Abstract
This study investigated the hypothesis that the chromosomal genotoxicity of inorganic mercury results from interaction(s) with cytoskeletal proteins. Effects of Hg2+ salts on functional activities of tubulin and kinesin were investigated by determining tubulin assembly and kinesin-driven motility in cell-free systems. Hg2+ inhibits microtubule assembly at concentrations above 1 µM, and inhibition is complete at about 10 µM. In this range, the tubulin assembly is fully (up to 6 µM) or partially (~6–10 µM) reversible. The inhibition of tubulin assembly by mercury is independent of the anion, chloride or nitrate. The no-observed-effect-concentration for inhibition of microtubule assembly in vitro was 1 µM Hg2+, the IC50 5.8 μM. Mercury(II) salts at the IC50 concentrations partly inhibiting tubulin assembly did not cause the formation of aberrant microtubule structures. Effects of mercury salts on the functionality of the microtubule motility apparatus were studied with the motor protein kinesin. By using a “gliding assay” mimicking intracellular movement and transport processes in vitro, HgCl2 affected the gliding velocity of paclitaxel-stabilised microtubules in a clear dose-dependent manner. An apparent effect is detected at a concentration of 0.1 µM and a complete inhibition is reached at 1 μM. Cytotoxicity of mercury chloride was studied in V79 cells using neutral red uptake, showing an influence above 17 µM HgCl2. Between 15 and 20 µM HgCl2 there was a steep increase in cell toxicity. Both mercury chloride and mercury nitrate induced micronuclei concentration-dependently, starting at concentrations above 0.01 µM. CREST analyses on micronuclei formation in V79 cells demonstrated both clastogenic (CREST-negative) and aneugenic effects of Hg2+, with some preponderance of aneugenicity. A morphological effect of high Hg2+ concentrations (100 µM HgCl2) on the microtubule cytoskeleton was verified in V79 cells by immuno-fluorescence staining. The overall data are consistent with the concept that the chromosomal genotoxicity could be due to interaction of Hg2+ with the motor protein kinesin mediating cellular transport processes. Interactions of Hg2+ with the tubulin shown by in vitro investigations could also partly influence intracellular microtubule functions leading, together with the effects on the kinesin, to an impaired chromosome distribution as shown by the micronucleus test.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has long been recognised that mercury compounds display cytogenetic effects, e.g. resulting in altered distribution of chromosomes (Léonard 1988). Human epidemiological studies on carcinogenic effects of mercury have been performed in occupationally exposed persons, but no strong association with cancer risk could be found. Nevertheless, the sparse epidemiological data pointed to possible associations with neoplasias of the lung, kidney or central nervous system (Boffetta et al. 1993, 1998).
The U.S. National Toxicology Program (1993) has conducted long-term carcinogenicity studies with mercury chloride in F-344 rats and B6C3F1 mice upon oral administration (gavage). Focal hyperplasia and squameous cell papillomas of the forestomach were observed. In male rats, there were thyroid follicular adenomas and carcinomas. There was also evidence for an increased incidence of squameous forestomach papillomas in female rats and renal adenomas and carcinomas in male mice, but this was considered equivocal. All increased tumour incidences were observed at high doses, in excess of the maximal tolerated dose. No renal tumours appeared in rats, although renal toxicity was noticed (Dieter et al. 1992).
On this basis, no conclusions were drawn by official bodies with regard to a categorisation of mercury compounds for carcinogenicity (Cross et al. 1995; Schoeny 1996; DFG 1999).
Mercury compounds, in general, fail to induce point mutations in bacteria but exert chromosomal genotoxicity such as chromosomal aberration, clastogenicity, and aneugenic effects (De Flora et al. 1994; Al-Sabti1994; Akiyama et al. 2001; Thier et al. 2003). When the U.S. Environmental Protection Agency reviewed the genetic toxicology data of mercury compounds, it arrived at the conclusion that data for clastogenicity, in the absence of mutagenicity, supported the categorisation of inorganic and methyl mercury as materials that produce carcinogenic effects only at high, toxic doses. However, the evidence for clastogenicity, coupled with information on metabolism and distribution, resulted in a judgement of a moderate degree of concern that inorganic mercury might act as a human germ cell mutagen (Schoeny 1996).
Mercury compounds are known to interact with tubulin and to affect tubulin assembly (Vogel et al. 1989; Duhr et al. 1993; Liliom et al. 2000). Hence, in previous publications (Thier et al. 2003; Stoiber et al. 2004) we have advanced the theory that the chromosomal genotoxicity of mercury may result, at least in part, from interaction(s) with cytoskeletal proteins. The present study further investigates this possibility. In particular, interactions of inorganic mercury salts with tubulin and kinesin, their genotoxic effects, and dose-response-relationships are demonstrated. Effects of exposure to mercury salts on functional activities of tubulin and kinesin were investigated by measurements of tubulin assembly and kinesin-driven motility in cell-free systems. As endpoint of chromosomal genotoxicity of mercury salts, the micronucleus test in vitro, including CREST analysis, was used.
Materials and methods
Chemicals
The test substance mercury(II) chloride (CAS No. 7487-94-7) was purchased from Sigma-Aldrich (Taufkirchen, Germany), and mercury (II) nitrate (CAS No. 7783-34-8) was from Fluka (Buchs, Switzerland). Reagents for buffer solutions came from Sigma-Aldrich (magnesium nitrate), Fluka (sodium chloride, sodium hydroxide, magnesium chloride, and EGTA, Merck (Darmstadt, Germany, glycerol), Roth (Karlsruhe, Germany; dithiotreitol/DTT) and Serva (Heidelberg, Germany; imidazole and PIPES). Biochemical reagents were from the following suppliers: Roth (GTP), Roche Diagnostics (Mannheim, Germany; ATP as sodium salt), and Sigma. Uranyl acetate for negative staining was purchased from Merck. Reagents for immunofluorescence studies came from Fisher Scientific (Loughborough, UK; bovine albumin), GIBCO (Paisley, UK; PBS), Merck (paraformaldehyde), Roth (Triton X 100), and Sigma (anti-β-tubulin antibody, cat. No. C-4585). For cell culture, we used DMEM (Biochrom, Berlin, Germany), PBS, FCS (GIBCO/Biochrom), trypsin-EDTA (Invitrogen, Karlsruhe, Germany) and accutase (PAA, Linz, Austria, for cell detachment). Vincristine (VCR), methyl methane sulfonate (MMS), neutral red (NR), acridine orange, propidium iodide, and 4’,6’-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich (Taufkirchen, Germany). The primary antibody for the CREST analysis, so-called “positive control centromere serum”, was obtained from DPC Biermann (Bad Nauheim, Germany); the second antibody, FITC conjugated anti-human IgG, was from Sigma. Dimethyl-sulfoxide (DMSO), ethanol, methanol, acetone, acetic acid and Tween 20 were products of Merck (Darmstadt, Germany). Acridine orange working solution was prepared as follows: 5 ml acridine orange stock solution (1 mg/ml aq. dest.) were mixed with 7 ml 0.3 M KH2PO4, 7 ml 0.3 M Na2HPO4 and 80 ml distilled water. All salts were first dissolved in water before they were added to buffer solutions or cell culture medium.
Microtubule assembly: turbidity assay
Microtubule protein (tubulin containing microtubule-associated proteins, MAPs) was isolated from porcine brain by two cycles of temperature-dependent assembly-disassembly (Shelanski et al. 1973) using the following buffer system: 0.02 M Pipes, 0.08 M NaCl, 0.5 mM MgCl2, 1 mM EGTA, and 1 mM DTT. To avoid side reactions of mercury(II) with some of the buffer components during the assembly tests, the microtubule sediment obtained after the second disassembly/reassembly cycle was resuspended in 100 mM PIPES, 0.5 mM Mg(NO3)2, and 1 mM NaN3. With exception of NaN3, this buffer (“assembly buffer”) was used throughout all experiments.
Microtubule assembly is induced by adding GTP (final concentration: 0.5 mM) to 1 mg/ml microtubule protein and shifting the temperature to 37°C. This assembly is accompanied by changes of turbidity which may be recorded at 360 nm, as described by Gaskin et al. (1974). After 20 min, the turbidity increases and reaches a plateau level (steady-state of assembly/disassembly). The corresponding absorbance is taken as a reference value to quantify the effects of metal salts. Assembly antagonists or disassembly effectors (e.g. Ca2+ ions or cold) prevent assembly or cause degradation. To check the reversibility of microtubule assembly, the measurement was continued for additional 20 min during decreasing the temperature to 4°C.
Transmission electron microscopy
Negative staining of microtubules formed during the tubulin assembly assay in presence of mercury(II) chloride (at the IC50) was performed using 1% uranyl acetate in water. The samples were visualised with a Zeiss EM 902 A transmission electron microscope (for details, see Unger et al. 1988).
Microtubule disassembly: video-enhanced microscopic assay
Microtubules (0.8 mg/ml tubulin) formed in assembly buffer by 20-min incubation at 37°C were stabilised with paclitaxel (10 µM final concentration). This suspension was 10-fold diluted with water containing 10 µM paclitaxel and 5 or 10 µM HgCl2, and transferred onto glass slides. The disassembly of individual microtubules was followed over 15 min by differential interference contrast (DIC) video enhanced microscopy.
Microtubule motility: video-enhanced microscopic gliding assay
Kinesin was isolated from porcine brain homogenates by a combined procedure of ion exchange chromatography, microtubule affinity-binding in the presence of tripolyphosphate, and gel filtration (Kuznetsov and Gelfand 1986). For the gliding assays, paclitaxel-stabilised microtubules, formed from MAP-free tubulin that had been prepared according to Weingarten et al. (1975), were used.
The gliding assay was performed according to Böhm et al. (2000). The following changes were made: at first, the kinesin (5 µl, 1 mg/ml) was allowed to attach to an 18×18 mm area of a glass slide. After 10 min, non-bound kinesin was removed by washing with 100 mM imidazole buffer (pH 6.8), containing 0.5 mM Mg(NO3)2. Thereafter, 10 µl of the microtubule suspension (final tubulin concentration 40 µg/ml), in the same buffer supplemented with 0.5 mM Na2ATP, and the metal salts were added and the area was covered by a cover-slip. Gliding activity of microtubules was monitored by video-enhanced differential interference contrast microscopy as described by Weiss and Maile (1992), using an Axiophot microscope (Zeiss) equipped with the image processing system Argus 20 (Hamamatsu). Gliding velocities were directly measured using the “speed function” of the Argus image processor.
Cytotoxicity assays
Cytotoxicity of mercury chloride was determined in V79 hamster lung fibroblast cells by means of the neutral red assay (Borenfreund et al. 1988). The results were used to define a suitable range of concentrations (below and near cytotoxicity) for the subsequent micronucleus (MN) assay.
Briefly, 10,000 V79 cells were plated in 0.2 ml DMEM (with 10% FCS) per well in 96-well tissue-culture plates (Costar from VWR International, Bruchsal, Germany) and allowed to grow for 24 h at 37°C. Then the test substance was added to culture medium at various concentrations. After 18 h the medium was replaced by medium containing neutral red (NR, 50 µg/ml), and incubation was continued for 3 h at 37°C. The cells were then washed five times with PBS (1x) and fixed with 0.2 ml fixative (glacial acetic acid/water/ethanol, 1:49:50) per well, which brings the dye NR into solution upon 20 min of shaking (600 rpm). The absorbance of NR was measured at 540 nm using a plate photometer (340 ATC, SLT).
Furthermore, proliferation studies were performed in normal cell culture flasks (12.5 cm2) with V79 cells cultured in DMEM containing 5% FCS and various concentrations of mercury(II) chloride. Every 24 h after seeding a constant number of 5*104 cells into the flasks, one representative sample for each concentration was treated with accutase and analysed for cell density and viability with the CASY cell counter system.
Micronucleus (MN) assay and CREST analysis
The MN assay was performed according to Matsuoka et al. (1992). Initially, 300,000 V79 cells were seeded into 25 cm2 flasks (Greiner, Frickenhausen, Germany) and cultured for 48 h at 37°C. Then the cells (in medium containing 10% FCS) were treated with the mercury or control substances (positive controls: VCR and MMS) for 1.5 cell cycles (at 37°C) for 18 h. Cells were harvested by disaggregation with trypsin/EDTA (0.25% trypsin in PBS with 0.02% EDTA) and resuspended in complete medium with 20% FCS after centrifugation at 200 g for 10 min. Cells were then subjected to hypotonic conditions with 0.4% KCl, and to fixation with methanol/acetic acid (3:1; four cycles and centrifugation in between). Some drops of the fixed cell suspensions (0.5 ml) were put on glass slides and allowed to dry. Shortly before microscopic analysis, the cells were stained with 50 µl acridine orange working solution. The slides were examined using a fluorescence microscope (Leitz DMBR) at 400-fold magnification and filter settings with excitation at 440–490 nm and emission at 520 nm. The scoring criteria for MN followed those of Countryman and Heddle (1976) and Fenech et al. (1993). In total, 7,000–16,000 cells per concentration were evaluated. This necessitated at least two parallel sets of experiments. Test substance concentrations examined in the MN assay were generally below the cytotoxicity threshold determined by the neutral red assay.
A distinction between aneugens and clastogens was achieved by CREST analysis, according to Renzi et al. (1996) and Russo et al. (1992) with some modifications. Vincristine (VCR at 10 nM) and methyl methane sulfonate (MMS at 25 µg/ml) served as aneugenic and clastogenic positive controls, respectively. The test conditions were those used in the standard MN assay except that the cells were seeded directly onto sterile slides and treated in Quadriperm dishes (Viva Science, Hannover, Germany). After incubation with the test compounds for 18 h (at 37°C under 5% CO2), the slide preparation was as follows: slides were rinsed 2 min with 1x PBS and kept in 0.075 M KCl for about 15 min (at 37°C). For fixation of the cells, slides were placed for 30 min in methanol (−20°C) and for 10 min in acetone (−20°C). Then, fixed cells were placed 5 min in PBS with 0.1% Tween 20. Fifty microlitres of the primary antibody (CREST serum diluted 1:50 with PBS/0.2% Tween 20) were put on the slides and incubated for 24 h (37°C, 5% CO2). This was followed by rinsing twice with PBS/0.1% Tween 20 and an incubation for 1 h with the second antibody (FITC conjugated anti-Human IgG, diluted 1:100 in PBS/0.5% Tween 20). Then, slides were rinsed twice with PBS/0.1% Tween 20 before doublestaining was performed with 100 µl staining solution (1 µg/ml DAPI + 0.1 mg/ml propidium iodide, mixed at 3:1 v/v).
Slides were examined with a Leitz DMBR (Leica, Wetzlar, Germany) fluorescence microscope at 400-fold or 1,000-fold magnification. For the fluorochromes used for staining, appropriate filter settings were chosen: excitation at 340–380 nm for DAPI and 515–560 nm for propidium iodide and emissions at 425 and 590 nm, respectively. In cells stained with CREST serum and FITC-labeled antibodies, the presence of a kinetochore in the micronucleus was determined using fluorescein filter settings (excitation at 490 nm, emission 525 nm) after a micronucleated cell had been located on the slide. For each concentration 1,000 cells per slide were counted, totalling 4,000 cells on four slides per treatment group. The MN were assigned “CREST positive”, “CREST negative” and “unclear” (when classification was not clear due to high background fluorescence or a very weak signal). A positive CREST reaction reveals that the MN consists of one or more complete chromosomes, primarily indicating an aneugenic effect (Miller and Adler 1990; Schuler et al. 1997).
Immunofluorescence studies
Cells were seeded on coverslips and incubated for 24 h with culture medium containing 5% FCS and mercury(II) chloride. After fixation with formaldehyde, the cells were treated with Cy3-labelled monoclonal anti-β-tubulin antibody (SIGMA) according to the manufacturer’s instructions. Mounted cover-slips were examined at magnification 100× using an Axiovert 135 TV fluorescence microscope (Carl Zeiss, Jena, Germany).
Results
Microtubule assembly: turbidity assay
The “tubulin assembly assay” is based on the observation that, under defined conditions, tubulin assembles and disassembles dependent on the ambient temperature. Physiological temperatures lead to polymerisation of the tubulin dimers and to microtubule formation. This process is reversible at low temperature. Microtubule assembly and disassembly were observed spectrophotometrically during consecutive temperature cycles (4, 37, 4°C). Turbidity (absorbance) at 360 nm is increased upon formation of microtubules. If tubulin assembly is inhibited, e.g. by HgCl2, the turbidity at 37°C is reduced compared with the control. At a certain concentration, the assembly may be completely inhibited (no rise in absorption). Denaturation of tubulin followed by aggregation and precipitation may occur at higher concentrations of (some) chemicals and is reflected by a change in curve shape during the period of warming, compared with the control. The rise in absorption resulting from denaturation is not reversible, resulting in higher absorbances at 4 C compared with control samples.
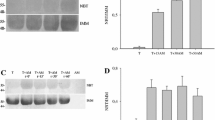
Mercury(II) inhibits the microtubule assembly at concentrations above 1 µM (Fig. 1). The inhibition is complete at about 10 µM. In this range the assembly is fully (up to 6 µM) or partially (~6–10 µM) reversible. Higher doses of mercury(II) cause the formation of protein-mercury- aggregates (Fig. 1a). The dose-response curve is shown in Fig. 1b. The inhibition of tubulin assembly is independent of the anions chloride or nitrate. The no-observed-effect-concentration for inhibition of microtubule assembly in vitro is 1 µM mercury(II); the IC50 is 5.8 µM.
Effect of mercury(II) chloride on tubulin assembly (a). Relative absorbance of the tubulin assembly mixture (compared to control) after incubation of tubulin with various concentrations of mercury(II) salts upon heating for 20 min to 37°C and additional cooling to 4°C. In the resulting dose-dependence curve (b) each data point represents the average of at least six individual tests (means∓S.D.)
Transmission electron microscopy
The in vitro assembly of microtubules, the possible formation of aberrant structures and their dynamic behaviour was investigated by electron microscopy. This technique was used as a confirmation of the results of the turbidity assay.
Electron micrographs for samples incubated with 5 µM mercury chloride showed no apparent deviations of the microtubule structures, compared with the control (data not shown). This means that mercury(II) salts, at concentrations partly inhibiting tubulin assembly, did not cause the formation of aberrant microtubule structures. Furthermore, it was demonstrated that complete inhibition by Hg2+ (10 µM) was reversible and not accompanied by the formation of tubulin clusters, other irregular structures or even denaturation. Normal microtubules were also formed following the addition of the chelator DTT (data not shown).
Microtubule disassembly
Mercury(II) has the potential to completely destroy microtubules (Keates and Yott 1984; Miura et al. 1984). Figure 2 shows the dynamics of this process. Microtubule decomposition occurs only at 10 µM HgCl2, starting after 10 min, but not at 5 µM HgCl2. In accordance with the literature, the effective molar ratio of mercury/tubulin for microtubule decomposition is higher than the one needed for the inhibition of microtubule assembly.
Gliding assay
Additionally, possible effects of mercury salts on motility functions of the microtubule system were studied by a so-called gliding assay using kinesin. In this assay, paclitaxel-stabilised microtubules were moved across a kinesin-coated glass surface mimicking intracellular movement and transport processes (see Bonacker et al. 2004).
HgCl2 affects the gliding velocity of paclitaxel-stabilised microtubules in a clear dose-dependent manner, apparent at a concentration of 0.1 µM, and reaching complete inhibition of motility at 1 µM (Fig. 3).
Cytotoxicity assay and effects on cell proliferation
Cytotoxicity of HgCl2 was studied in V79 cells. Reduction of neutral red (NR) uptake by an 18-h treatment with the test agent starts at concentrations above 17 µM HgCl2. Between 15 and 20 µM HgCl2 there is an intense decrease in NR-absorbance, indicative of cell toxicity. Concentrations above 25 µM HgCl2 reduce the NR uptake almost completely (Fig. 4). Studies assessing inhibition of cell proliferation by HgCl2 for longer culture periods essentially confirmed the results of NR-assay: the no-observed-effect concentration was 10 µM in this experiment, marked decreases in cell number were seen at 15 µM, and at 50 µM HgCl2 cell proliferation was completely suppressed (Fig. 5).
Micronucleus assay and CREST analysis
In terms of chromosomal genotoxicity, relevant interactions of chemicals with cytoskeletal macromolecules are reflected by the MN assay. Aneugenic compounds causing spindle or kinetochore damage lead to the formation of MN containing complete chromosomes. Clastogens may induce structural chromosome breaks. On the basis of preliminary observations (Thier et al. 2003) mercury salts were investigated regarding their ability to induce MN in V79 hamster fibroblasts. Distinction of aneugenic and clastogenic mechanisms was achieved by CREST analysis.
Both mercury chloride and mercury nitrate induced micronuclei concentration-dependently starting at concentrations above 0.01 µM mercury(II). Maximal MN induction was seen at 0.1 µM HgCl2 or 1 µM Hg(NO3)2 (Fig. 6a). In general, the CREST analysis argued in favour of a multiplicity of mechanisms of MN induction by mercury ions, with some prevalence of aneugenic over clastogenic effects (Fig. 6b).
Immunofluorescence studies
The effects of high concentrations of mercury(II) on the cytoskeleton were verified in V79 cells by immunofluorescence staining of the microtubule network. Disruption of the microtubule network is visualised by labelling with β-tubulin antibody conjugated with Cy3 fluorophore.
Up to 100 µM mercury(II), no dramatic structural effect on the microtubule network was detected in V79 cells. At concentrations of about 100 µM HgCl2 the microtubular network in some cells was destroyed, whereas the other cells remained intact. At 250 µM mercury(II) all cells lost integrity of their cytoskeleton. This was independent of absence or presence of 5% FCS (Fig. 7).
Discussion
Dose response relationships play a major role in risk assessment and regulatory toxicology since they allow the identification of no-observed effect concentrations of chemical exposure (Bolt 2003). Also with regard to genotoxicity and carcinogenicity, threshold mechanisms may be operative at different stages (Hengstler et al. 2003). There is a continuing debate regarding “indirect mechanisms of genotoxicity” leading to thresholds in dose-effect relationships (Kirsch-Volders et al. 2000, 2003; Pratt and Barron 2003). This present debate is focussed on chromosomal genotoxicity, and in particular to mechanisms leading to aneuploidy and clastogenicity (Kirsch-Volders et al. 2000; Bolt et al. 2004).
As far as the genotoxicity of mercury compounds is concerned, Léonard (1988) primarily addressed effects on chromosome distribution, e.g. aneugenicity. Later, De Flora et al. (1994) reviewed clastogenic effects of mercury compounds, and attributed these to the generation and activity of reactive oxygen. This view is seconded by others (Schurz et al. 2000; Rao et al. 2001). In cellular in vitro systems, mercury(II) compounds cause glutathione depletion that facilitates toxic effects of redox cyclings (De Flora et al. 1994), whereas ascorbic acid prevents genotoxicity of mercury(II) chloride (Rao et al. 2001). This points to a relevance of reactive oxygen mechanisms which are being viewed as connected to clastogenicity.
Our present data on micronuclei formation in V79 cells demonstrate both clastogenic (CREST-negative) and aneugenic effects of mercury(II), with some preponderance of aneugenicity (Fig. 5b). This, in turn, supports the original view of Léonard (1988) that mechanisms affecting chromosome distribution are also relevant for the chromosomal genotoxicity of mercury compounds. Taking the dose-response data of the present investigation into account, the following staggering of effects by Hg2+ is noted:
-
>0.01 µM: concentration-dependent induction of micronuclei in V79 cells
-
>0.1 μM: concentration-dependent inhibition of kinesin-mediated microtubule motility in a cell-free environment
-
>1 µM: inhibition of microtubule formationin a cell-free system
-
At 10 µM: disassembly of microtubules in a cell-free system, visualised by video contrast microscopy
-
>15 µM: overt cytotoxicity, indicated by decrease of neutral red uptake in V79 cells and inhibition of cell proliferation
-
>15 µM: decomposition of the microtubule network in IMR-32 cells (Stoiber et al. 2004)
-
>100 µM: decomposition of the microtubule network in V79 cells
In general, it is difficult to compare the concentrations of Hg2+ required for distinct effects in cellular vs. cell-free systems. On the one hand, cellular transport processes must be taken into account. On the other hand, the availability of binding sites for Hg2+ should be much different in cellular vs. cell-free systems. For some effectors (e.g. colchicin) it is known that interference with tubulin assembly is more critical than interference with disassembly. Such a phenomenon has also been described for mercury compounds (Miura et al. 1984), and it has been postulated that binding to assembled tubulin and associated microtubule-associated proteins (MAP) would provide protection against effectors.
However, taken as a whole the present data appear consistent with the concept that part of the chromosomal genotoxicity of Hg2+ could be due to the impairment of the functionality of the motor protein kinesin and/or the microtubules, leading to disturbances in chromosome distribution. Aneugenic compounds may act by affecting proper tubule formation or chromosomal segregation and subsequently lead to chromosomal loss. Recent studies have provided evidence for the presence of thresholds for the induction of chromosome non-disjunction and chromosome loss by microtubule inhibitors (Elhajouji et al. 1995; Decordier et al. 2002). The theoretical basis for the existence of such thresholds has been reviewed by Kirsch-Volders et al. (2000, 2003).
In essence, there is now compelling evidence that aneugenicity and clastogenicity are mechanisms that lead to chromosomal genotoxicity by inorganic mercury. Plausible underlying mechanisms are interactions with motor protein functions, leading to aneugenicity, and generation of reactive oxygen, leading to clastogenicity. Both mechanisms are not based on direct DNA interaction of the chemical and are likely associated with thresholds. However, the present data also show that such thresholds for the chromosomal genotoxicity of mercury salts could be very low.
References
Akiyama M, Oshima H, Nakamura M (2001) Genotoxicity of mercury used in chromosome aberration tests. Toxicol In Vitro 15:463–467
Al-Sabti K (1994) Micronuclei induced by selenium, mercury, methylmercury and their mixtures in binucleated blocked fish erythrocyte cells. Mutat Res 320:157–163
Boffetta P, Merler E, Vainio H (1993) Carcinogenicity of mercury and mercury compounds. Scand J Work Environ Health 19:1–7
Boffetta P, Garcia-Gomez M, Pompe-Kirn V, Zaridze D, Bellander T, Bulbulyan M, Caballero JD, Ceccarelli F, Colin D, Dizdarevic T, Espanol S, Kobal A, Petrova N, Sallsten G, Merler E (1998) Cancer occurrence among European mercury miners. Cancer Causes Control 9:591–599
Bolt HM (2003) Genotoxicity—threshold or not? Introduction of cases of industrial chemicals. Toxicol Lett 140/141:43–51
Bolt HM, Foth H, Hengstler JG, Degen GH (2004) Carcinogenicity categorisation of chemicals—new aspects to be considered. Toxicol Lett 151:29–42
Bonacker D, Stoiber T, Böhm KJ, Unger E, Degen GH, Thier R, Bolt HM (2004) Chromosomal genotoxicity of nitrobenzene and benzonitrile. Arch Toxicol 78:49–57
Borenfreund E, Babich H, Martin-Alguacil N (1988) Comparisons of two in vitro cytotoxicity assays—the neutral red (NR) and tetrazolium MTT test. Toxicol in Vitro 2:1–6
Böhm KJ, Stracke R, Unger E (2000) Speeding up kinesin-driven microtubule gliding in vitro by variation of cofactor composition and physicochemical parameters. Cell Biology International 24:335–341
Countryman PI, Heddle JA (1976) The production of micronuclei from chromosome aberrations in irradiated cultures of human lymphocytes. Mutation Res 41:321–332
Cross HJ, Smillie MV, Chipman JK, Fletcher AC, Levy LS, Spurgeon A, Fairhurst S, Howe A, Mason H, Northage C, Wright A (1995) Mercury and its inorganic divalent compounds. Criteria document for an occupational exposure limit. HSE Books, Health and Safety Executive, London, UK. ISBN 0-7176-10144
Decordier I, Dillen L, Cundari E, Kirsch-Volders M (2002) Elimination of micronucleated cells by apoptosis after treatment with inhibitors of microtubules. Mutagenesis 17:337–344
De Flora S, Bennicelli C, Bagnasco M (1994) Genotoxicity of mercury compounds. A review. Mutat Res 317:57–79
DFG (1999) Quecksilber und anorganische Quecksilberverbindungen. In: Greim H (ed) Gesundheitsschädliche Arbeitsstoffe. Toxikologisch-arbeitsmedizinische Begründungen von MAK-Werten. 28. WILEY-VCH, Weinheim, Lieferung, pp 1–42
Dieter MP, Boorman GA, Jameson CW, Eustis SL, Uraith LC (1992) Development of renal toxicity in F344 rats gavaged with mercuric chloride for 2 weeks, or 2, 4, 6, 15, and 24 months. J Toxicol Environ Health 36:319–340
Duhr EF, Pendergrass JC, Slevin JT, Haley BE (1993) HgEDTA complex inhibits GTP interactions with the E-site of brain beta-tubulin. Toxicol Appl Pharmacol 122:273–280
Elhajouji A, Van Hummelen P, Kirsch-Volders M (1995) Indications for a threshold of chemically-induced aneuploidy in vitro in human lymphocytes. Environ Mol Mutagen 26:292–304
Fenech M (1993) The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res 285:35–44
Gaskin F, Cantor CR, Shelanski ML (1974) Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol 89:737–755
Hengstler JG, Bogdanffy MS, Bolt HM, Oesch F (2003) Challenging dogma: thresholds for genotoxic carcinogens? The case of vinyl acetate. Annu Rev Pharmacol Toxicol 43:485–520
Keates RAB, Yott B (1984) Inhibition of microtubule polymerization by micromolar concentrations of mercury(II). Can J Biochem Cell Biol 62 814–818
Kirsch-Volders M, Aardema M, Elhajouji A (2000) Concept of threshold in mutagenesis and carcinogenesis. Mutation Res 464:3–11
Kirsch-Volders M, Vanhauwaert A, Eichenlaub-Ritter U, Decordier I (2003) Indirect mechanisms of genotoxicity. Toxicol Lett 140/141:63–74
Kuznetsov SA, Gelfand VI (1986) Bovine brain kinesin is a microtubule-activated ATPase. Proc Natl Acad Sci U S A 83:8530–8534
Léonard A (1988) Mechanisms in metal genotoxicity: the significance of in vitro approaches. Mutation Res 198:321–326
Liliom K, Wagner G, Pacz A, Cascante M, Kovacs J, Ovadi J (2000) Organization-dependent effects of toxic bivalent ions microtubule assembly and glycolysis. Eur J Biochem 267:4731–4739
Matsuoka A, Yamazaki N, Suzuki T, Hayashi M, Sofuni T (1992) Evaluation of the micronucleus test using a Chinese hamster cell line as an alternative to the conventional in vitro chromosomal aberration test. Mutation Res 272:223–236
Miller BM, Adler ID (1990) Application of antikinetochore antibody staining (CREST staining) to micronuclei in erythrocytes induced in vivo. Mutagenesis 5:411–415
Miura K, Inokawa M, Imura N (1984) Effects of methylmercury and some metal ions on microtubule networks in mouse glioma cells and in vitro tubulin polymerization. Toxicol Appl Pharmacol 73:218–231
National Toxicology Program (1993) Toxicology and carcinogenesis studies of mercuric chloride (CAS no. 7487-94-7) in F344 rats and B6C3F1 mice (gavage studies) Natl Toxicol Program Tech Rep Ser 408:1–260
Pratt IS, Barron T (2003) Regulatory recognition of indirect genotoxicity mechanisms in the European Union. Toxicol Lett 140/141:53–62
Rao MV, Chinoy NJ, Suthar MB, Rajvanshi MI (2001) Role of ascorbic acid on mercuric chloride-induced genotoxicity in human blood cultures. Toxicol In Vitro 15:649–654
Renzi L, Pacchierotti F, Russo A (1996) The centromere as a target for the induction of chromosome damage in resting and proliferating mammalian cells: assessment of mitomycin C-induced genetic damage at kinetochores and centro-meres by a micronucleus test in mouse splenocytes. Mutagenesis 11:133–138
Russo A, Stocco A, Majone F (1992) Identification of kinetochore-containing (CREST+) micronuclei in mouse bone marrow erythrocytes. Mutagenesis 7:195–197
Schoeny R (1996) Use of genetic toxicology data in U.S. EPA risk assessment: the mercury study report as an example. Environ Health Perspect 104[Suppl 3]: 663–673
Schuler M, Rupa DS, Eastmond DA (1997) A critical evaluation of centromeric labeling to distinguish micronuclei induced by chromosomal loss and breakage in vitro. Mutation Res 392:81–95
Schurz F, Sabater-Vilar M, Fink-Gremmels J (2000) Mutagenicity of mercuric chloride and mechanism of cellular defence: the role of metal-binding proteins. Mutagenesis 15:525–530
Shelanski ML, Gaskin F, Cantor CR (1973) Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A 70:765–768
Stoiber T, Degen GH, Bolt HM, Unger E (2004) Interaction of mercury(II) with the microtubule cytoskeleton in IMR-32 neuroblastoma cells. Toxicol Lett 151:99–104
Thier R, Bonacker D, Stoiber T, Böhm KJ, Wang M, Unger E, Bolt HM, Degen G (2003) Interaction of metal salts with cytoskeletal motor protein systems. Toxicol Lett 140/141:75–81
Unger E, Böhm KJ, Müller H, Grossmann H, Fenske H, Vater W (1988) Formation of double-walled microtubules and multilayered tubulin sheets by basic proteins. Eur J Cell Biol 46:98–104
Vogel DG, Margolis RL, Mottet NL (1989) Analysis of methyl mercury binding sites on tubulin subunits and microtubules. Pharmacol Toxicol 64:196–201
Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW (1975) A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A 72:1858–1862
Weiss DG, Maile W (1992) Principles, practice and applications of video-enhanced contrast microscopy. In: Shotton DM (ed) Electronic light microscopy. Wiley-Liss, New York, pp 105–140
Acknowledgements
The studies were supported by CEFIC (CEFIC/LRI: CC-1FOAR-0003). The dedicated stewardship by Dr. A. Sarrif is very much appreciated. The authors thank H. Wolfram for performing part of the in vitro assays. The Ph.D. thesis of D.B., submitted to the Faculty of Science of the Heinrich-Heine University, Düsseldorf, Germany, contains part of the data of the present publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonacker, D., Stoiber, T., Wang, M. et al. Genotoxicity of inorganic mercury salts based on disturbed microtubule function. Arch Toxicol 78, 575–583 (2004). https://doi.org/10.1007/s00204-004-0578-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-004-0578-8