Abstract
Ochratoxin A (OTA) is a widespread mycotoxin produced by several species of fungi. OTA induces a tubular-interstitial nephropathy in humans and in animals. It has been implicated as one of the aetiological agents involved in the development of endemic nephropathy. OTA-induced oxidative stress and apoptosis may play key roles in the development of chronic tubulointerstitial nephritis connected to the long-term exposure to this food contaminant. We studied the effects of low doses of OTA on kidney cells. Wistar rats were treated with 120 μg OTA/kg bodyweight daily, for 10, 30 or 60 days. Toxin concentration in kidney was proportional to the time of exposure, and amounted to 547.2, 752.5 and 930.3 ng OTA/g kidney tissue after 10, 30 and 60 days, respectively. OTA treatment caused an increased number of cells undergoing apoptosis in both proximal and distal epithelial kidney cells. The apoptotic cells were visualised using the TUNEL assay and staining with haematoxylin and eosin in situ. The number of apoptotic cells in rats treated for 10, 30 and 60 days increased by 5-, 6.4- and 12.7-fold, respectively, compared with the control cells. However, DNA electrophoresis did not show characteristic fragmentation (DNA laddering). The oxidative stress was evident via increased malondialdehyde formation. The concentration of lipid peroxides showed an increase (36%), but the activity of superoxide dismutase decreased (26%) in 60-day treated rats. In spite of the observed biochemical and morphological changes in the kidney cells, renal functional status was preserved to the end of experiment. This study demonstrates that a combination of morphologic and biochemical markers can be used to monitor early cell death in OTA-induced renal injury. We have shown that the exposure to the relatively low OTA concentrations has activated apoptotic processes and oxidative damage in kidney cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ochratoxin A (OTA) is a widespread mycotoxin produced by several species of fungi of species Aspergillus and Penicillium (Van der Merwe et al. 1965). OTA is a structural analogue of phenylalanine and consists of a chlorinated dihydro-isocumarin moiety linked through its 7-carboxyl group by an amide bond to l-phenylalanine. The toxin contaminates animal feed and human food. It was also found in human sera in areas where endemic nephropathy (EN) occurs (Cooper 1979; Hult et al. 1982; Peraica et al. 1999), but also elsewhere (Maaroufi et al. 1995). OTA is a potent nephrotoxic (Krogh 1980), genotoxic (Creppy et al. 1985) and carcinogenic (Kanisawa and Suzuki 1978) agent, and it affects normal blood coagulation (Galtier et al. 1979) and immune response (Creppy et al. 1983). It has been implicated as one of the aetiological agents involved in the development of EN (Cooper 1979).
Some forms of human interstitial nephropathies seem to be related to enhanced OTA exposure (Maaroufi et al. 1995; Čvoriščec et al. 1998; Peraica et al. 1999). Fifty-percent of human end-stage renal diseases are of unknown origin, and current data suggest that chemicals, including mycotoxins such as OTA, probably play a significant role in those cases where no aetiology is documented (Bach et al. 1996; Rastegar and Kashgarin 1998).
The toxin acts on different sites of nephron function, including renal blood flow, glomerular function and tubular epithelia (Krogh 1980; Gekle and Silbernagl 1993; 1994; Gekle et al. 1998). Exposure to relatively low concentrations of OTA for several years has resulted in accumulation of toxin in various tissues, with maximal accumulation in proximal tubule cells. Zingerle et al. (1997) have discovered that 70% of the ingested OTA was reabsorbed in proximal tubules. It has been shown that two-thirds of OTA present in glomerular filtrate would be reabsorbed in proximal tubules and only one-third in distal tubules. Therefore, these high concentrations of toxin might produce various toxic effects including inhibition of protein synthesis (Creppy et al. 1979), stimulation of lipid peroxidation (Rahimtula et al. 1988; Omar et al. 1990) and other changes in the kidney cell integrity before the other tissues are attacked. Chronic effects of OTA in kidneys are usually related to primary lesions of proximal tubules, followed by spontaneous damage of glomeruli and involution of the interstitia (Krogh 1980). In 1977, Kanisawa and colleagues studied acute OTA intoxication, and after several consecutive daily doses produced massive acidophilic degeneration with necrosis and desquamation of epithelium in proximal tubules (Kanisawa et al. 1977).
Experimental evidence indicates that impairments in renal cell function observed after the exposure to a low concentration of OTA are different from those seen with a high OTA concentration (Gekle et al 1998).
EN is a chronic renal disease with a high prevalence in a geographically limited area of Croatia. It has also been recorded in some part of Bosnia, Serbia, Bulgaria and Romania. Despite numerous studies conducted to date, the aetiology of this disease has not been clarified. Pathological studies of the kidney in the early stage of EN have shown that renal tubules are the primary sites of the pathologic process with an interstitial tissue reaction, whereas glomerular alterations are of a secondary character (Čvoriščec et al. 1998).
Numerous studies have reported that OTA induced oxidative stress in the dose-dependent manner. It stimulates lipid peroxidation by complexing Fe3+ and facilitating its reduction (Omar et al. 1990). OTA has been reported to increase malondialdehyde (MDA) formation in vitro upon incubation with rat liver microsomes in the presence of NADPH (Rahimtula et al. 1988). However, Hoehler et al. (1997) and Gautier et al. (2001) did not find increased MDA concentration in kidney, liver and plasma of rats treated with lower doses of OTA.
During normal aerobic metabolism or biotransformation of xenobiotics reactive oxygen species (ROS) are produced. Low levels of ROS play an important role in signal transduction, cell proliferation, apoptosis, immunity and defence mechanisms (Jacobson 1996; Schulze-Osthoff et al. 1997; Vogt et al. 1998). In normal tissues there is balance between the production and scavenging of ROS. Oxidative stress occurs when the level of cellular antioxidant depletion exceeds the level of ROS production. ROS include superoxide and hydrogen peroxide that can generate the more potent hydroxyl radical in the presence of transition metals. Tissue injury inflicted by higher concentration of ROS may be the result of the oxidative damage to critical cellular targets, such as protein, lipid or DNA (Halliwell and Gutteridge 1985; Wills 1987; Jacobson 1996).
The genotoxic effects, inhibition of DNA synthesis and mitosis, as well as histopathological effects on the nuclei of OTA-treated cells (Wei and Sulik 1993), may be explained by OTA-inflicted DNA damage, which includes DNA adduct formation and DNA single-strand breaks. OTA–DNA adducts in kidney tissue of intoxicated mice has been reported (Pfohl-Leszkowicz et al. 1993). The degree of OTA-induced DNA adduct formation is dose- and time-dependent. Moreover, OTA–DNA adducts have been found in a biopsy of kidney tissue from a patient suffering from chronic interstitial nephropathy and having high OTA concentration in blood (Maaroufi et al. 1994). Evidence that OTA-induced renal tubular lesions were in fact partially due to apoptosis has been demonstrated in vitro (Seegers et al. 1994a).
OTA-induced cell death has been reported both in vivo and in vitro, in rat renal tubules (Albassam et al. 1987), in developing mouse embryos (Wei and Sulik 1993), in hamster kidney, in HeLa cells (Seegers et al. 1994a) and in human lymphocytes (Seegers 1994b). The type of cell death observed in the affected kidneys and the teratogenic OTA effects still remain unclear.
Cell death can generally proceed via necrosis or apoptosis (Vaux et al. 1994). Necrosis is characterised by the formation of lesions in the plasma membrane. However, initiation of apoptosis can result from multiple stimuli, including heat, toxins, free radicals, growth factors and cytokines (Vaux et al. 1994; Jacobson 1996; Vogt et al. 1998). Apoptosis is the predominant form of physiological cell death. Apoptosis causes cell death in a way that differs morphologically and biochemically from necrosis. Much evidence has indicated that alterations in the control of cell death/survival contribute to the pathogenesis of many human diseases (Thompson 1995).
The mechanism of cell death in host response to OTA has not been fully characterised. The objective of the present study was to determine the sequence of events leading to cell death produced by exposure to relatively low doses of OTA. In this work we measured a marker of oxidative stress (MDA), superoxide dismutase (SOD) activity, the total antioxidant status (TAS) concentration, and the level of apoptosis in rat kidneys.
Materials and methods
Chemicals
All biochemicals, including enzymes, coenzymes and substrates were of analytical grade and were purchased from Boehringer (Mannheim, Germany) and Sigma Chem. Co. (St. Louis, MO, USA). Ochratoxin A was from Sigma.
Animals and treatment
Male Wistar rats, weighing 240–280 g, that were used in the experiments were bred in the Animal Unit of the Faculty of Pharmacy and Biochemistry, Zagreb, Croatia. The animals were given food and water ad libitum, the food consisting of standard laboratory pellets, declared not to contain mycotoxin (PLIVA d.d., Zagreb, Croatia). Rats were allocated to control (n=8) or treated (n=8) groups. For the OTA/10 days group, a daily dose of ochratoxin A 120 μg/kg bodyweight (OTA was dissolved in a volume of 0.5 ml neutral olive oil) was administered to experimental animals each morning by gastric intubation for 10 days. OTA/30 days group and OTA/60 days group received the same daily dose for 30 or 60 days, respectively. Control animals received a daily dose of 0.5 ml neutral olive oil for 30 days. Previously, we assessed the effect of olive oil. No significant differences in studied biochemical parameters were observed between the controls receiving saline and those receiving olive oil.
Principles of good laboratory animal care were followed throughout, as well as the Croatian Guidelines for the protection of animals according to Organisation for Economic Co-operation and Development Guidelines.
Samples
Rats were killed by cervical dislocation after light ether anaesthesia. Kidneys were placed in ice-cold saline, the cortex was dissected out, minced and placed in cold 0.14 M KCl medium supplemented with phenylmethylsulphonyl fluoride (PMSF) as a proteinase inhibitor, pH 7.4. An Ultra-Turrax homogenisor was used for preparing the homogenates (100 g/l) of kidney tissue. Protein concentration in kidney homogenates were determined by the method of Lowry et al. (1951).
Blood was taken from the aorta while the rats were under light ether anaesthesia, and serum was separated by centrifugation at 3000 rpm for 10 min and was stored at –20°C until analysis.
Twenty-four-hour urine was collected while the animals kept in metabolic cages. Using test strips, the urine was tested for the presence of blood pigment, urobilinogen, bilirubin, glucose, protein, ketones, nitrite, leukocyte and pH.
Renal status and level of apoptosis, lipid peroxidation, activity of SOD and concentrations of TAS and OTA were determined. DNA electrophoresis and morphological methods were used for detection of apoptosis. The apoptotic cells were visualised using the TUNEL assay (terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling) and were stained with haematoxylin and eosin in situ.
DNA electrophoresis
DNA was isolated from kidney tissue by the phenol–chloroform method for tissues (Sambrook et al. 1989). DNA concentration was ascertained by UV spectophotometry at 260 nm and 2 μg per 10 μl aliquots of DNA were electophoresed on a 1% agarose gel. The gel was stained briefly with ethidium bromide, and was visualised and photographed under UV transillumination.
TUNEL assay
Kidney tissue was fixed in 10% buffered neutral formalin solution, dehydrated, and embedded in paraffin. Paraffin sections (4 μm) were mounted on polylysine-coated glass slides (Polysine, Menzel-Gläser, Freiburg, Germany) and deparaffinised. TUNEL was carried out using dUTP- fluorescein isothiocyanate (FITC), according to the instructions of the manufacturer (In Situ Cell Death Detection Kit; Boehringer Mannheim).
Morphological characterisation
Renal tissues, used for histological examination, were fixed in formalin, dehydrated and paraffin-embedded. Thin sections of paraffin-embedded tissues were stained with haematoxilin and eosin before microscopy examination.
Lipid peroxidation determinations
Lipid peroxidation (LPO) was assayed by quantifying malondialdehyde (MDA) in the form of thiobarbituric acid-reactive substances (TBARS), using the thiobarbituric acid reaction (Ohkawa et al. 1979). TBARS were measured in the homogenate of kidney cortex at 532 nm.
Superoxide dismutase
The activity of SOD was measured by colorimetric assays (Ransod; Randox Laboratories Ltd, Crumlin, UK). This method employs xanthine and xanthine oxidase to generate superoxide radicals that react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride to form a red formazan dye. SOD activity is then measured by the degree of inhibition of this reaction.
Total antioxidant status (TAS)
TAS was measured by colorimetric assay kit (Randox Laboratories). ABTS (2,2′-azino-di-[3-ethylbenzothiazoline sulfonate]) is incubated with a peroxidase (metmyoglobin) and H2O2 to produce the radical cation ABTS+, a relatively stable blue-green colour, which is measured at 600 nm. Antioxidants in the added sample cause suppression of this colour production to a degree that is proportional to their concentration.
Measurement of OTA concentration
OTA concentration in serum, urine and kidney cortex was assayed by high-performance liquid chromatography (Peraica et al. 1999).
Statistics
The data are presented as mean values ±SD. Significance of differences was tested by Wilcoxon/Mann-Whitney test. Values were considered significantly different if P<0.05 (Samuels 1989).
Results
In this study we have examined the effects of relatively low concentrations of OTA (120 μg/kg bodyweight per day) on the level of lipid peroxidation and apoptosis in rat kidney tissues. Furthermore, SOD activity, TAS concentration in the kidneys and biochemical parameters significant for the characterisation of renal status have been determined.
The results show that OTA was well absorbed and that its concentration in all samples increased with the time of exposure. Figure 1 displays concentrations of OTA in the sera, homogenates of kidneys and urine of experimental animals during the course of the study. The increase between days 10 and 60 amounted to 2.3, 1.7 and 10.2 times for serum, kidney tissue and urine, respectively. Toxin concentration in renal cortex and outer medulla amounted to 547, 752 and 930 ng OTA/g tissue after 10, 30 and 60 days, respectively.
Results of the routine urine examination have shown no significant damage to kidney functions in any of the treated groups of rats (results not shown). Previous research has proven that the concentrations of urea and creatinine in serum and urine, as well as the respective clearance tests remained within the normal range, thus indicating preservation of the kidney function even in the group of experimental animals that were treated for 60 days (Petrik et al. 2002).
However, it has been mentioned that OTA could stimulate lipid peroxidation. Increased concentrations of lipid peroxides have been found so far in animals treated with high doses only (Omar et al. 1990; Rahimtula et al. 1988).
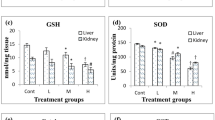
Our results showed a significant increase (36%) of lipid peroxide concentration in kidney tissue in the group of animals that were treated with OTA for 60 days, while the catalytic activity of SOD was substantially decreased (−26%) in the same group of animals. The TAS concentration did not change during the experiment (Table 1).
We suspected that the observed increased concentration of lipid peroxides, along with decreased SOD activity, were capable of inducing some injuries visible at the cellular level. We therefore studied the morphological changes in the kidney tissue.
Figure 2 shows representative samples of the tubule and interstitial cells of rat kidneys stained with haematoxylin–eosin. In the treated animals, cells undergoing apoptosis were observed. Apoptosis was manifested as cell shrinkage and chromatin condensation. Only a few apoptotic cells were found in rats treated for 10 days but, in the animals treated for 30 and 60 days, more apoptotic cells could be observed, along with a number of cells with karyomegaly.
Morphological evidence for renal cell apoptosis in rats following ochratoxin A (OTA) treatment and in controls. OTA was administered by gastric intubation at 120 µg/kg bodyweight per day for 10, 30 or 60 days. Representative microscopy images of thin sectioned fixed, paraffin-embedded rat kidneys are shown (haematoxylin and eosin, ×630). Apototic cells (arrowheads) and karyomegaly (arrows) are seen in kidney of OTA-treated rats
It has been well documented that apoptosis is associated with DNA fragmentation, resulting from activation of endonucleases induced during the apoptotic process. We have performed DNA electrophoresis in order to find the characteristic DNA ladder pattern on the agarose gel.
Figure 3 represents electrophoretic separation of genomic DNA isolated from the kidney tissue on 1% agarose gel. The characteristic fragmentation of DNA was not noticed in the samples tested. We observed a light DNA smear in samples from animals treated for 60 days. The DNA smear most probably resulted from random DNA digestion connected with necrosis, or accidental degradation that occurred during the isolation of genomic DNA.
Therefore, we performed more sensitive TUNEL staining in situ to check our morphological results. Figure 4 represents the fluorescent marking of breaks in the DNA chain (TUNEL method) in slices of rat kidneys (magnification ×630). The upper left photomicrograph of Fig. 4 presents a representative slide of a control animal (no positive TUNEL reaction). However, apoptosis in epithelial cells of the proximal tubule can be seen in animals treated with OTA for 10 days, (TUNEL-positive nuclei are fluorescent and stained yellow-green). It can also be seen that the number of TUNEL-positive cells increased in the OTA/30 days and OTA/60 days groups. The bottom left panel of Fig. 4 shows the results in the OTA/30 days group, with two distal tubule cells in apoptosis, and fluorescent apoptotic bodies. On the photomicrograph of an animal treated for 60 days with OTA (OTA/60 days), more epithelium cells of the proximal tubule in apoptosis are visible, together with fluorescent cell nuclei.
Fluorescence microscopy of kidney cells from rats treated with ochratoxin A (OTA) and controls. OTA was administered by gastric intubation at 120 µg/kg bodyweight per day for 10, 30 or 60 days. Representative photomicrographs of sections stained with FITC–dUTP by the TUNEL technique are shown (×630). The TUNEL-positive nuclei indicating apototic cells (arrowheads) are stained yellow-green
Data presented here indicate that apoptosis is associated with the early changes in renal tissue during OTA administration. Our determinations of apoptosis relied on multiple criteria: morphological staining (haematoxylin and eosin), and nuclear chromatin staining with the TUNEL assay.
Quantitative results of morphological changes of cells in rat kidney are presented in Table 2. The table shows the number of apoptotic cells in a vertical longitudinal section of kidney tissue stained with haematoxylin and eosin. In the control group, the number of cells in apoptosis varied per section, ranging from 1 to 11, with an average of 4.75 for eight sections of rat kidneys examined.
The number of apoptotic cells in kidney tissue of OTA-treated animals showed increased values of 5, 6.4 and 12.8 times that of controls in the OTA/10 days, OTA/30 days and OTA/60 days groups, respectively. In this work, we have demonstrated a higher than normal level of apoptotic cell death in kidney tissues of rats treated with OTA for 10, 30 and 60 days. Apoptosis occurred in both proximal and distal epithelial cells, but with higher frequency in the proximal tubule cells.
Discussion
OTA contaminates foods of plant and animal origin; it is exceptionally stable and accumulates in human and animal tissues.
The kidney is the main target organ for toxic effects of OTA (Kuiper-Goodman and Scott 1989; Krogh 1992; Gekle and Silbernagl 1994). The high sensitivity of kidneys is partially explained by the toxicokinetics of OTA.
Aetiology and mechanism of EN development and of approximately 50% of tubulointerstitial nephritis in the world are not known. OTA, together with some other factors, can play a significant role in the aetiological mosaic. Delayed pathogenesis, lack of specific symptoms and impossibility of early diagnosis are characteristic of this nephropathy.
The mechanism of OTA cytotoxicity mainly depends on the quantity of toxin and the length of time that the organism was exposed to the toxin. That is why signs of damage to the kidney function after being given relatively small concentrations of OTA (similar to natural exposure in endemic areas) differ from those occurring during the exposure of experimental animals to higher concentrations of OTA. Primary unspecified effects of OTA, such as inhibition of the synthesis of macromolecules (Creppy et al. 1979), stimulation of lipid peroxidation (Rahimtula et al. 1988) or disintegration of mitochondria (Aleo et al. 1991), happen only at relatively high experimental concentrations of the toxin (Gekle et al. 1998). Low concentrations of OTA affect cells in a specific way. Interactions of the toxin with secondary messengers and other cell targets, which can activate mitogen-activated protein kinase (Sauvant et al. 1998) or inhibit certain carriers (Gekle and Silbernagl 1994) and disturb the normal cell homeostasis, are assumed. There are literature reports that some damage to kidney cells occurs primarily in the proximal tubules, distal tubule and interstitium (Krogh 1980) following a long-term exposure to low OTA concentrations.
The objective of this study was to create the conditions that precede chronic damage caused by OTA and to characterise such status with the biochemical and morphological tests. We have examined the effects of the relatively low concentrations of OTA on the level of lipid peroxidation and apoptosis in the rat kidney tissues.
According to our research results, there is a linear correlation between the measured concentrations of OTA and the toxin exposure period. The toxin concentration is determined in the cortex and external medulla of the kidney due to the localisation of the cells of proximal and distal tubules in these regions. It has already been reported that OTA distribution in kidney tissue greatly depends on the manner of toxin application. Following the acute administration of toxin to Wistar rats, the highest concentrations were measured in the papilla and inner medulla. However, during the chronic toxin exposure of adult rats, OTA concentrations were significantly higher in the cortex and external medulla (Kane et al. 1986; Fuchs et al. 1988; Schwerdt et al. 1996).
Since our experiments were conducted in the form of a subchronic intoxication model, OTA concentrations were higher in serum than in kidneys. In orally treated rats, about 99% of OTA was bound to plasma proteins, foremost to albumin (Fuchs and Hult 1992; Krogh 1992). Although we measured the highest absolute concentrations of OTA in kidney tissue in the OTA/60 days group, the rate of accumulation was the highest in the initial phase (10-day group) and we observed a substantial accumulation of toxin in the kidney tissue. The concentrations found in urine were proportional to the time of exposure (82.81, 129.45 and 921.50 ng OTA/ml for groups OTA/10 days, OTA/30 days and OTA/60 days, respectively). It has been suggested that OTA uses the same transport mechanism in luminal and in basolateral membranes of proximal tubule cells as p-aminohippurate (Sokol et al. 1988; Gekle and Silbernagl 1994; Welborn et al. 1998; Tsuda et al. 1999).
The concentrations of urea and creatinine in serum and urine and their respective clearances were not significantly altered in the treated animals, not even in the OTA/60 days group. Routine examination of urine, using test strips, confirmed the fact that there was no greater damage to the kidney, most probably because these biochemical indicators do not reveal smaller changes. The results indicated preservation of the functional state of kidneys, which is in agreement with our earlier results (Petrik et al 2002). However, it is known that the functional reserve of the kidney is great and that the kidney might be substantially damaged before impairments to its functions can be noticed by conventional biochemical diagnostics.
OTA activates peroxidation processes in the cell, stimulates lipid peroxidation by complexing Fe3+, and lessens reduction of the complex, which has been noticed so far only in cases of relatively large doses of toxin (Omar et al. 1990; Rahimtula et al. 1988).
We observed a significantly increased (36%) concentration of lipid peroxides in the kidney tissue of rats treated with OTA for 60 days. According to Gekle et al. (1998), OTA stimulates lipid peroxidation only at large concentrations, so it can be supposed that our finding of 930 ng OTA/g kidney tissue approaches the critical concentration. However, in the process of lipid peroxidation, a significant part is played by the cell homeostatic mechanisms, most of all through the antioxidative status of kidney cells. Catalytic activity of SOD is significantly decreased (−26%) in the 60-day group of animals, indicating an increase of prooxidative in comparison to antioxidative events in the cell. SOD is part of an antioxidant system that prevents the formation of new free radical species. However, TAS concentration, which refers to the general antioxidative status of the organism, was not changed in the kidneys in any group of animals tested (Table 1).
It has been reported that OTA also provokes apoptosis in HeLa and HaK cells (Seegers et al. 1994a). Oxidative stress is considered to play the key role in the process of apoptosis. General manifestations under these circumstances are an accumulation of oxidised proteins and lipids, which additionally accelerate the creation of reactive oxygen intermediaries, a change in the cells' redox potential and interruption of transport processes (Kroemer 1997). This may result in increased atrophy of cells through apoptosis.
Apoptosis can be detected through characteristic morphological changes, such as aggregation of chromatin and creation of apoptotic bodies, fragmentation of DNA, expression of proteins important for apoptosis, proteolysis of apoptosis specific substrates (creation of neo-epitope) or exposure of phosphatidyl serine on the external side of cell membrane.
We determined the number of apoptotic cells in a vertical longitudinal section of kidney tissue (Fig. 2, Table 2) in OTA-treated groups and in the control group of animals. We found on average 4.75 apoptotic cells in control animals and considered this as the level of physiologic apoptosis in rat kidney. The number of apoptotic cells increased with the time of exposure to OTA, such that in the OTA/10 days group the increase was 5-fold, in OTA/30 days group 6.4-fold, and in the OTA/60 day group 12.8-fold higher. The increased cell death of epithelial cells can play a significant role in the development of chronic changes in kidney parenchyma.
The electrophoretic separation of genomic DNA, isolated from the kidney tissue, did not show the characteristic fragmented DNA products. However, this technique is not sensitive enough for identification of a relatively low number of apoptotic cells. Therefore, we used the more sensitive TUNEL technique for detection apoptosis, and we found TUNEL-positive cells (Fig. 4). Results obtained by TUNEL technique are in good correlation with results of our morphologic studies.
Our results are in agreement with several in vitro and in vivo studies. Morphological changes or activation of enzymes participating in the apoptotic process have been found in a number of cell lines. In 1994, Seegers and colleagues found that OTA at 20 μg/ml caused nuclear and nucleolar changes characteristic of apoptosis in hamster kidney and HeLa cells (Seegers et al. 1994a). The morphological and biochemical lesions were distinct; in hamster kidney cells formation of micronuclei predominated, but in HeLa cells apoptotic body formation was more prevalent. Ueno et al. (1995) found that OTA induced apoptotic nuclear changes and DNA fragmentation in HL-60 human promyelotic leukaemia cells. In cultured human proximal tubule cells OTA, in low concentrations, induced time- and concentration-dependent activation of caspase 3 (Schwerdt et al. 1999). Activation of caspase 3 is crucial for the development of apoptosis. Gekle et al. (2000) found that OTA at nanomolar concentrations induced c-jun amino-terminal kinase (JNK) activation and apoptosis in MDCK-C7 cells. Horvath et al. (2002) also showed induced apoptosis in the myc-transfected cell line. In studies in vivo, Atroshi et al. (2000) showed the presence of intracellular apoptotic bodies in livers of mice treated with OTA for 2 weeks only.
A number of descriptive studies have suggested a correlation between exposure to OTA and EN, and have found a correlation between the geographical distribution of EN and high incidence of mortality from urothelial urinary tract tumours. According to International Agency for Research on Cancer, OTA is classified in group 2B, i.e., potentially carcinogenic to humans (IARC 1993). Under our experimental conditions, no morphological changes have been observed in rat kidney tissues that would point to malignant transformations.
Karyomegaly in renal parenchyma was also shown in rats treated with OTA. When OTA was administered for a long period, the apoptotic-like cells and carcinogenic effects were documented (Maaroufi et al. 1999). Epithelial cells with karyomegaly were found in our study for the animals treated for 60 days.
However, it is not clear how OTA activated the process of apoptosis in our experiments. It is possible that during long exposure (OTA/60 days group) acceleration of the apoptosis process predominated due the damage of the mitochondrial membrane caused by increased free radicals concentration. In the 60-day group of animals the concentration of lipid peroxides was significantly increased.
On the other hand, it is well known that treatment with 10 mg OTA/kg body mass for 4 days causes glomerular changes and necrosis of epithelial cells of the kidney proximal tubule in rats (Kanisawa et al. 1977). For rats, the LD50 is 22–44 mg OTA/kg body mass. In rats that had been exposed to relatively high concentrations of OTA (5 mg/kg food, which corresponds to 360 μg OTA/kg body mass a day) for 3–4 months, kidney damage developed, followed by tubular degeneration and later interstitial fibrosis, then atrophy and sclerosis of glomeruli (Krogh 1980).
A great deal of reference data and our results show that the mechanism of OTA cytotoxicity is related to the dose and time of exposure. Sensitivity of certain cells, tissues and organs differs substantially. In general, one can distinguish four phases of cell response to in vivo exposure to the toxin: (1) preserved homeostasis with reversible changes, (2) activation of apoptotic process, (3) stimulation of both apoptosis and necrosis, and (4) mainly necrotic changes, which occur when high OTA concentrations persist in kidney tissue.
Lieberthal and Levine (1996) showed the same developmental stages of necrotic and apoptotic cell death induced by ischaemic and toxic injuries in renal tissue. Their hypothesis also suggests that tubular epithelial cells may die by either necrosis or apoptosis, depending upon the severity of the injury to which the cells are exposed.
The results of our study show that exposure to relatively low OTA doses caused oxidative stress and apoptosis. OTA significantly stimulated apoptosis in both proximal and distal tubule cells of the kidney. Due to the additional antimitotic impact of OTA, the process of apoptosis was accelerated and could play an important role in the development of chronic tubulointerstitial kidney damage.
It has been thought so far that OTA nephrotoxicity develops primarily through mechanisms that end in proximal tubule cell necrosis. Our results point to the conclusion that in the initial development phase of tubulointerstitial nephropathy and, with exposure to relatively low concentrations of OTA, a significant role could be played by the accelerated apoptotic process.
References
Albassam MA, Yong SI, Bhatnagar R, Shrma AK, Prior MG (1987) Histopathologic and electron microscopic studies on the acute toxicity of Ochratoxin A in rats. Vet Pathol 24:427–435
Aleo MD, Wyatt RD, Schnellmann RG (1991) Mitohondrial dysfunction is early event in ochratoxin A but not in oosporein toxicity to rat renal proximal tubules. Toxicol Appl Pharmacol 107:73–80
Atroshi F, Biese I, Saloniemi H (2000) Significance of apoptosis and its relationship to antioxidants after ochratoxin A administration in mice J Pharm Pharmaceut Sci 3:281–291
Bach PH, Morin J-P, Pfaller W (1996) Nephrotoxicity—What we have learned and what we still need to know! TEN 3:4–13
Cooper P (1979) The kidney and ochratoxin A. Food Cosmet Toxicol 17:406–408
Creppy EE, Luginer AAJ, Fasiolo F, Heller K, Röschenthaler R, Dirheimer G (1979) In vitro inhibition of yeast phenylalanine-tRNA synthetase by ochratoxin A. Chem Biol Interact 24:257–262
Creppy EE, Stromer FC, Röschenthaler R, Dirheimer G (1983) Effects of two metabolites of ochratoxin A, (4R)-4-hydroxy ochratoxin A and ochratoxin α, on the immmune response in mice. Infect Immun 39:1015–1018
Creppy EE, Kane A, Dirheimer G, Lafarge-Frayssinet C, Mousset S (1985) Genotoxicity of ochratoxin A in mice: DNA single-stand breaks. Evaluation in spleen, liver and kidney. Toxicol Lett 28:29–35
Čvoriščec D, Čeović S, Boršo G, Stavljenić Rukavina A (1998) Endemic nephropathy in Croatia. Clin Chem Lab Med 36:271–277
Fuchs R, Hult K(1992) Ochratoxin A in blood and its pharmacokinetic properties. Food Chem Toxicol 30:201–204
Fuchs R, Appelgren LE, Hagelberg S and Hult K (1988) Carbon-14-Ochratoxin A distribution in the Japanese quail (Coturnix coturnix japonica) monitored by whole body autoradiography. Poultry Sci 67:707–714
Galtier P, Boneu B, Charpenteau J, Bodin G, Alvinerie M, More J (1979) Physiopathology of haemorrhagic syndrome related to ochratoxin A intoxication in rats. Food Cosmet Toxicol 17:49–53
Gautier JC, Holzhaeuser D, Markovic J, Gremaud E, Schilter B, Turesky RJ (2001) Oxidative damage and stress response from ochratoxin A exposure in rats. Free Radic Biol Med 10:1089–1098
Gekle M, Silbernagl S (1993) Mechanism of ochratoxin A-induced reduction of glomerular filtration rate. J Pharmacol Exp Ther 276:316–321
Gekle M, Silbernagl S (1994) The role of the proximal tubule in ochratoxin A nephrotoxicity in vivo: toxodynamic and toxokinetic aspects. Ren Physiol Biochem 17:40–49
Gekle M, Sauvant C, Schwerdt G, Silbernagl S (1998) Tubulotoxic mechanisms of ochratoxin A. Kidney Blood Press Res 21:277–279
Gekle M, Schwerdt G, Freudinger R, Mildenberger S, Wilflingseder D, Pollack V, Dander M, Schramek H (2000) Ochratoxin A induced JNK activation and apoptosis in MDCK-C7 cells at nanomolar concentration. J Pharmacol Exp Ther 293:837–844
Halliwell B, Gutteridge JMC (1985) Free radicals in biology and medicine. Clarendon Press, Oxford, pp 206–245
Hoehler D, Marquardt RR, Frohlich AA (1997) Lipid peroxidation as one mode of action in ochratoxin A toxicity in rats and chicks. Can J Anim Sci 77:287–292
Horvath A, Upham BL, Ganev V, Trosko JE (2002) Determination of the epigenetic effects of ochratoxin in a human kidney and a rat liver epithelial cell line. Toxicon 40:273–282
Hult K, Pleština R, Habazin-Novak V, Radić B, Čeović S (1982) Ochratoxin A in human blood and Balkan endemic nephropathy. Arch Toxicol 51:313–321
IARC (International Agency for Research on Cancer) (1993) Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. In: Monographs on the evaluation of carcinogenic risk to humans, Vol. 56. IARC Press, Lyon, pp 489
Jacobson MD (1996) Reactive oxygen species and programmed cell death. Trends Biochem Sci 21:83–86
Kane A, Creppy EE, Röschenthaler R, Dirheimer G (1986) Changes in urinary and renal tubular enzymes caused by subchronic administration of ochratoxin A. Toxicology 42:233–243
Kanisawa M, Suzuki S (1978) Induction of renal and hepatic tumors in mice by ochratoxin A, a mycotoxin. Gann 69:599–600
Kanisawa M, Suzuki S, Kozuka Y, Yamazaki M (1977) Histopathological studies on the toxicity of ochratoxin A in rats. I. Acute oral toxicity. Toxicol Appl Pharmacol 42:55–64
Kroemer G (1997) The proto-oncogene Bcl-2 and its role in regulating apoptosis Nat Med 3:614–620
Krogh P (1980) Causal association of mycotoxic nephropathy. In: Preusser HJ (ed) Medical mycology. Zbl Bakt [Suppl] 8:291–299
Krogh P (1992) Role of ochratoxin A in disease causation. Food Chem Toxic 30:213–224
Kuiper-Goodman T, Scott PM (1989) Risk assessment of the mycotoxin ochratoxin A. Biomed Environ Sci 2:179–248
Lieberthal W, Jevine JS (1996) Mechanisms af apoptosis and its potential role in renal tubular cell injury. Am J Physiol 271:F477–F488
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurment with the Folin phenol reagent. J Biol Chem 193:265–275
Maaroufi K, Pfhol-Leszkowicz A, Achour A, el May M, Grosse Y, Hammami M, Ellouz F, Creppy EE, Bacha H (1994) Ochratoxin A genotoxicity, relation to renal tumors. ArchInst Pasteur Tunis 71:21–31
Maaroufi K, Achour A, Betbeder AM, Hammami M, Ellouz F, Creepy EE, Bacha H (1995) Foodstuffs and human blood contamination by the mycotoxin ochratoxin A: correlation with chronic interstitial nephropathy in Tunisia. Arch Toxicol 69:552–558
Maaroufi K, Zakhama A, I Baudrimont, Achour A, Abid S, Ellouz F, Dhouib S, Creepy EE, Bacha H (1999) Karyomegaly of tubular cells as early stage marker of the nephrotoxicity induced by ochratoksin A in rats. Hum Exp Toxicol 18:410–415
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Omar RF, Hasinoff BB, Mejilla F, Rahimtula AD (1990) Mechanism of ochratoxin A stimulated lipid peroxidation. Biochem Pharmacol 40:1183–1191
Peraica M, Domijan AM, Fuchs R, Lucić A, Radić B (1999) The occurrence of ochratoxin A in blood in general population of Croatia. Toxicol Lett 110:105–112
Petrik J, Čepelak I, Barišić K, Vrkić N, Žanić Grubišić T (2002) Endogenous digitalis-like factors and endothelin-1 during experimental toxic nephropathy. Period Biol 104:77–80
Pfohl-Leszkowicz A, Grosse Y, Castegnaro M, Nicolov IG, Chernozemski IN, Bartsch H, Betbeder A.M, Creppy EE, Dirheimer G (1993) Ochratoxin A related DNA adducts in urinary tract tumors of Bulgarian subjects. In: Phillips DH, Castegnaro M, Bartsch H (eds) Postlabelling methods for detection of DNA adducts. IARC Scientific Publication no. 124. IARC Press, Lyon, pp 141–147
Rahimtula AD, Bereziat JC, Bussacchini-Griot V, Bartsch H (1988) Lipid peroxidation as a possible cause of ochratoxin A toxicity. Biochem Pharmacol 37:4469–4477
Rastegar A, Kashgarian M (1998) The clinical spectrum of tubulointerstitial nephritis. Kidney Int 54:313–327
Sambrook J, Fritsch EE, Maniatis T (1989) Isolation of DNA from mammalian cells: protocol I. In: Molecular cloning. A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor NY, pp 9.16–9.19
Samuels ML (1989) Statistics for the life sciences. Maxwell Macmillan, Singapore, pp 245–258
Sauvant C, Silbernagl S, Gekle M (1998) Exposure to ochratoxin A impairs organic anion transport in proximal tubule-derived opossum kidney cells. J Pharmacol Exp Ther 287:13–20
Schulze-Osthoff K, Bauer MK, Vogt M, Wesselborg S (1997) Oxidative stress and signal transduction. Int J Vitam Nutr Res 67:336–342
Schwerdt G, Bauer K, Gekle M, Silbernagl S (1996) Accumulation of Ochratoxin A in rat kidney in vivo and in cultivated renal epithelial cells in vitro. Toxicology 114:177–185
Schwerdt G, Freudinger R, Mildenberger S, Silbernagl S, Gekle M (1999) The nephrotoxin ochratoxin A induces apoptosis in cultured human proximal tubule cells. Cell Biol Toxicol 15:405–415
Seegers JC, Bohmer LH, Kruger MC, Lottering M-L, De Kock M (1994a) A comparative study of ochratoxin A-Induced apoptosis in hamster kidney and HeLa cells. Toxicol Appl Pharmacol 129:1–11
Seegers JC, Lottering M-L, Garlinski JP (1994b) The mycotoxin ochratoxin A causes apoptosis-associated DNA degradation in human lymphocytes. Med Sci Res 22:417–419
Sokol PP, Ripich G, Holohan PD, Ross CR (1988) Mechanism of ochratoxin A in transport in kidney. J Pharmacol Exp Ther 246:460–466
Thompson CB (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462
Tsuda M, Sekine T, Takeda M, Cha SH, Kanai Y, Kimura M, Endou H (1999) Transport of ochratoxin A by renal multispecific organic anion transporter 1. J Pharmacol Exp Ther 289:1301–1305
Ueno Y, Umemori K, Niimi E-C, Tanuma S-I, Nagata S, Sugamata S, Ihara T, Sekijima M, Kawai K-I, Ueno I, Tashiro F (1995) Induction of apoptosis by T-2 toxin and other natural toxins in HL-60 human promyelotic leukaemia cells. Nat Toxins 3:129–137
Van der Merwe KJ, Steyn PS, Fourrie L, Scott DB, Theron JJ (1965) Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature 205:1112–1113
Vaux DL, Haecker G, Strasser A (1994) An evolutionary perpesctive on apoptosis. Cell 76:777–781
Vogt M, Bauer K, Ferrari K, Schulze-Osthoff K (1998) Oxidative stress and hypoxia/reoxigenation tigger CD95 (APO-1/Fas) ligand expression in microglial cells. FEBS Lett 429:67–72
Wei X, Sulik KK (1993) Pathogenesis of craniofacial and body wall malformations induced by ochratoxin A in mice. Am J Med Genet 47:862–871
Welborn JR, Groves CE, Wright SH (1998) Peritubular transport of ochratoxin A by single rabbit renal proximal tubules. J Am Soc Nephrol 9:1973–1982
Wills ED (1987) Evaluation of lipid peroxidation in lipids and biological membranes. In: Snell K, Mullock B (eds) Biochemical toxicology: a practical approach. IRL Press, Oxford, pp 127–152
Zingerle M, Silbernagl S, Gekle M (1997) Reabsortion of the nephrotoxin ochratoxin A along the rat nephron in vivo. J Pharmacol Exp Ther 280:220–224
Acknowledgements
These studies were supported by grants from the Ministry of Science and Technology of Croatia, Project no. 006 311.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petrik, J., Žanić-Grubišić, T., Barišić, K. et al. Apoptosis and oxidative stress induced by ochratoxin A in rat kidney. Arch Toxicol 77, 685–693 (2003). https://doi.org/10.1007/s00204-003-0501-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-003-0501-8