Abstract
The aim of this study was to assess the effects of chronic exposure to cadmium (Cd) on the structure and function of kidneys, as well as to establish the body burden of Cd at which the changes occur. For this purpose we have created an experimental model using rats intoxicated with Cd administered in drinking water at the concentration of 5 or 50 mg Cd/l for 6, 12 and 24 weeks. The degree of kidney damage was evaluated biochemically and histopathologically. Sensitive biomarkers of Cd-induced proximal tubular injury such as urinary total N-acetyl-β-d-glucosaminidase (NAG-T) and its isoenzyme B (NAG-B), and alkaline phosphatase (ALP) were used. Cd content in the kidney increased with the level and duration of exposure leading to dose- and time-dependent structural and functional renal failure. In rats exposed to 5 mg Cd/l, first symptoms of injury of the main tubules of long and short nephrons (structural damage to epithelial cells, increased urinary activities of NAG-T and NAG-B) were noted after 12 weeks of the experiment. The damage occurred at a low kidney Cd concentration amounting to 4.08±0.33 µg/g wet weight (mean ±SE) and a urinary concentration of 4.31±0.28 µg/g creatinine. On exposure to 50 mg Cd/l, damage to the main tubules (blurred structure of tubular epithelium, atrophy of brush border, partial fragmentation of cells with release of nuclei into tubular lumen as well as increased urinary activities of NAG-T, NAG-B and ALP) was already evident after 6 weeks with the kidney Cd concentration of 24.09±1.72 µg/g wet weight. In rats exposed to 50 mg Cd/l, a lack of regular contour of glomeruli was noted after 12 weeks, whereas after 24 weeks thickening of capillary vessels and widening of filtering space were evident. After 24 weeks of exposure to Cd, increased urea concentration in the serum with simultaneous decrease in its level in the urine, indicating decreased clearance of urea, and increased excretion of total protein were observed, but endogenous creatinine clearance remained unaffected. At the lower exposure, symptoms of structural, but not functional, damage to the glomeruli were also evident after 24 weeks of the experiment. Our results provide evidence that chronic exposure to Cd dose-dependently damages (structurally and functionally) the whole kidney. The injury affects the main resorptive part (proximal convoluted tubules and straight tubules) and the filtering part (glomeruli) of the nephron. But the target site for Cd action is the main tubule. We hypothesize that the threshold for Cd effects on the kidney is less than 4.08±0.33 µg/g wet kidney weight and greater than 2.40±0.15 µg/g (at this Cd concentration no symptoms of kidney damage were noted), and it may be close to the latter value. A very important finding of this study is that Cd acts on the whole kidney, especially on the main tubules, even at relatively low accumulation in this organ. It confirms the hypothesis that humans environmentally exposed to Cd, especially smokers, are at risk of tubular dysfunction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is one of the most toxic heavy metals, having a very strong ability to accumulate in the living organisms especially in the kidneys, and an extremely long biological half-life. The kidney has been considered the critical organ for Cd toxicity following long-term exposure in humans and experimental animals (Kjellström 1986; WHO 1992; Hac et al. 1998). Cadmium-induced kidney injury is characterized first of all by proximal tubular dysfunction, which is believed to be irreversible at advanced stages (WHO 1992; Mitsumori et al. 1998; Roels et al. 1999).
Recent papers show that tubular damage may appear in environmentally exposed subjects at lower levels of Cd exposure than previously anticipated and that the kidney cortex Cd concentration of 50 µg/g that is accepted as safe for the general population can be too high (Järup et al. 2000; Noonan et al. 2002). The level of Cd above which early effects occur is unknown. Thus, special attention has been paid to evaluation of safe levels of Cd exposure in humans, and to search for sensitive biomarkers allowing detection of early changes at the stage when they are still reversible (Mueller et al. 1998; Jin et al. 1999; Price at al. 1999; Roels et al. 1999).
The nephrotoxicity of Cd has been extensively studied and widely reported in occupationally (Kahan et al. 1992; Bernard et al. 1995; Järup et al. 2000) and environmentally (Jin et al. 1999; Price et al. 1999; Järup et al. 2000) exposed human subjects, as well as in various experimental models (Aughey et al. 1984; Nordberg et al. 1994; Mitsumori et al. 1998; Ohta et al. 2000). However, reports concerning the simultaneous assessment of the structure and function of this organ and Cd accumulation are rare. Moreover, there are some discrepancies regarding levels of exposure and the kidney Cd concentrations at which kidneys are injured as well as regarding the severity of damage (Aughey et al. 1984; Uriu et al. 2000). There is still too little knowledge on kidney status in conditions of low-level long-term exposure.
Therefore, we have created an experimental model using rats chronically treated with Cd at relatively low and relatively high levels in which we have assessed the effect of Cd on the structure and function of the kidney. The degree of damage to the kidney was evaluated biochemically, histopathologically and histoenzymatically. The biochemical and histopathological results have been reported in the first part of the paper while the histoenzymatic results appear in the second part.
Materials and methods
Experimental protocol
The study was carried out on 108 inbred male 2-month-old Wistar rats of initial body weight 180–200 g. The animals were housed in conventional conditions at a temperature of 22±1°C, with a relative humidity of 50±10% and a 12-h/12-h light/dark cycle. They had unlimited access to drinking water (redistilled water or aqueous solutions of CdCl2) and rat chow (LSM dry diet; Fodder Manufactures, Motycz, Poland). Cd concentration in the diet was assessed in our laboratory to be 0.211 mg/kg.
The rats were allocated randomly to the three experimental groups of 36 animals each. Two groups received aqueous solutions of CdCl2 at the concentration of 5 or 50 mg Cd/l as the only drinking fluid. Control rats drank redistilled water (uncontaminated with Cd). To assess Cd intake, 24-h consumption of drinking water was measured during the whole course of the experiment. After 24-h urine collection in metabolic cages and overnight fasting, 12 animals of each group were killed under anaesthesia with Vetbutal (pentobarbital sodium and pentobarbital 5:1, 30 mg/kg body weight, i.p.) at 6, 12 and 24 weeks of the experiment. Blood was collected (with and without anticoagulant) from the heart, and both kidneys were removed. The kidneys were washed thoroughly in ice-cold physiological saline (0.9% NaCl), weighed and cut into a few pieces, which were assigned to Cd analysis, biochemical and morphological studies. In the serum, concentrations of creatinine, total protein and urea were measured. Cd, creatinine, total protein and urea concentrations were determined in the urine, as well as total N-acetyl-β-d-glucosaminidase (NAG-T) and its isoenzyme B (NAG-B), and alkaline phosphatase (ALP) activities. In the whole blood, Cd concentration was assayed as well.
The study was approved by the Local Ethics Committee for animal experiments in Bialystok. The research complied with the current laws of Poland, and procedures involving the animals and their care conformed to the institutional guidelines, in compliance with national and international laws and Guidelines for the Use of Animals in Biomedical Research (Giles 1987).
Chemicals
For chemical and biochemical examinations, ultra-pure water received from water purification Milli-Q system (Millipore Corporation, Milford, MA, USA) was used. All reagents and chemicals were of analytical grade purity or higher. For Cd analysis, trace pure nitric acid (HNO3; Merck, Darmstadt, Germany) and a stock of atomic absorption standard solution of Cd (Sigma, St. Louis, MO, USA) were used.
Estimation of Cd concentration in the blood, kidney and urine
The whole blood collected in heparinized tubes was wet-digested with HNO3. Slices of the kidneys were dry-mineralized in an electric oven and the ash was dissolved in 1 M HNO3. Urine samples, without mineralization, were diluted with ultra-pure water. Cd concentration was determined by flameless atomic absorption spectrometry method (atomic absorption spectrophotometer model Z-5000; Hitachi, Tokyo, Japan) with electrothermal atomization in a graphite cuvette according to the manufacturer's recommendation with our own modification. The cathode lamp of Cd (Photron, Narre Warren, Australia) was operated under standard conditions using its respective resonance line of 228.8 nm. The detection limit was 0.08 µg Cd/l. Internal quality control was employed to keep the measurement process reliable.
Serum and urinary markers of renal toxicity
The activities of NAG-T and NAG-B (EC 3.2.1.52) in urine were determined colourimetrically according to the method of Zwierz et al. (1981), using p-nitrophenyl-N-acetyl-β-d-glucosamide (Sigma) as substrate. ALP (EC 3.1.3.1) activity was measured colourimetrically by a kinetic method (based on liberation of p-nitrophenol from p-nitrophenyl phosphate), using a commercially available diagnostic test (POCh, Gliwice, Poland). The concentrations of creatinine and urea in serum and urine were determined colourimetrically using a diagnostic laboratory test (POCh). Creatinine clearance was calculated in order to evaluate the glomerular filtration rate. The concentration of total protein was estimated according to the method of Lowry et al. (1951) with bovine albumin (Sigma) as a standard. All colourimetrical determinations were made using a Hitachi U-3010 spectrophotometer.
Histopathological studies
Slices of the kidneys of five rats from each experimental group were fixed in Carnoy's fluid or in Grander's fluid. The slices fixed in Carnoy's fluid were embedded in paraffin, cut into 5-µm sections and routinely stained with hematoxylin and eosin (H+E). The slices fixed in Grander's fluid were subjected to periodic acid-Schiff (PAS) reaction, performed by the method of McManus-Mowry as described by Pearse (1972). The reaction was pereformed to show the vessels and membranes of Bowman's capsule of glomeruli as well as the brush border and basement membranes of particular sections of nephrons.
All specimens were photographed under a Polyvar-Reichert light microscope.
Statistical analysis
Experimental groups were compared using a one-way analysis of variance (ANOVA) by the Kruskal-Wallis ranks test. A linear Pearson's correlation analysis was performed to evaluate the relationship between Cd concentrations and the biochemical parameters in serum and urine. P-values <0.05 were considered significant. Statistical tests were performed using Statistica version 5.0 (StatSoft, Tulsa, OK, USA).
Results
Consumption of drinking water and Cd intake
The addition of Cd to the drinking water diminished fluid consumption in a concentration-dependent manner during the whole experiment (Fig. 1). Average Cd intake, calculated on the basis of fluid consumption, was 157.6±1.4 µg/24 h (range 320.5–678.4 µg/kg body weight per 24 h) for 5 mg Cd/l, and 913.6±9.0 µg/24 h (range 1963.9–4487.0 µg/kg body weight per 24 h) for 50 mg Cd/l.
Consumption of drinking water of rats exposed to Cd (0, 5 or 50 mg/l water) during the 24-week experiment. Each point represents mean water consumption during 1 week of the experiment. The calculations are for 36 animals during the first 6 weeks, for 24 during weeks 7–12, and for 12 during weeks 13–24. There were statistically significant differences (P<0.001) among all groups at each consecutive week
Body weight gain and kidney weight
During the experiment an increase in the body weight of all the rats was noted. The rats treated with 5 mg Cd/l reached the weights of the control. Exposure to 50 mg Cd/l resulted in a reduction in body weight gain after 6 weeks (by 7%, P<0.05) and 12 weeks (by 22%, P<0.001), but the effect was no further observed after 24 weeks of the experiment. Kidney weight and relative kidney weight (kidney weight expressed as a percentage of body weight) were not changed by any treatment, except for a slight increase (by 8%, P<0.01) in the relative kidney weight in the rats treated with 50 mg Cd/l for 12 weeks, which was caused by the decrease in body weight gain (data not shown).
Cd concentration in the blood, kidney and urine
The administration of Cd resulted in a marked dose- and time-dependent increase in its concentration in the blood (Fig. 2), kidney (Fig. 3) and urine (Fig. 4). The kidney Cd concentration in the rats exposed to 5 mg Cd/l was 61, 87 and 187 times higher (P<0.001) compared with that in the respective control group after 6, 12 and 24 weeks, respectively. In the animals treated with 50 mg Cd/l, the increase in the kidney Cd concentration was 617, 943 and 1154 times higher after 6, 12 and 24 weeks of the experiment, respectively. In control rats an increase in Cd concentration in the kidney was also observed (Fig. 3).
Cd concentration in blood (µg/l) of rats exposed to Cd (0, 5 or 50 mg/l water) for up to 24 weeks. Each bar represents mean ±SE of 12 animals. Statistically significant differences: *P<0.05 in comparison with appropriate control; † P<0.05 for comparison of 5 and 50 mg Cd/l at the same period of exposure; § P<0.05 for comparison of 6 and 24 weeks of exposure at the same level; ¶ P<0.05 for comparison of 12 and 24 weeks of exposure at the same level
Cd concentration in kidney (µg/g wet weight) of rats exposed to Cd (0, 5 or 50 mg/l water) for up to 24 weeks. Each bar represents mean ±SE of 12 animals. Statistically significant differences: *P<0.05 in comparison with appropriate control; † P<0.05 for comparison of 5 and 50 mg Cd/l at the same period of exposure; ‡ P<0.05 for comparison of 6 and 12 weeks of exposure at the same level; § P<0.05 for comparison of 6 and 24 weeks of exposure at the same level; ¶ P<0.05 for comparison of 12 and 24 weeks of exposure at the same level
Cd concentration in urine (µg/g creatinine) of rats exposed to Cd (0, 5 or 50 mg/l water) for up to 24 weeks. Each bar represents mean ±SE of 12 animals. Statistically significant differences: *P<0.05 in comparison with appropriate control; † P<0.05 for comparison of 5 and 50 mg Cd/l at the same period of exposure; § P<0.05 for comparison of 6 and 24 weeks of exposure at the same level; ¶ P<0.05 for comparison of 12 and 24 weeks of exposure at the same level
High correlation coefficients were noted between Cd concentrations in the blood, kidney and urine (Table 1).
Serum and urinary markers of renal toxicity
The administration of 5 mg Cd/l for 6 weeks had no effect on the urinary NAG-T and NAG-B activities (Fig. 5). However, after 12 and 24 weeks increases in NAG-T (by 41%, P<0.05 and 95%, P<0.001, respectively; Fig. 5) and NAG-B (by 71%, P<0.05 and 104%, P<0.001, respectively; Fig. 5) activities were noted in comparison to the respective control groups. On exposure to 50 mg Cd/l, increases in NAG-T and NAG-B activities (by 244% and 113%, respectively, P<0.001) were already observed after 6 weeks, but longer term Cd administration did not cause further increase in their excretion (Fig. 5).
Total N-acetyl-β-d-glucosaminidase (NAG-T) and isoenzyme NAG-B activities in urine (IU/l) of rats exposed to Cd (0, 5 or 50 mg/l water) for up to 24 weeks. Each bar represents mean ±SE of 12 animals. Statistically significant differences: *P<0.05 in comparison with appropriate control; † P<0.05 for comparison of 5 and 50 mg Cd/l at the same period of exposure; ‡ P<0.05 for comparison of 6 and 12 weeks of exposure at the same level; § P<0.05 for comparison of 6 and 24 weeks of exposure at the same level; ¶ P<0.05 for comparison of 12 and 24 weeks of exposure at the same level
Exposure to 5 and 50 mg Cd/l for 6 and 12 weeks had no effect on the total protein and urea concentrations in the serum and urine. Creatinine concentration in the serum and creatinine clearance were also unchanged, while the urinary activity of ALP significantly increased (P<0.05), but only in the group receiving 50 mg Cd/l (data not shown). In the rats exposed to 5 mg Cd/l for 24 weeks the concentrations of total protein and urea in the serum and urine were unchanged, while the urinary activity of ALP increased (by 22%, P<0.01; Table 2). In the animals intoxicated with 50 mg Cd/l for 24 weeks, a significant increase was observed in the concentration of total protein (by 51%, P<0.001), and in the urinary ALP activity (by 71%, P<0.001) (Table 2). The concentration of urea in the serum increased (by 38%, P<0.001), while that in the urine decreased (by 26%, P<0.001; Table 2). Serum creatinine concentration and creatinine clearance were not changed by any treatment (Table 2).
High positive correlations between Cd concentration in the kidney or urine, and the urinary NAG-T, NAG-B and ALP activities were noted (Table 1). The concentration of urea in the serum, as well as total protein urinary excretion, also positively correlated with Cd concentration in the kidney or in urine, while for the urinary urea levels a negative correlation was observed (Table 1).
Kidney histopathology
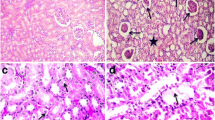
The kidneys of all control rats had a normal structure of cortex (Fig. 6A) and medulla. After 6 weeks of the experiment no structural lesions were evident with exposure to 5 mg Cd/l. After exposure to 50 mg Cd/l for 6 weeks, a blurred structure of tubular epithelium, atrophy of brush border, and partial fragmentation of cells with release of nuclei into tubular lumen were noted in the main tubules (formed by proximal convoluted tubules and straight tubules). Cytoplasm of the tubular cells had micro- and medium-granular forms. The damaged tubules were located in the cortex as well as in the circummedullary region. In the main tubules of rats intoxicated with 5 mg Cd/l for 12 weeks, structural damage to the epithelial cells (degenerative changes) occurred, whereas in the animals treated with the higher Cd level the injury became more prominent than after 6 weeks. At both levels of the exposure, practically in the whole region of the kidney cortex, we observed oedema, interruption of borders of lining epithelial cells, and the presence of acidophilic micro-granular mass as well as nuclei of epithelial cells released into tubular lumen. Most of the main tubules revealed a lack of regular contour and a similar picture was observed in the glomeruli of rats exposed to 50 mg Cd/l. The remaining tubules (i.e. distal convoluted tubules, connecting tubules and papillary ducts) had no, or only marginal, structural changes. After 24 weeks of exposure to both Cd levels, dose-dependent damage to the whole nephron was observed (Fig. 6B, C). The injury affected mainly the major resorptive part (proximal convoluted tubules and straight tubules) and the filtering part (glomeruli) of the nephron, and became less advanced further away from these sections. The structural damage to the tubules was of similar type and location but was more advanced, dose-dependently, than after 12 weeks of the exposure. Necrosis of epithelial cells and apoptotic-like changes were evident in the proximal convoluted tubules and straight tubules. Thickened capillary vessels and widened filtering spaces were observed in the renal glomeruli. The damage to the glomeruli was more pronounced in the rats exposed to 50 mg Cd/l than in those receiving 5 mg Cd/l.
Histopathological evaluation of the effect of Cd on rat kidney. A Normal structure of tubules (arrows) and glomeruli (barbed arrows) in kidney cortex in control. B Damaged kidney structure in rats exposed to 5 mg Cd/l for 24 weeks. C Kidney structure after exposure to 50 mg Cd/l for 24 weeks. The damage to the renal tubules is more serious than at 5 mg Cd/l (H&E, ×460)
In control rats a positive carmine diffuse PAS reaction was located in the renal glomeruli, walls of blood vessels, brush border and basement membranes of tubules of particular nephrons (Fig. 7A). Circummedullary nephrons were characterized by especially well-saturated brush borders. On the surfaces of the epithelial cells of the distal convoluted tubules, PAS reaction was minimal. A similar picture was observed in the thick and collecting tubules and papillary ducts. In the rats exposed to 5 and 50 mg Cd/l for 6 weeks, a marked increase in the carmine product of the reaction was noted in the brush border of the proximal convoluted tubules, straight tubules and renal glomeruli. In the rats intoxicated with 50 mg Cd/l, a focal loss of PAS reaction was found in the brush border of some main tubules. After exposure to 5 and 50 mg Cd/l for 12 weeks, a considerable decrease in the intensity of colour reaction or even its total fade were evident in the brush border of the epithelial cells in the majority of the main tubules. In the renal glomeruli, the intensity of PAS reaction was similar to that observed after 6 weeks. The intensity, character and location of PAS reactions after 24 weeks (Fig. 7B, C) of the experiment resembled those noted in the corresponding groups after 12 weeks.
Discussion
The aim of the present study was to assess early changes in the structure and function of the kidney in rats chronically exposed to Cd as well as to determine the body burden of Cd at which they occurred. The degree of kidney damage was evaluated biochemically and histopathologically. Sensitive biomarkers of Cd-induced proximal tubular injury such as urinary NAG-T and its isoenzyme B (NAG-B), and ALP were measured.
In the rats intoxicated with 5 and 50 mg Cd/l, the body burden of Cd, reflected in its urinary concentration and accumulation in the kidney, increased with the level and duration of treatment. In this paper we have presented the urinary Cd excretion and measured in urine biochemical indicators of kidney status only after 6, 12 and 24 weeks of exposure (i.e. periods of exposure after which blood and kidneys were removed for biochemical or histopathological examination), but in the rats exposed for 24 weeks these variables were monitored during the whole experiment (once a week). Cd concentration in the urine (recognized as the best indicator of exposure to this metal) of rats exposed to 5 mg Cd/l ranged from 2.26 to 10.97 µg/g creatinine, and in those exposed to 50 mg Cd/l ranged from 8.10 to 26.76 µg/g creatinine, depending on time-point of measurement during the 24-week period of exposure. After 24 weeks of exposure to 50 mg Cd/l, Cd concentration in urine was relatively high, but it most probably resulted from its increased loss by damaged kidneys. Between the 13th and 22nd week of exposure, the urinary Cd concentrations were markedly lower than after 24 weeks. When the kidney damage occurs, increased urinary excretion of Cd is observed at the same time. Indeed, in the rats exposed to 50 mg Cd/l a gradual increase in Cd excretion occurred from the 18th week. The enhanced urinary Cd excretion most probably was a cause of the decrease in the blood concentration of the metal that was noted in these animals between the 12th and 24th week of the exposure.
Since Cd concentrations in the blood, kidney and urine of rats continuously intoxicated with Cd are comparable with levels reported by some authors in humans exposed environmentally or occupationally to Cd, including smokers (Koyama et al. 1992; WHO 1992; Bem et al. 1993; Chalkley et al. 1998; Rydzewski et al. 1998; dell'Omo et al. 1999; Khassouani et al. 2000), we think that our experimental model can reflect the situation of exposure to Cd that may take place in human life.
The rats exposed to 50 mg Cd/l developed a stronger aversion to drinking than those that received 5 mg Cd/l because water containing the higher Cd concentration had a bad taste. In interpreting the results from rats exposed to 50 mg Cd/l, it has to be taken into consideration that Cd could cause dehydration (as a result of aversion to drinking), which in turn would mask a real influence on Cd concentrations and the biochemical parameters in the serum (blood) and urine. On the basis of results obtained, it is difficult to recognize whether and to what extent the reduced water intake influenced the measured variables. To minimize this effect, the urinary concentrations of Cd, total protein and urea have been expressed in calculation relative to creatinine concentration.
The excretion of Cd via kidneys and its accumulation in this organ resulted in structural and functional damage to the whole kidney. The injury was dose- and time-dependent, and localized mainly in the main resorptive part (proximal convoluted tubules and straight tubules) and in the filtering part (glomeruli) of the nephron.
Cadmium-induced nephrotoxicity is thought to be caused by the release of Cd2+ ions from the extracellular Cd–metallothionein complex (Cd–Mt). After absorption, Cd is transported by the blood initially in the form bound to high molecular weight proteins, especially albumin. The Cd bound to albumin is taken up predominantly by the liver where Cd2+ ions are released from albumin and induce synthesis of low molecular weight protein — metallothionein (Mt). Mt binds and retains Cd2+ ions in the liver. A small quantity of Cd–Mt is released into the blood from hepatocytes, and is efficiently transported through the glomerular membrane to the tubular fluid in the kidney. Next the Cd–Mt complex is taken up from the tubular filtrate by pinocytotic vesicles in the brush border of the proximal tubular cells, and is transported into lysosomes where it is degraded. The free Cd2+ ions released are transported to the cytoplasm where they induce synthesis of Mt molecules, which bind and retain Cd in the kidney for a very long time. Part of the Cd–Mt complexes of tubular fluid is degraded in tubular lumen before reabsorption. Cd2+ ions, released into tubular lumen, are responsible for damage to the membrane proteins of renal tubular cells (Kjellström 1986; Nordberg et al. 1994).
The increased urinary excretion of cytotoxicity marker enzymes such as NAG-T, NAG-B and ALP indicate the tubular Cd toxicity. ALP originates from the brush border, while NAG is located mainly in lysosomes of proximal tubular epithelial cells. NAG-T is present in the kidney and urine as two major isoenzymes — isoenzyme A (NAG-A), which reflects an increased lysosomal activity, and isoenzyme B (NAG-B), which is the lesional form of NAG. Isoenzyme A resides in the soluble intralysosomal compartment, and is released into urine by exocytosis during the physiological turnover of the cells. NAG-B is an intralysosomal membrane-bound enzyme, which is released into urine when disruption of lysosomal membranes occurs. NAG-T and especially NAG-B were reported as highly sensitive indicators of Cd tubular toxicity, and thus they were recommended for the biological monitoring of exposure to this heavy metal (Bernard et al. 1995; Mueller et al. 1998; Jin et al. 1999; Price et al. 1999). The Cd-induced increase in NAG-T and NAG-B urinary activities considerably preceded the occurrence of changes in other biochemical markers of kidney status. Moreover, we noted high positive correlations between the urinary NAG-T and NAG-B activities and the urinary, as well as the kidney, Cd concentrations. On exposure to 5 mg Cd/l, increased urinary NAG-T and NAG-B activities were observed after 12 weeks, while at the higher exposure level increased activities were already evident after the first week (data not shown). At both levels of exposure, all of the biochemical variables describing kidney status measured in the serum and/or urine were unchanged at the time when an increase in the urinary activities of NAG-T and NAG-B had been first noted. The large increase in the urinary NAG-T activity with simultaneous modest elevation that of NAG-B suggests that the Cd-induced increase in NAG-T was caused by isoenzymes other than NAG-B, apparently NAG-A. It can result from an increased lysosomal activity and leakage of NAG-A and other NAG isoenzymes by damaged lysosomal membranes. This phenomenon will be taken into account in our future studies.
The PAS reaction demonstrates substances involved in the creation of the structure of biological membranes, including glycoproteins and glycosaminoglycans. The reaction stains the structures connected with adhesiveness and tightness of membranes such as capillary loops, brush borders of the main tubules and basement membranes. The focal decrease in the intensity of PAS reaction that was evident in the brush border of the main tubules of some rats exposed to 50 mg Cd/l for 6 weeks could indicate damage to the reabsorptive surface of epithelial cells. The marked decrease in the product of PAS reaction, or even its total lack, observed in the brush border of tubular epithelial cells after 12 and 24 weeks of exposure to 5 and 50 mg Cd/l reflects serious impairment of reabsorptive processes in the main tubules, or cessation of the mechanisms of active transport.
The main tubules, due to their high reabsorptive activity, are especially sensitive to injury under Cd influence (Kjellström 1986). The early effects of Cd action on the proximal convoluted tubules and straight tubules were observed at relatively low kidney accumulation (2.69–6.94 µg/g wet weight) and urinary concentration (3.22–5.93 µg/g creatinine) of Cd. This suggests that Cd can affect kidneys even at relatively low accumulation levels, and that the kidney cortex Cd concentration (the ratio of Cd concentration in the kidney cortex to its concentration in the whole kidney is about 1.25) of 50 µg/g, which is accepted as safe for the general population, can be too high and should be revised. Degenerative changes in proximal tubular epithelial cells at low Cd exposure have recently been reported by Uriu et al. (2000). On the basis of results obtained, we hypothesize that the threshold for the kidney effects of Cd is less than 4.08±0.33 µg/g wet kidney weight and higher than 2.40±0.15 µg/g (at this Cd concentration no changes were noted in the kidney structure and biochemical indicators of kidney status, including NAG-T and NAG-B activities), and it may be close to the latter value.
In the rats intoxicated with 50 mg Cd/l, the damage to the main tubules was considerably prominent and occurred earlier than in the rats exposed to 5 mg Cd/l. Moreover, with exposure to 50 mg Cd/l symptoms of the structural and functional glomerular damage occurred. However, this effect of Cd was observed at markedly higher kidney Cd concentrations than those at which tubular injury took place.
The increased serum urea concentration with the simultaneous decrease in its urinary level (indicating its decreased clearance), and the enhanced urinary excretion of total protein, together with the structural changes observed on exposure to 50 mg Cd/l, show glomerular functional disturbances in spite of creatinine clearance being unaffected. The enhancement in the urinary total protein concentration could partially result from decreased reabsorption of low molecular weight proteins by injured tubules. The glomerular structural damage was also revealed in the rats exposed to 5 mg Cd/l, but in these animals there was no evidence of functional disturbances.
In our experiment, the rats exposed to 50 mg Cd/l for 24 weeks had less serious kidney damage than in the study by Aughey et al. (1984) at the same level and duration of exposure. Those authors observed early pathological changes around weeks 4–6 of treatment with Cd. After 12 weeks signs of tubular necrosis, interstitial fibrosis and glomerular epithelial cells hypertrophy were present in small areas of the cortex, whereas after 24 weeks the renal cortex showed clear evidence of tubulo-interstitial nephritis at the concentration of 60 µg Cd/g wet weight (Aughey et al. 1984).
In conclusion, chronic intoxication with Cd results in damage to the whole kidney, the main tubules being the target for Cd action. Severity of renal toxicity increases with Cd accumulation in the kidney, which depends on the level and duration of exposure. A very important finding of this study is that Cd affects the kidney, especially the main tubules, even at relatively low accumulation levels in this organ. Our results confirm the hypothesis that humans environmentally exposed to Cd, especially smokers, are at risk of tubular dysfunction. Moreover, they seem to suggest that the critical Cd concentrations in the kidney cortex that are accepted for the general population (50 µg/g) and occupationally exposed subjects (200 µg/g) are too high and should be revised.
References
Aughey E, Fell GS, Scott R, Black M (1984) Histopathology of early effects of oral cadmium in the rat kidney. Environ Health Perspect 54:153–161
Bem EM, Piotrowski JK, Turzyńska E (1993) Cadmium, zinc, and copper levels in the kidneys and liver of the inhabitants of north-eastern Poland. Pol J Occup Med Environ Health 6:133–141
Bernard A, Thielmans N, Roels H, Lauwerys R (1995) Association between NAG-B and cadmium in urine with no evidence of a threshold. Occup Environ Med 52:177–180
Chalkley SR, Richmond J, Barltrop D (1998) Measurement of vitamin D3 metabolites in smelter workers exposed to lead and cadmium. Occup Environ Med 55:446–452
dell'Omo M, Muzi G, Piccinini R, Gambelunghe A, Morucci P, Fiordi T, Ambrogi M, Abbritti G (1999) Blood cadmium concentrations in the general population of Umbria, central Italy. Sci Total Environ 226:57–64
Giles AR (1987) Guidelines for the use of animals in biomedical research. Thromb Haemost 58:1078–1984
Hac E, Krzyzanowski M, Krechniak J (1998) Cadmium content in human kidney and hair in the Gdansk region. Sci Total Environ 224:81–85
Järup L, Hellstrom L, Alfven T, Carlsson MD, Grubb A, Persson B, Pettersson C, Spang G, Schutz A, Elinder CG (2000) Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup Environ Med 57:668–672
Jin T, Nordberg G, Wu X, Ye T, Kong Q, Wang Z, Zhuang F, Cai S (1999) Urinary N-acetyl-β-d-glucosaminidase isoenzymes as biomarkers of renal dysfunction caused by cadmium in a general population. Environ Res 81:167–173
Kahan E, Derazne E, Rosenboim J, Ashkenazi R, Ribak J (1992) Adverse health effects in workers exposed to cadmium. Am J Ind Med 21:527–537
Khassouani CE, Soulaymani R, Mauras Y, Allain P (2000) Blood cadmium concentration in the population of the Rabat area, Morocco. Clin Chim Acta 302:155–160
Kjellström T (1986) Renal effects. In: Friberg L, Elinder CG, Kjellström T, Nordberg GF (eds) Cadmium and health: a toxicological and epidemiological appraisal, vol 2. CRC Press, Boca Raton Florida, pp 21–109
Koyama H, Satoh H, Suzuki S, Tohyama C (1992) Increased urinary cadmium excretion and its relationship to urinary N-acetyl-β-d-glucosaminidase activity in smokers. Arch Toxicol 66:598–601
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mitsumori K, Shibutani M, Sato S, Onodera H, Nakagawa J, Hayashi Y, Ando M (1998) Relationship between the development of hepato-renal toxicity and cadmium accumulation in rats given minimum to large amounts of cadmium chloride in the long-term: preliminary study. Arch Toxicol 72:545–552
Mueller PW, Price R, Finn W (1998) New approaches for detecting thresholds of human nephrotoxicity using cadmium as an example. Environ Health Perspect 106:227–230
Noonan CW, Sarasua SM, Campagna D, Kathman SJ, Lyberger JA, Mueller PW (2002) Effects of exposure to low levels of environmental cadmium on renal biomarkers. Environ Health Perspect 110:151–155
Nordberg GF, Jin T, Nordberg M (1994) Subcellular targets of cadmium nephrotoxicity: cadmium binding to renal membrane proteins in animals with or without protective metallothionein synthesis. Environ Health Perspect 102 [Suppl 3]:191–194
Ohta H, Yamauchi Y, Nakakita M, Tanaka H, Asami S, Seki Y, Yoshikawa H (2000) Relationship between renal dysfunction and bone metabolism disorder in male rats after long-term oral quantitative cadmium administration. Ind Health 38:339–355
Pearse AGE (1972) Histochemistry: theoretical and applied. JA Churchill, London, p 2
Price RC, Patel S, Chivers I, Milligan P, Taylor SA (1999) Early markers of nephrotoxicity: detection of children at risk from environmental pollution. Ren Fail 21:303–308
Roels HA, Hoet P, Lison D (1999) Usefulness of biomarkers of exposure to inorganic mercury, lead, or cadmium in controlling occupational and environmental risk of nephrotoxicity. Ren Fail 21:251–262
Rydzewski B, Sułkowski W, Miarzyńska M (1998) Olfactory disorders induced by cadmium exposure: a clinical study. Int J Occup Med Environ Health 11:235–245
Uriu K, Kaizu K, Qie YL, Ito A, Takagi I, Suzuka K, Inada Y, Hashimoto O, Eto S (2000) Long-term oral intake of low-dose cadmium exacerbates age-related impairment of renal function reserve in rats. Toxicol Appl Pharmacol 169:151–158
WHO (World Health Organisation) (1992) Environmental health criteria 134. Cadmium. International Programme on Chemical Safety (IPCS), WHO, Geneva
Zwierz K, Gindzieński A, Głowacka D, Porowski T (1981) The degradation of glycoconjugates in the human gastric mucous membrane. Acta Med Acad Sci Hung 38:145–152
Acknowledgements
The present study was supported by the Grant (No. 4PO5D 012 19) from the Committee for Scientific Research (KBN, Poland).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brzóska, M.M., Kamiński, M., Supernak-Bobko, D. et al. Changes in the structure and function of the kidney of rats chronically exposed to cadmium. I. Biochemical and histopathological studies. Arch Toxicol 77, 344–352 (2003). https://doi.org/10.1007/s00204-003-0451-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-003-0451-1