Abstract
A psychrotolerant bacterial strain of Pseudomonas sp. (P. palleroniana GBPI_508), isolated from the Indian Himalayan region, is studied for analyzing its potential for degrading bisphenol A (BPA). Response surface methodology using Box–Behnken design was used to statistically optimize the environmental factors during BPA degradation and the maximum degradation (97%) was obtained at optimum conditions of mineral salt media pH 9, experimental temperature 25 °C, an inoculum volume of 10% (v/v), and agitation speed 130 rpm at the BPA concentration 270 mg L−1. The Monod model was used for understanding bacterial degradation kinetics, and 37.5 mg−1 half saturation coefficient (KS) and 0.989 regression coefficient (R2) were obtained. Besides, the utmost specific growth rate µmax was witnessed as 0.080 h−1 with the GBPI_508 during BPA degradation. Metabolic intermediates detected in this study by GC–MS were identified as valeric acid, propionic acid, diglycolic acid, and phenol. The psychrotolerant bacterial strain of Pseudomonas sp. (P. palleroniana GBPI_508), isolated from the Indian Himalayan region has shown good potential for remediation of BPA at variable conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) belongs to diphenylmethane derivatives and it is used as an additive for the production of polycarbonate plastics and epoxy resins, which account for nearly 64% of BPA demand in 2018 (Almeida et al. 2018). Its production has been estimated to be more than 550,000 tons annually (Staples et al. 1998). BPA does not occur naturally, but became a part of the environment due to high production, consumption and introduction into the environment through effluent discharge from manufacturing area, municipal wastewater treatment plants, and also through domestic effluents, leaching from landfills, combustion of domestic waste, and degradation of plastics in the environment (Corrales et al. 2015; Flint et al. 2012; Staples et al. 1998). BPA contamination from plastics has been widely reported since 2007 (Vandenberg et al. 2007) and has drawn the attention of the regulatory bodies and the scientific community due to their widespread occurrences, distribution, endocrine-disrupting effects, and severe toxicity (Lee et al. 2019). Due to structural similarity with endogenous hormones, compounds like BPA can mimic the physiological functions in the body and are also known as endocrine-disrupting chemicals (EDCs). These compounds are also reported as mutagenic and carcinogenic (Chai et al. 2005). The toxicity of BPA is reported against fish, algae and also humans. It can be toxic for aquatic life even at very low levels (0.175–1.6 μg L−1). These figures are expected to increase in the near future due to continuous discharge of contaminants into natural water resources (such as rivers, ponds, lakes, etc.). Accordingly, unless BPA is satisfactorily treated in wastewater treatment plants (WWTPs), BPA released in both WWTP effluents and waste sludge (e.g., biosolids) will contaminate the natural environments leading to significant ecological risks.
Most conventional water treatment methods, such as adsorption, coagulation, ultrafiltration, and reverse osmosis, concentrate pollutants. Other methods, such as sedimentation, filtration, chemical treatments, and membrane technologies, have high operating costs and could release toxic secondary pollutants into the ecosystem. So, the development of low-cost and effective technologies for wastewater treatment is needed, which can add the value after addition in already existing technologies.
Bioremediation is a natural and low-cost process for the removal of contaminants from the water where microorganisms have been used for removal of target pharmaceutical and personal care products (PPCP)/xenobiotic compounds. Several BPA-degrading bacteria have been isolated, including the unidentified Gram-negative MV1 and WH1 strains (Lobos et al. 1992; Ronen and Abeliovich 2000) Sphingomonas bisphenolicum strain AO1, Achromobacterxylosoxidans strain B-16 (Zhang et al. 2007), Pseudomonas paucimobilis strain FJ-4, Pseudomonas sp. (Kamaraj et al. 2014; Masuda et al. 2007), Streptomyces sp. strain, Sphingomonas sp. Strains (Oshiman et al. 2007; Sakai et al. 2007), Novosphingobium sp. strain TYA-1, Bacillus sp. (Li et al. 2012) and Cupriavidusbasilensis JF1, Enterobacter gergoviae strain BYK-7 (Badiefar et al. 2015), and Bacillus megaterium strain ISO-2 (Suyamud et al. 2018a) which have shown significant potential of degrading BPA. The bioremediation process can target the contaminants present in low amount in wastewater and freshwater bodies, and the intermediates generated during this process are mostly less toxic or nontoxic to the environment. Hence, the present study was designed to identify the BPA degradation potential of bacteria, isolated from Indian Himalayan region, along with the understanding of the kinetics of the degradation process. These microorganisms have been reported for their unique traits such as tolerance to a wide range of temperature and pH along with their biodegradation potential under low temperature environment (Dhakar and Pandey 2020, 2016).
Materials and methods
Chemicals, bacteria and culture conditions
Bisphenol A (≥ 99% pure) was purchased from Merck Limited, Worli, Mumbai, India; ammonium sulfate (NH4)2SO4), potassium dihydrogen phosphate (KH2PO4), sodium dihydrogen phosphate (NaH2PO4), magnesium sulfate heptahydrate (MgSO4∙7H2O), sodium chloride (NaCl), zinc chloride (ZnCl2), and calcium chloride dihydrate (CaCl2∙2H2O) from SRL Chemicals Limited, Mumbai, India; and tryptone yeast extract (TYE) (casein enzyme hydrolysate 5.0 g/L and yeast extract 3.0 g/L), agar, corn starch, (NH4)2SO4, potassium chloride (KCl), ferrous sulfate heptahydrate (FeSO4∙7H2O), tributarin, copper sulfate pentahydrate (CuSO4 0.5H2O), zinc sulfate heptahydrate (ZnSO4 0.7H2O), sodium molybdate (Na2MoO4), magnesium sulfate monohydrate (MnSO4∙H2O), and boric acid (H3BO3) from Hi-media Limited, Mumbai, India and SRL Chemicals Limited, Mumbai, India.
Bacterial strains such as GBPI_Hb0, GBPI_Hb5, GBPI_Hb14, GBPI_Hb61, GBPI_CDB143, GBPI_CDB149, GBPI_507, GBPI_508, B0, GBPI_506, GBPI_CDB84, GBPI_CDB87, GBPI_Hb1, GBPI_Hb149 and GBPI_CDB94 (Table S1) were taken from the Microbiology Laboratory of the Institute (G. B. Pant national institute of Himalayan environment), which was originally isolated from high altitudes in the Indian Himalayan region (Pandey et al. 2019). The bacterial culture was maintained in TYE agar at 25 °C. Mineral salt media (MSM) were used for screening the bacteria responsible for the degradation of BPA. MSM composition of the growth medium consisted of (g L−1): ammonium sulfate ((NH4)2SO4), 0.06 g; magnesium sulfate heptahydrate (MgSO4∙7H2O), 0.3 g; calcium chloride dihydrate (CaCl2∙2H2O), 0.05 g; zinc chloride (ZnCl2), 3 mg; and sodium chloride (NaCl), 0.03 g., with agar for Petri plate experiments and without agar for shake flask experiments.
Screening of bisphenol A-degrading bacteria
Bacteria were cultured on tryptone yeast medium and then inoculated into MSM which was earlier sterilized in an autoclave for 20 min at 121 °C. Different BPA concentrations (10, 50, 70, 100 and 270 mg L−1) dissolved in miliQ water were added to a flask containing MSM with agar and then transferred into Petri plate for solidification. When the plates were solidified, one loop full of the overnight culture was used to streak in the plate containing MSM medium with different concentrations of BPA. After streaking, the plates were kept in an incubator at 25 °C for 48 h for the observation of bacterial growth. The strain which showed the highest growth in the MSM media containing BPA was selected as a predominant BPA-degrading strain. The plate without the BPA was set as the control. After the selection of bacterial strain, the shake flask experiments were performed for analyzing its biodegradation capacity against BPA.
Bacterial growth assessment
The bacterial strain, recorded with maximum growth in the presence of BPA in plate experiments, was used for raising the culture suspension for further experiments. The bacterial cells were inoculated in mineral salt media (MSM) agar plates and incubated at 25 °C for 24 h. The bacterial culture was taken for making bacterial suspension by maintaining the optical density of 0.3 at 600 nm. This bacterial solution was used for performing shake flask experiments for BPA degradation, where the main experimental variables were media pH, BPA concentration, agitation speed, inoculum volume and experimental temperature conditions. During the experiments performed under controlled conditions, the samples were collected at every 24 h up to 96 h and used for the estimation of microbial growth and BPA degradation potential.
Bacterial growth kinetics during BPA degradation
During batch experiments, the bacterial growth kinetics were analyzed using Monod model (Monod 1949) to determine the kinetic parameters. Equation (1) was used for understanding the bacterial growth in the presence of different BPA concentrations in the shake flask.
A linear form of Eq. (1) can be represented as Eq. (2):
where µ is the specific growth rate, µmax is the maximum specific growth rate, S is the substrate concentration and Ks is the substrate saturation constant at half µmax. The kinetic model parameters estimated by various factors such as inoculum size 10% (v/v), compound concentrations (100 ppm), duration (96 h), and growth of bacteria were selected to study the biodegradation of BPA.
µ was calculated using Eq. (3) (Bitton 1994):
The values of 1/µ and 1/S were calculated and plotted to obtain µmax and Ks.
Design of experiments using response surface method (RSM)
Mini tab 18 was used for designing the experiments through the response surface method (RSM) Box–Behnken design (BBD). It is a second-order multivariate technique based on a three-level fractional factorial design consisting of a full 22 factor scattered in a balanced incomplete block design. The BBD is one of the most common designs used in RSM, has the same predictability in all directions and is helpful for investigating the behavior of the response surface for the response function (Y) using the second-order polynomial equation (Ferreira et al. 2007). Five experimental factors selected for designing the experiments included pH (X1), agitation speed (X2), the concentration of the compound (X3), inoculum size (X4) and incubation temperature (X5) with three levels for each factor (Table 1). The detailed experimental design with the total of 40 runs is shown in Table 2S. In these experiments, the BPA degradation capability of bacteria was checked in MSM for analyzing its potential to use BPA as a sole source of carbon and energy. In the system involving five variables, a mathematical relationship of the response (BPA % degradation) of these variables was approximated using the polynomial Eq. (4) (Box and Behnken 1960):
where Y is the predicted response value, b0 is the constant, bi is the linear coefficient and bij is the quadratic coefficient, and Xi and Xj are the variables.
Analytical methods
The bacterial cell growth was determined at 600 nm using UV–Vis spectrophotometer (Shimadzu, Japan) and expressed as optical density (OD). Before chromatographic analysis, each sample was centrifuged at 15000 g at 4 °C for 10 min using refrigerated centrifuge (Hi Mac CR-22G) and then filtered through a 0.22 µm pore size membrane filter (Axiva). The BPA concentration was determined using reverse-phase high-pressure liquid chromatography (RP-HPLC) (Shimadzu LC10) equipped with a photodiode detector (PDA) and a C18 column (HP 250 mm × 4.6 mm × 5 µm). The mobile phase consists of ortho-phosphoric acid (0.1%) and acetonitrile in the ratio of 45:5(v/v) which was used at a flow rate of 1 mL/min. The column temperature was maintained at 25 °C and the sample injector volume was 20 µL. The BPA was detected at 280 nm at 7.5 min retention time.
The degradation products were analyzed using GC/MS (QP 2010 mass spectrometer: Shimadzu, Japan). The GC–MS analysis was carried out using HP5 MS column (Agilent, USA) and the oven temperature program was started from 60 °C (hold time 5 min), increased to 180 °C (hold time 3 min), then to 250 °C (hold time 1 min) and finally up to 280 °C with the hold time of 1 min, and injector temperature maintained at 280 °C. Helium was used as carrier gas at a flow rate of 1 mL/min and the sample injection volume was 5 µL. The GC–MS interface was maintained at 260 °C at 57.4 kPa. In the full scan mode, electron ionization mass spectra in the range of 40–400 (m/z) were recorded at electronic energy of 70 eV. The structure of degradation intermediates was confirmed by comparing with that of the data available in the GC–MS spectral library (Wiley, NIST).
Enzyme assessment
As enzymes are mainly responsible for degradation of xenobiotics in case of bacterial degradation, for understanding about the responsible enzymes, their qualitative assessment was carried out during the BPA degradation process using selected bacterial species. As the selected bacterial strain produces extracellular enzymes, namely amylase, lipase, and laccase (Jain et al. 2017), their presence was tested in the presence of 270 mg/L BPA. The point inoculation method was used for the inoculation of bacteria in a Petri plate. The amylase production was analyzed using the medium containing corn starch 5 g/L, yeast extract 5 g/L, (NH4)2SO4 2.5 g/L, MgSO4.7H2O 0.2 g/L, KH2PO4 3 g/L, and CaCl2.2H2O 0.25 g/L (Burhan et al. 2003). The plates were inoculated with 24 h fresh culture and then incubated at 25 °C. After incubation, agar plates were flooded with Gram’s iodine for observing the zone of clearance around the bacterial colony. For lipase production, NaNO33.0 g/L, K2HPO4 0.1 g/L, MgSO4.7H2O 0.5 g/L, KCl 0.5 g/L, FeSO4.7H2O 0.01 g/L, and yeast extract 5.0 g/L were taken in the medium containing 1% tributyrin and 2% agar (Jain et al. 2017). The plates were then inoculated with 24 h fresh culture. The zone of clearance for lipolytic enzyme, developed around the colony, was recorded. Kirk and Farrell (modified) medium, supplemented with ABTS (2, 2′-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid), was used for the screening of ligninolytic enzymes. The media composition was 2.0 g/L malt extract, 2.0 g/L glucose, 2.0 g NH4NO3/L, 0.26 g/L Na2HPO4,0.26 g/L KH2PO4,0.5 g/L,MgSO4 (7H2O),0.01 g/L, CuSO4 (5H2O),0.006 g/L,CaCl2.2H2O,0.005 g/L, FeSO4(7H2O),0.0005 g/L, ZnSO4(7H2O),0.00002 g/L,Na2MoO4,0.00009 g/L, MnSO4·H2O, and 0.00007 g/L H3BO3 (Nicole et al. 1992).
Results and discussion
Screening of BPA-degrading bacteria
All the 15 bacterial strains were screened for analyzing their BPA degradation capability qualitatively through Petri plate experiments. The performance of P. palleroniana strain GBPI_508 was found to be the best; it was able to grow well in MSM agar plates in the presence of different BPA concentrations ranging from 10 to 270 mg/L (Table S1). The highest number of colonies appeared on the plates containing 10 mg L−1 BPA, followed by 50, 100 mg L−1. In contrast, few colonies appeared on the plates containing 270 mg L−1 BPA. Decreasing biomass with increase in BPA concentrations reflected the toxicity of BPA against the bacterium P. palleroniana GBPI_508. Similar results have been reported in a previous study by Vijayalakshmi et al. (2018).
Statistical optimization of BPA degradation using RSM
RSM was used to determine the combined effect of five variables and their interactions. A statistical optimization approach using BBD was used to study the linear, interactive, and square effects of various parameters on the BPA degradation capacity of P. palleronianaGBPI_508. 100% degradation was observed with 50 mg/L BPA concentration as reflected in run number 17, 39, 30, 33 and 34. In case of the highest BPA taken for the study, i.e., 270 mg/L, a maximum of 97% degradation was observed as indicated in run number 4 (Table 2S). The regression equation generated through the study is shown in Eq. (5):
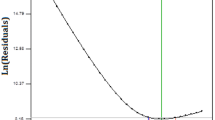
The parity plot, shown in Fig. 1(a), represents the adequacy of the model as the calculated values of % BPA removal (%Rcalc) were found closer to the experimental values (%Rexpt) (Table 2S), which was also confirmed by a normal probability plot, where the normality of the residuals of the data was analyzed (Fig. 1b) (Vijayalakshmi et al. 2018). The results of the second-order response surface model fitting in the form of analysis of variance (ANOVA) are shown in Table 3S. The surface model was significant with F value of 13.29 and a p value < 0.05. The larger F value along with the smaller p value is indicative of the high significance of the corresponding coefficient (Dahiru 2011). The adequacy of the model as indicated by the determination coefficient (R2 = 0.9140) suggested 91.40% of the variability in the response, which was attributed to the independent variables. The value of the adjusted determination coefficient was also very high to advocate for the high significance of the model. The regression coefficients, standard error coefficient, t value and p value for all linear, square and interaction effects of the variables are shown in Table 3S. It showed that the effects of media pH, temperature, and BPA concentration were more significant than those of agitation speed and inoculum volume for the degradation process (Fig. 2). Among these parameters, the linear effects (X1, X2, and X5), the square effects (X32, X42 and X52) and the interactive effects (X1X2) are the influential ones.
Effect of experimental conditions on biodegradation of BPA
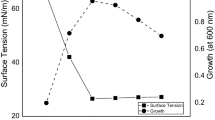
The effect of all the independent factors on BPA degradation (%) is shown in Fig. 3 and the RP-HPLC data, analyzed at optimized conditions at every 24 h interval up to 96 h, are shown in Fig. 4(a) and (b). P. palleronianaGBPI_508 could grow and degrade BPA over a wide range of pH and temperature. Maximum BPA degradation was observed at a media pH 9. The inoculum volume of 10% (v/v) was found best for the degradation study. Lower inoculum volume had decreased the degradation efficiency of P. palleronianaGBPI_508. There was a gradual increase in BPA degradation by P. palleroniana GBPI_508 with the increase in temperature from 15 to 25 °C and the maximum degradation was observed at 25 °C. An agitation speed of 130 rpm was best in terms of degradation potential, and with the decrease or increase in agitation speed, the rate of degradation decreased as shown in Fig. 4(a), and (d).The ability of P. palleroniana GBPI_508 to survive at both high and low BPA concentrations was determined and their behaviors are presented in Fig. 3(h). The bacterial strain was able to degrade 97% of 270 mg L−1 BPA up to 96 h without much supplementation in mineral salt media.
Growth kinetics of Pseudomonas palleroniana (GBPI Hb_508)
The substrate saturation constant (Ks) of 37.5 mg/L, maximum specific growth rate (µmax) of 0.014644 day−1 and regression coefficient (R2) of 0.989 were obtained for the Monod model (Fig. 5 and Table 4S), which shows the efficiency of the model for describing cell growth and nutrient (BPA) uptake by P. palleroniana GBPI Hb_508. The regression coefficient (R2) is believed to be better to detect the suitability of any model for the process if its value is closer to 1 (Annuar et al. 2008; Reardon et al. 2002). Reardon et al. (2002) had also observed a similar type of Ks value during the degradation of BPA using P. putida F1. Thus, the Monod model was able to describe the cell growth and BPA uptake kinetics of the bacterial strain under study in a mineral salt medium.
BPA metabolites produced during biodegradation
BPA was found to be degraded at different conditions and at higher concentration (270 mg/L); maximum removal was observed at 9 pH, 25 °C, 10% (v/v) inoculum volume and 130 rpm agitation speed. The samples were analyzed through mass spectrometry for understanding the intermediate compounds generated during the process. The intermediates identified during BPA biodegradation at 96 h are beta-famesene, chamazulene, bisabolol oxide, Z)-ene-yne-dicyclo ether, hexadecenoic acid, methyl ester, and n-pentadecanoic acid methyl ester along with some content of BPA (Table 2). The intermediate compounds depict the involvement of biotransformation of compounds along with degradation of BPA. Both oxidation and reduction of BPA during degradation can be understood by observing the intermediates; however, the elucidation of the exact mechanism of BPA degradation by this Himalayan strain P. palleroniana GBPI_508 would require the comprehensive expression and functional genetic analysis of strain and its integration with the metabolome data related to the production of different types of catabolic intermediates as identified through the study.
Discussion
The optimum conditions for the degradation of BPA are shown in Fig. 2. The temperature was observed as the most important factor for BPA degradation, followed by BPA concentration, media pH, and inoculum volume. At high BPA concentration, the highest concentration was observed at 9 pH. Vijayalakshmi et al. (2018) had also observed high BPA degradation at higher pH, i.e., 10 pH, where approximately 97% BPA degradation was observed using Pseudomonas aeruginosa. The biodegradation of BPA was highly affected by inoculum volume, indicating the importance of the size of the inoculum in the degradation process. Such conditions possibly minimize the length of the log phase and low number of bacterial cells release less amount of enzymes involved in the degradation process. Inoculum size higher than 10% (v/v) did not increase BPA degradation by P. palleronianaGBPI_508, which might be due to the reduction of dissolved oxygen and increased competition toward nutrients (Eltoukhy et al. 2020). (Zhang et al. (2007) also reported increase in the degradation of BPA with the increasing size of inoculum of Achromobacter xylosoxidans strain B-16 isolated from compost leachate of municipal solid waste.
Temperature is one of the most important parameters that affect any microbial process. The growth rate of microorganisms becomes slow below or above the optimum growth temperature because of a reduced rate of cellular production (Malinverno and Martinez 2015; Margesin and Schinner 1997). The degradation decreased above 25 °C (Fig. 3d). (Eltoukhy et al. (2020) also found similar results during the degradation of BPA using Pseudomonas putida strain YC-AE1 where the maximum degradation was observed between 25 and 30 °C. Thermal stability and activity of enzymes, which are responsible for the degradation capacity of bacteria, are dependent on the temperature due to which there is always an optimum temperature for bacterial activity (Engqvist 2018). Agitation speed affects the degradation rate and the bacterial growth. 130 rpm agitation speed was found suitable in the current study similar to Vaidya et al. (Vaidya et al. 2018), who had reported a similar observation while studying the degradation of polycyclic aromatic hydrocarbon chrysene, where maximum degradation was observed at 150 rpm. Agitation speed affects the oxygen concentration during the degradation process, and oxygen is required for the catalytic activities of bacteria where molecular oxygen is involved in the degradation of compounds (Leahy and Colwell 1990).
Compounds like BPA are present at very low concentration (at ppb level) in the environment (Peng et al. 2015; Yamanaka et al. 2008) and harmful at that level also, although in some areas this compound is also reported at high concentration. Suyamud et al. (2018b) reported the capacity ofBacillus megaterium strain ISO-2, to degrade 5 mg L−1 of BPA within 72 h in mineral salt medium supplemented with yeast extract. Sphingomonas bisphenolicum strain AO1 was reported to degrade 100 mg L−1 BPA to undetectable level within 48 h in minimal medium with 1% glucose (Oshiman et al. (2007). Yu et al. (2019) found approximately 84% BPA degradation up to 72 h while comparing the co-culture of Sphingomonas sp. (Sph-2) and Pseudomonas sp. Although faster BPA degradation rate has generally been reported for microbial consortium as compared to the BPA-degrading lonely strains (Eio et al. 2014), interestingly, P. palleroniana GBPI_508 demonstrated high BPA degradation potential while used as an individual.
P. palleroniana GBPI_508 had shown the production of lipase enzyme in qualitative plate assays in the presence of BPA in MSM agar plate at 25 °C. This indicates the possibility of the involvement of the lipase enzyme in the degradation of BPA or other intermediate compounds. It might be attributed to the fact that the lipase enzyme has the capacity to break the bonds present in close ring structures of organic compound (Karigar and Rao 2011). The bacteria showed negative response for amylase and laccase enzymes. The intermediates produced during the degradation process were found to be different from that reported in earlier studies (Lobos et al. 1992; Masuda et al. 2007; Vijayalakshmi et al. 2018), although the genes responsible for the degradation of BPA, i.e., bisd A and bisd B were found to be present in P. palleronianaGBPI_508 (http://www.ncbi.nlm.nih.gov/BLAST/). It has been reported that bisdA and bisdB genes encoding ferredoxin and cytochrome P450 were responsible for BPA degradation in Sphingomonas bisphenolicum strain AO1 (Sasaki et al. 2005a, 2005b). The cytochrome P450 family of heme monooxygenases is found in virtually all living organisms, these enzymes catalyze the oxidation of a wide range of endogenous compounds in biosynthetic and biodegradation pathways, as well as xenobiotics such as drugs and environmental contaminants (Wong 1998). The bacterial P450s generally utilize an electron transport chain, consisting of an FAD-containing NADH-dependent oxidoreductase, and reduction is mediated by an iron–sulfur (2Fe-2S) ferredoxin (Gray 1992). The presence of these enzymes shows the possibility of their involvement in the degradation of BPA while using P. palleroniana GBPI_508, but the different intermediate compounds show the possibility of different pathways, which needs to be further monitored.
Conclusions
This present study reports the ability of Pseudomonas palleroniana strain (GBP_508) to survive in the presence of higher concentration of BPA in mineral salt media and the best performance was observed at 270 mg L−1 BPA concentration with MSM pH 9 at 25 °C temperature and 130 rpm agitation speed up to 96 h. The GC–MS data and the Monod model equation indicate the possibility of using this bacterial strain in the remediation of BPA at a wide range of variations in the environment. The conditions optimized using Box–Behnken design and an empirical statistical model equation will be useful in further upscaling the process. The identification of new (possible) catabolic intermediates may help in the identification of new genes and pathway(s) involved in BPA degradation in future studies.
References
Almeida S, Raposo A, Almeida-González M, Carrascosa C (2018) Bisphenol A: food exposure and impact on human health. Compr Rev Food Sci Food Saf 17:1503–1517. https://doi.org/10.1111/1541-4337.12388
Annuar MSM, Tan IKP, Ibrahim S, Ramachandran KB (2008) A kinetic model for growth and biosynthesis of medium-chain-length poly-(3 -hydroxyalkanoates) in Pseudomonas putida. Braz J Chem Eng 25:217–228. https://doi.org/10.1590/S0104-66322008000200001
Badiefar L, Yakhchali B, Rodriguez-Couto S, Veloso A, García-Arenzana JM, Matsumura Y, Khodabandeh M (2015) Biodegradation of bisphenol A by the newly-isolated Enterobacter gergoviae strain BYK-7 enhanced using genetic manipulation. RSC Adv 5:29563–29572. https://doi.org/10.1039/c5ra01818h
Bitton G (1994) Wastewater microbiology. Wiley-Liss Inc., Hoboken
Box GEP, Behnken DW (1960) Some new three level designs for the study of quantitative variables. Technometrics 2:455–475. https://doi.org/10.1080/00401706.1960.10489912
Burhan A, Nisa U, Gökhan C, Ömer C, Ashabil A, Osman G (2003) Enzymatic properties of a novel thermostable, thermophilic, alkaline and chelator resistant amylase from an alkaliphilic Bacillus sp. isolate ANT-6. Process Biochem 38:1397–1403. https://doi.org/10.1016/S0032-9592(03)00037-2
Chai W, Handa Y, Suzuki M, Saito M, Kato N, Horiuchi CA (2005) Biodegradation of bisphenol A by fungi. Appl Biochem Biotechnol 120:175–180. https://doi.org/10.1385/ABAB:120:3:175
Corrales J, Kristofco LA, Baylor Steele W, Yates BS, Breed CS, Spencer Williams E, Brooks BW (2015) Global assessment of bisphenol a in the environment: review and analysis of its occurrence and bioaccumulation. Dose-Response 13:1–29. https://doi.org/10.1177/1559325815598308
Dahiru T (2011) P value, a true test of statistical significance? A cautionary note. Ann Ibadan Postgrad Med 6:21–26. https://doi.org/10.4314/aipm.v6i1.64038
Dhakar K, Pandey A (2016) Wide pH range tolerance in extremophiles: towards understanding an important phenomenon for future biotechnology. Appl Microbiol Biotechnol 100:2499–2510. https://doi.org/10.1007/s00253-016-7285-2
Dhakar K, Pandey A (2020) Microbial ecology from the Himalayan cryosphere perspective. Microorganisms 8:1–17. https://doi.org/10.3390/microorganisms8020257
Eio EJ, Kawai M, Tsuchiya K, Yamamoto S, Toda T (2014) Biodegradation of bisphenol A by bacterial consortia. Int Biodeterior Biodegrad 96:166–173. https://doi.org/10.1016/j.ibiod.2014.09.011
Eltoukhy A, Jia Y, Nahurira R, Abo-Kadoum MA, Khokhar I, Wang J, Yan Y (2020) Biodegradation of endocrine disruptor bisphenol A by Pseudomonas putida strain YC-AE1 isolated from polluted soil, Guangdong. China BMC Microbiol. https://doi.org/10.1186/s12866-020-1699-9
Engqvist MKM (2018) Correlating enzyme annotations with a large set of microbial growth temperatures reveals metabolic adaptations to growth at diverse temperatures 06 biological sciences 0605 microbiology 06 biological sciences 0601 biochemistry and cell biology. BMC Microbiol 18:1–14. https://doi.org/10.1186/s12866-018-1320-7
Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão GC, da Silva EGP, Portugal LA, dos Reis PS, Souza AS, dos Santos WNL (2007) Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta 597:179–186. https://doi.org/10.1016/j.aca.2007.07.011
Flint S, Markle T, Thompson S, Wallace E (2012) Bisphenol A exposure, effects, and policy: a wildlife perspective. J Environ Manage 104:19–34. https://doi.org/10.1016/j.jenvman.2012.03.021
Gray RD (1992) The molecular basis of electron transfer in cytochrome P450 enzyme systems. Front Biotransform 7:321–350
Jain R, Pandey A, Pasupuleti M, Pande V (2017) Prolonged production and aggregation complexity of cold-active lipase from Pseudomonas proteolytica (GBPI_Hb61) isolated from cold desert Himalaya. Mol Biotechnol 59:34–45. https://doi.org/10.1007/s12033-016-9989-z
Kamaraj M, Sivaraj R, Venckatesh R (2014) Biodegradation of bisphenol A by the tolerant bacterial species isolated from coastal regions of Chennai, Tamil Nadu, India. Int Biodeterior Biodegrad 93:216–222. https://doi.org/10.1016/j.ibiod.2014.02.014
Karigar CS, Rao SS (2011) Role of microbial enzymes in the bioremediation of pollutants: a review. Enzyme Res. https://doi.org/10.4061/2011/805187
Leahy JG, Colwell RR (1990) Microbial degradation of hydrocarbons in the environment. Microbiol Rev 54:305–315. https://doi.org/10.1128/mmbr.54.3.305-315.1990
Lee E, Ku S, Jung M, Lee S (2019) Simple and rapid detection of bisphenol A using a gold nanoparticle-based colorimetric aptasensor. Food Chem 287:205–213. https://doi.org/10.1016/j.foodchem.2019.02.079
Li G, Zu L, Wong PK, Hui X, Lu Y, Xiong J, An T (2012) Biodegradation and detoxification of bisphenol A with one newly-isolated strain Bacillus sp. GZB: kinetics, mechanism and estrogenic transition. Bioresour Technol 114:224–230. https://doi.org/10.1016/j.biortech.2012.03.067
Lobos JH, Leib TK, Su TM (1992) Biodegradation of bisphenol A and other bisphenols by a gram-negative aerobic bacterium. Appl Environ Microbiol 58:1823–1831
Malinverno A, Martinez EA (2015) The effect of temperature on organic carbon degradation in marine sediments. Sci Rep 5:1–10. https://doi.org/10.1038/srep17861
Margesin R, Schinner F (1997) Effect of temperature on oil degradation by psychrotrophic yeast in liquid culture and in soil. FEMS Microb Ecol 24:243–249
Masuda M, Yamasaki Y, Ueno S, Inoue A (2007) Isolation of bisphenol A-tolerant/degrading Pseudomonas monteilii strain N-502. Extremophiles 11:355–362. https://doi.org/10.1007/s00792-006-0047-9
Monod J (1949) The growth of bacterial cultures. Ann Rev Microbiol 3(1):371–394
Nicole M, Chamberland H, Geiger JP, Lecours N, Valero J, Rio B, Ouellette GB (1992) Immunocytochemical localization of laccase L1 in wood decayed by Rigidoporus lignosus. Appl Environ Microbiol 58:1727–1739. https://doi.org/10.1128/aem.58.5.1727-1739.1992
Oshiman KI, Tsutsumi Y, Nishida T, Matsumura Y (2007) Isolation and characterization of a novel bacterium, Sphingomonas bisphenolicum strain AO1, that degrades bisphenol A. Biodegradation 18:247–255. https://doi.org/10.1007/s10532-006-9059-5
Pandey A, Jain R, Sharma A, Dhakar K, Kaira GS, Rahi P, Dhyani A, Pandey N, Adhikari P, Shouche YS (2019) 16S rRNA gene sequencing and MALDI-TOF mass spectrometry based comparative assessment and bioprospection of psychrotolerant bacteria isolated from high altitudes under mountain ecosystem. SN Appl Sci 1:278. https://doi.org/10.1007/s42452-019-0273-2
Peng YH, Chen YJ, Chang YJ, Shih YH (2015) Biodegradation of bisphenol A with diverse microorganisms from river sediment. J Hazard Mater 286:285–290. https://doi.org/10.1016/j.jhazmat.2014.12.051
Reardon KF, Mosteller DC, Rogers JB, DuTeau NM, Kim KH (2002) Biodegradation kinetics of aromatic hydrocarbon mixtures by pure and mixed bacterial cultures. Environ Health Perspect 110:1005–1011. https://doi.org/10.1289/ehp.02110s61005
Ronen Z, Abeliovich A (2000) Anaerobic-aerobic process for microbial degradation of tetrabromobisphenol A. Appl Environ Microbiol 66:2372–2377. https://doi.org/10.1128/AEM.66.6.2372-2377.2000
Sakai K, Yamanaka H, Moriyoshi K, Ohmoto T, Ohe T (2007) Biodegradation of bisphenol A and related compounds by Sphingomonas sp. strain BP-7 isolated from seawater. Biosci Biotechnol Biochem 71:51–57. https://doi.org/10.1271/bbb.60351
Sasaki M, Akahira A, Oshiman KI, Tsuchido T, Matsumura Y (2005a) Purification of cytochrome P450 and ferredoxin, involved in bisphenol A degradation, from Sphingomonas sp. strain AO1. Appl Environ Microbiol 71:8024–8030. https://doi.org/10.1128/AEM.71.12.8024-8030.2005
Sasaki M, Maki JI, Oshiman KI, Matsumura Y, Tsuchido T (2005b) Biodegradation of bisphenol A by cells and cell lysate from Sphingomonas sp. strain AO1. Biodegradation 16:449–459. https://doi.org/10.1007/s10532-004-5023-4
Staples CA, Dorn PB, Klecka GM, O’Block ST, Harris LR (1998) A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 36:2149–2173. https://doi.org/10.1016/s0045-6535(97)10133-3
Suyamud B, Inthorn D, Panyapinyopol B, Thiravetyan P (2018a) Biodegradation of Bisphenol A by a newly isolated Bacillus megaterium strain ISO-2 from a polycarbonate industrial wastewater. Water Air Soil Pollut 229:347–359. https://doi.org/10.1007/s11270-018-3983-y
Suyamud B, Inthorn D, Panyapinyopol B, Thiravetyan P (2018b) Biodegradation of bisphenol A by a newly isolated Bacillus megaterium strain ISO-2 from a polycarbonate industrial wastewater. Water Air Soil Pollut 229:348–359. https://doi.org/10.1007/s11270-018-3983-y
Vaidya S, Devpura N, Jain K, Madamwar D (2018) Degradation of chrysene by enriched bacterial consortium. Front Microbiol 9:1–14. https://doi.org/10.3389/fmicb.2018.01333
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV (2007) Human exposure to bisphenol A (BPA). Reprod Toxicol 24:139–177. https://doi.org/10.1016/j.reprotox.2007.07.010
Vijayalakshmi V, Senthilkumar P, Mophin-Kani K, Sivamani S, Sivarajasekar N, Vasantharaj S (2018) Bio-degradation of bisphenol A by Pseudomonas aeruginosa PAb1 isolated from effluent of thermal paper industry: kinetic modeling and process optimization. J Radiat Res Appl Sci 11:56–65. https://doi.org/10.1016/j.jrras.2017.08.003
Wong L-L (1998) Cytochrome P450 monooxygenases. Curr Opin Chem Biol 2:263–268. https://doi.org/10.1101/gad.986602.te
Yamanaka H, Moriyoshi K, Ohmoto T, Ohe T, Sakai K (2008) Efficient microbial degradation of bisphenol A in the presence of activated carbon. J Biosci Bioeng 105:157–160. https://doi.org/10.1263/jbb.105.157
Yu K, Yi S, Li B, Guo F, Peng X, Wang Z, Wu Y (2019) An integrated meta-omics approach reveals substrates involved in synergistic interactions in a bisphenol A (BPA)—degrading microbial community. Microbiome 7:1–13
Zhang C, Zeng G, Yuan L, Yu J, Li J, Huang G, Xi B, Liu H (2007) Aerobic degradation of bisphenol A by Achromobacter xylosoxidans strain B-16 isolated from compost leachate of municipal solid waste. Chemosphere 68:181–190. https://doi.org/10.1016/j.chemosphere.2006.12.012
Acknowledgements
Authors are grateful to the Director GBP-NIHE for extending their facilities and the Department of Science and Technology-Water Technology Initiative (DST-WTI) [DST/TM/WTI/2K15/63C] for financial support. We are also thankful to AIRF-JNU for providing facilities for GC–MS analysis.
Funding
This research work was supported by Department of Science and Technology-Water Technology Initiative, DST/TM/WTI/2K15/63C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thathola, P., Agnihotri, V., Pandey, A. et al. Biodegradation of bisphenol A using psychrotolerant bacterial strain Pseudomonas palleroniana GBPI_508. Arch Microbiol 204, 272 (2022). https://doi.org/10.1007/s00203-022-02885-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02885-y