IL-4, IL-5, KC and MIP-1α, respectively (n=18 in each group).
Cytokine and chemokine protein levels in lung tissue supernatants
The frozen lungs were homogenized with 10 mM potassium phosphate buffer (pH 7.4) containing 0.1 mM ethylenediaminetetraacetic acid (Sigma, St. Louis, MO, USA), 0.1 mM phenylmethanesulfonyl fluoride (Nacalai Tesque, Kyoto, Japan), 1 μM pepstatin A (Peptide Institute, Osaka, Japan), and 2 μM leupeptin (Peptide Institute), as described previously (Takano et al 2002). The homogenates were then centrifuged at 105,000× g for 1 h. The supernatants were stored at −80 °C. The ELISAs for IL-4, IL-5, KC and MIP-1α in the lung tissue supernatants were conducted in the same manner as described above for the BAL supernatants.
Statistical analysis
Values are expressed as mean±SEM. Student’s t-test was used to test differences between the groups (Stat view Version 4.0; Abacus Concepts Inc., Berkeley, CA, USA). When significances were detected, post hoc comparisons within each group were evaluated using the Turkey–Kramer test (Stat view). Significance was assigned to P values smaller than 0.05.
Results
Effect of pulmonary exposure to DEP on airway inflammation in NC/Nga mice
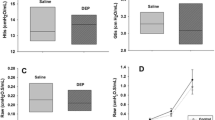
To elucidate the effect of repeated pulmonary exposure to DEP on the respiratory systems in NC/Nga mice, we investigated the cellular profile of BAL fluid 24 h after the last intratracheal instillation with vehicle or DEP. The DEP challenge induced significant increases in the numbers of total cells, neutrophils and mononuclear cells as compared with the vehicle challenge (P<0.01: Table 1). The number of eosinophils was not different between the groups.
Effect of pulmonary exposure to DEP on lung expression of cytokines and chemokines in NC/Nga mice
To investigate the effect of pulmonary exposure to DEP on lung expression of cytokines and chemokines, we compared the protein levels of IL-4, IL-5, KC and MIP-1α in BAL supernatants (Table 2) and lung tissue supernatants (Table 3) between the experimental groups 24 h after the last intratracheal instillation. The DEP challenge significantly elevated the levels of IL-4, KC and MIP-1α (P<0.01) in BAL supernatants as compared with the vehicle challenge (Table 2). The levels of IL-5 were also greater in the DEP group than in the vehicle group; however, the difference did not reach statistical significance (Table 2). The DEP exposure also significantly elevated the levels of IL-4 (P<0.05), KC and MIP-1α (P<0.01) in lung tissue supernatants as compared with vehicle exposure (Table 3). The levels of IL-5 were not different between the two experimental groups (Table 3).
Discussion
The present study has shown that weekly intratracheal administration of DEP (100 μg/body) causes the recruitment of inflammatory cells such as neutrophils and mononuclear cells to the airways in NC/Nga mice. DEP enhances lung expression of IL-4, KC and MIP-1α.
We have previously investigated a weekly pulmonary exposure to DEP on several strains of mice using the same protocol. Weekly intratracheal inoculation of DEP (100 μg/body) for six weeks did not influence lung inflammation, including neutrophil and eosinophil infiltration, as compared to vehicle inoculation in ICR mice (Takano et al 1997). We have also extended the experiments in order to elucidate strain differences in murine airway inflammation among several strains with or without antigen sensitization. In our previous study, we have also evaluated the effects of repeated challenge with DEP (50 μg/body) on airway inflammation in C3H/He and Balb/c mice, but no significant differences between the DEP and vehicle group were detected in either strain (Miyabara et al 1998). The Balb/c mouse is low responder for specific IgG production to antigen (ovalbumin), whereas the C3H/He mouse is a high responder (Takeyoshi and Inoue 1992).
The Nc/Nga mouse is recognized as being a strain with a propensity for spontaneous development of dermatitis and IgE overproduction when it is raised under conventional conditions (Matsuda et al 1997; Matsumoto et al 1999; Tsudzuki et al 1997). In the present study, repeated DEP instillation induced significant recruitment of neutrophils and mononuclear cells to the airway as compared to vehicle instillation. The results indicate that Nc/Nga mice are more susceptible to DEP-induced airway inflammation than other strains of mice we have previously examined.
A potent neutrophil chemotactic and activity factor, IL-8, has been shown to be elevated during inflammation in experimental animal models and human diseases (Matsumoto et al 1997). KC is thought to be the mouse’s functional homolog of IL-8 in humans (Singer and Sansonetti 2004). Also, MIP-1α has been shown to possess chemotactic activity for inflammatory and immune effector cells including neutrophils and mononuclear cells (Driscoll 1994). Furthermore, significant elevations of the chemokines in BAL fluid have been demonstrated in allergic patients (Katoh et al 2000). In the present study, the concentrations of KC and MIP-1α in lung tissue and BAL supernatants were markedly greater in the DEP group than in the vehicle group. The increase in inflammatory cell influx into the airways caused by DEP exposure might be explained, at least partly, by the induction of these chemokine expressions.
Interestingly, the protein levels of IL-4 in both BAL fluid and lung tissue were significantly greater in the DEP group than in the vehicle group in the present study. The IL-4 mediates essential proinflammatory functions in allergic reactions, including induction of the IgE isotype switch, expression of vascular cell adhesion molecule-1, and promotion of eosinophil transmigration across endothelium (Steinke and Borish 2001). In addition, IL-4 contributes to airway obstruction in asthma through the hypersecretion of mucus (Dabbagh et al 1999). Furthermore, IL-4 can drive the differentiation of naïve T helper type 0 (Th0) into Th2 lymphocytes. The Th2 lymphocytes induce orchestration of allergic inflammation (Steinke and Borish 2001). Significant higher local expression levels of IL-4 in the DEP group indicate that the enhanced expression of the cytokine can precede induction and/or enhancement of allergic airway inflammation thereafter.
Finally, we did not confirm that there is a relationship between pulmonary exposure to DEP and incidence of dermatitis in NC/Nga mice. The incidence of dermatitis was not significantly different between the two experimental groups (vehicle group, 10/18; DEP group, 13/18). We are investigating the possibility of enhancing the effects of DEP or DE exposure on dermatitis in NC/Nga mice with antigen sensitization for a longer experimental period than in the present study.
Overall, our findings demonstrate that repeated pulmonary exposure to DEP can induce airway inflammation in NC/Nga mice. The enhanced airway inflammation is concomitant with the increased lung expression of MIP-1α and KC. In addition, DEP alone can elicit lung expression of IL-4 protein. The results indicate a link between PM exposure and exaggerated manifestations in subjects with allergic disorders.
References
Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA (1999) IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol 162:6233–6237
Diaz-Sanchez D, Garcia MP, Wang M, Jyrala M, Saxon A (1999) Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J Allergy Clin Immunol 104:1183–1188
Dockery DW, Pope CA III (1994) Acute respiratory effects of particulate air pollution. Annu Rev Public Health 15:107–132
Driscoll KE (1994) Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res 20:473–490
Eichenfield LF, Hanifin JM, Beck LA, Lemanske RF Jr, Sampson HA, Weiss ST, Leung DY (2003) Atopic dermatitis and asthma: parallels in the evolution of treatment. Pediatrics 111:608–616
Fujimaki H, Saneyoshi K, Shiraishi F, Imai T, Endo T (1997) Inhalation of diesel exhaust enhances antigen-specific IgE antibody production in mice. Toxicology 116:227–233
Fujimaki H, Nohara K, Kobayashi T, Suzuki K, Eguchi-Kasai K, Tsukumo S, Kijima M, Tohyama C (2002) Effect of a single oral dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin on immune function in male NC/Nga mice. Toxicol Sci 66:117–124
Hamid Q, Boguniewicz M, Leung DY (1994) Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest 94:870–876
Ichinose T, Takano H, Sadakane K, Yanagisawa R, Yoshikawa T, Sagai M, Shibamoto T (2004) Mouse strain differences in eosinophilic airway inflammation caused by intratracheal instillation of mite allergen and diesel exhaust particles. J Appl Toxicol 24:69–76
Katoh S, Matsumoto N, Fukushima K, Mukae H, Kadota JI, Kohno S, Matsukura S (2000) Elevated chemokine levels in bronchoalveolar lavage fluid of patients with eosinophilic pneumonia. J Allergy Clin Immunol 106:730–736
Laughter D, Istvan JA, Tofte SJ, Hanifin JM (2000) The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol 43:649–655
Leung DY, Bieber T (2003) Atopic dermatitis. Lancet 361:151–160
Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, Matsumoto M, Ushio H, Saito S, Askenase PW, Ra C (1997) Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol 9:461–466
Matsumoto T, Yokoi K, Mukaida N, Harada A, Yamashita J, Watanabe Y, Matsushima K (1997) Pivotal role of interleukin-8 in the acute respiratory distress syndrome and cerebral reperfusion injury. J Leukoc Biol 62:581–587
Matsumoto M, Ra C, Kawamoto K, Sato H, Itakura A, Sawada J, Ushio H, Suto H, Mitsuishi K, Hikasa Y, Matsuda H (1999) IgE hyperproduction through enhanced tyrosine phosphorylation of Janus kinase 3 in NC/Nga mice, a model for human atopic dermatitis. J Immunol 162:1056–1063
Miyabara Y, Yanagisawa R, Shimojo N, Takano H, Lim HB, Ichinose T, Sagai M (1998) Murine strain differences in airway inflammation caused by diesel exhaust particles. Eur Respir J 11:291–298
Sagai M, Furuyama A, Ichinose T (1996) Biological effects of diesel exhaust particles (DEP) III. Pathogenesis of asthma like symptoms in mice. Free Radic Biol Med 21:199–209
Singer M, Sansonetti PJ (2004) IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J Immunol 173:4197–4206
Spergel JM, Paller AS (2003) Atopic dermatitis and the atopic march. J Allergy Clin Immunol 112:S118–S127
Steinke JW, Borish L (2001) Th2 cytokines and asthma. Interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res 2:66–70
Takafuji S, Suzuki S, Koizumi K, Tadokoro K, Miyamoto T, Ikemori R, Muranaka M (1987) Diesel-exhaust particulates inoculated by the intranasal route have an adjuvant activity for IgE production in mice. J Allergy Clin Immunol 79:639–645
Takano H, Yoshikawa T, Ichinose T, Miyabara Y, Imaoka K, Sagai M (1997) Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice. Am J Respir Crit Care Med 156:36–42
Takano H, Yanagisawa R, Ichinose T, Sadakane K, Yoshino S, Yoshikawa T, Morita M (2002) Diesel exhaust particles enhance lung injury related to bacterial endotoxin through expression of proinflammatory cytokines, chemokines, and intercellular adhesion molecule-1. Am J Respir Crit Care Med 165:1329–1335
Takeyoshi M, Inoue T (1992) H-2 haplotype and sex-related differences in IgG response to ovalbumin in mice. Jikken Dobutsu 41:315–319
Tsudzuki M, Watanabe N, Wada A, Nakane Y, Hiroi J, Matsuda H (1997) Genetic analyses for dermatitis and IgE hyperproduction in the NC/Nga mouse. Immunogenetics 47:88–90
Woolcock AJ, Peat JK (1997) Evidence for the increase in asthma worldwide. Ciba Found Symp 206:122–134
van Zijverden M, de Haar C, van Beelen A, van Loveren H, Penninks A, Pieters R (2001) Coadministration of antigen and particles optimally stimulates the immune response in an intranasal administration model in mice. Toxicol Appl Pharmacol 177:174–178
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inoue, Ki., Takano, H., Yanagisawa, R. et al. Pulmonary exposure to diesel exhaust particles induces airway inflammation and cytokine expression in NC/Nga mice. Arch Toxicol 79, 595–599 (2005). https://doi.org/10.1007/s00204-005-0668-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-005-0668-2