Abstract
Probiotics are known to human kind since ages as they are important component in fermented milk products, however the use of probiotics in non-dairy product is a novel method for the delivery of probiotics. Delivery of probiotics through non-dairy products will be beneficial for consumers who are lactose intolerant who are deprived of benefits of probiotics by dairy products. This studies aim at developing novel vegetable juices containing probiotic bacteria. Three different strains of bacteria have been used, i.e. Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus delbrueckii in carrot, beetroot and tomato juice. The viability of the bacteria has been checked after a specific duration of time of fermentation by Koch’s plate count method. The vegetable used for juices (carrot, beetroot, tomato) consist of high amount of antioxidants like carotenoids in carrot, betaxanthins and betacyanins in beetroot, lycopene in tomato. These antioxidant provide numerous health benefits to human body. The antioxidant activity in the juices has been checked before and after fermentation by HPLC and spectroscopic methods. The three bacterial strains Lactobacillus plantarum, Lactobacillus acidophilus and Lactobacillus delbrueckii used in three types of juices including tomato juice, carrot juice and beetroot juice showed good growth except Lactobacillus acidophilus due to reasons like insufficient nutrients. The amount of sugars and acids of the three juices indicated that the fermentation process takes place at a good and satisfying rate. This product will be especially useful for the people who are lactose intolerant who cannot intake probiotics via milk and milk products. Vegetable juices also have almost zero fat content and high in fiber so the people who are on a fat free diet can consume this product.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotics can be defined as the live microorganisms which when consumed in adequate amount give various health benefits to the host (Butel 2013).
Dairy products such as yogurt, fermented sour milk and cheese are the pioneers in probiotic dairy products. The delivery of probiotics through dairy products helps them in tolerating harsh gastro intestinal conditions (Randheera et al. 2017). Some probiotic species like L. gallinarum PL53, L. paracasei PL120 and L. gallinarum PL149 have shown anti-campylobacter activity in vitro, while L. gallinarum PL149 showed good activity after adjusting to pH and good tolerance to bile salt compared to other isolates (Khan et al. 2020a). Apart from humans, probiotics are used in animals as well to increase the yield of animal products P. acidilactici strain isolated from Nilli Ravi buffalo ruminal gut can be used as animal probiotic (Khan et al. 2020b). Probiotics are isolated from various medium like Poultry dropping, fermented foods (yogurt and pickles) and human faeces (Khan et al. 2020).
Dairy products are not always fit for probiotic intake as many people are intolerant to lactose. To overcome this issue researches are being undertaken to provide probiotics through non-dairy mediums. One of the mediums is via vegetable and fruit juices. B. animalis subsp. lactis Bb12 encapsulated in a milk protein matrix are good in maintaining sensory properties in pineapple juice and pineapple juice is well suited for their viability (Horáčková et al. 2018). L. paracasei NFBC43338 is pressure as well as acid tolerant strain when used in orange and pineapple juice (Sheehan et al. 2007). Most often the fruits and vegetables are affected with post-harvest diseases which might leave fruits unfit for consumption. Soft rot affects Peaches. CH-Fe2O3 nano particles are effective in inhibiting soft rot in Peaches. 1% CH-Fe2O3 prevents fungal growth and stops respiration due to chitosan surface layer which results into reduced weight loss of fruit (Saqib et al. 2020). The CH-Fe2O3 nanoparticles are also effective against phytopathogen (Rhizopus oryzea), they restrained the fungal growth and spore formation with increase in chitosan concentration from 0.25 to 1% (Saqib et al. 2020). Nanoparticles such as CH-Fe2O3 are found effective in controlling pre and post-harvest diseases which can facilitate to prevent the fruit from losses. Antioxidants assist in quenching all of the body's inevitably free radicals, thereby improving health by lowering the risk of various diseases such as cancer and also helps in treating acute central nervous system injury (Hamid et al. 2010). Beetroots are one of the rich sources of antioxidants, one beetroot juice shot (70 ml) was found to contain high Total Antioxidant Capacity (TAC) (697.9 ± 1.6 μmol/70 ml) and Total Polyphenol (TP) content (68.4 ± 0.3 mg GAE/70 ml) (Wootton-Beard and Ryan 2011). Noni (Morinda citrifolia) juice fermented with B. longum exhibited a higher antioxidant capacity than unfermented juice (Wang et al. 2009). The fermented pomegranate juice exhibited an increased antioxidant capacity (Mousavi et al. 2013). Some researchers have also stated that L. acidophilus increased juice antioxidant activity more than L. plantarum (Fernandes Pereira and Rodrigues 2018). Apart from being rich in antioxidant lycopene tomato is an ideal model plant for studying climacteric fruit ripening. Overexpression of Solanum melongena COP1 (SmCOP1) in tomato delays fruit ripening by three to six days compared to wild type fruits (Naeem et al. 2019). Graphene oxide can increase tomato yield when used in correct concentration. It advances morphological development and biomass accumulation in the roots and shoots/stems of tomato seedlings and mature plants by upregulating the genes related to root development and auxin content (Guo et al. 2021). Carrot juice is high carotenoids. Carrot juice fermented with three Bifidobacterium strains (B. lactis Bb-12, B. bifidum B7.1 and B3.2) showed biochemical activities without nutrient supplementation; the strains produced excellent amount of lactic acid (15–17 mg/ml); however; during fermentation, 15–45% of carotenoids (α-carotene and β-carotene) were degraded depending on the strain used (Kun et al. 2008).
Lactobacillus plantarum, L. acidophilus, L. delbrueckii belong to the family of gram-positive bacteria. L. plantarum has the ability to survive gastric transit and colonize the gut and is safe to the consumer (de Vries et al. 2006). They also show high tolerance to industrial conditions and have capacity to survive at extended shelf life at refrigerated temperatures. In addition, L. plantarum show growth ability in the presence of preservatives used in food industries e.g., NaCl (Georgieva et al. 2009). A potential probiotic bacterium Lactobacillus plantarum PH04 has cholesterol-lowering effects (Nguyen et al. 2007). L. acidophilus has been isolated from meat, milk, fruits, vegetables and cereal products as it is a natural contaminant. It also possesses various antimicrobial compounds (Anjum et al. 2014). L. acidophilus 1 strain isolated from different sources of honey demonstrated antibacterial properties and are stable at low pH (3 and 5) and high temperature (90 and 121 °C) (Aween et al. 2012). The lactic acid bacteria L. casei, L. plantarum, and L. delbrueckii synthesize cells and produce lactic acid without nutrients in cabbage juice. L. casei, L. plantarum, and L. delbrueckii survive at low high acidic conditions in fermented cabbage juice during cold storage at 4 °C (Yoon et al. 2006).
Thus probiotics are essential for enhancing the shelf life and quality of the fruit juices. Therefore, it is necessary to evaluate the viability, antioxidant potential and chemical compounds for lactose intolerant consumers.
This study aims at developing novel vegetable juices containing probiotic bacteria. Three different strains of bacteria have been used, i.e. Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus delbrueckii in carrot, beetroot and tomato juice. The viability of the bacteria has been checked after a specific duration of time of fermentation by Koch’s plate count method. The vegetable used for juices (carrot, beetroot, tomato) consist of high amount of antioxidants like carotenoids in carrot, betaxanthins and betacyanins in beetroot, lycopene in tomato. These antioxidants provide numerous health benefits to human body. The antioxidant activity in the juices has been checked before and after fermentation by HPLC and spectroscopic methods. This product will be especially useful for the people who are lactose intolerant and who cannot intake probiotics via milk and milk products. Vegetable juices also have almost zero fat content and are high in fiber so the people who are on a fat-free diet can consume this product.
Materials and methods
Materials
Lactobacillus plantarum (Poznan University of Life Sciences, Department of Fermentation and Biosynthesis), Lactobacillus acidophilus (Poznan University of Life Sciences, Department of Fermentation and Biosynthesis), Lactobacillus delbrueckii (Poznan University of Life Sciences, Department of Fermentation and Biosynthesis), MRS broth (BTL, Poland), MRS agar (BTL, Poland), 0.85% sodium chloride (POCh, Poland), 1% metaphosphoric acid 5% ditiotreithol, Acetone, Acetone for HPLC, ABTS, potassium persulphate, methanol, Trolox.
Preparation of MRS broth and inoculum
100 ml of MRS broth was prepared for inoculation of bacteria. 5.12 g was taken in a bottle and filled up to the volume 100 ml; 10 ml was taken in tubes and autoclaved at 121 °C for 15 min. After sterilization it was cooled. Strains of Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus delbrueckii have been taken from freezer and added to the MRS broth medium. The above strain was incubated at 30 °C for 24 h and 37 °C for 48 h, respectively. The inoculum was centrifuged at 1188×g for 10 min, The excess broth was discarded and the bacterial culture were washed with 0.85% NaCl, 5 ml of 0.85% NaCl has been added to the culture and mixed, The bacterial culture was ready to be used in the vegetale juices.
Preparation of juices
Three types of juices were used in this experiment: tomato juice, beetroot juice and carrot juice. Tomato juice was readily brought from the market which was already sterilised. Beetroot and carrot have been peeled and juices have been prepared in the laboratory using juicer. The latter two juices were taken in a volume of 200 ml in glass bottles. Beetroot juice has been pasteurized at 80 °C for 10 min, whereas carrot juice has been pasteurized at 70 °C for 10 min (one of the reasons for the decrease in the amount of vitamin C could be the fact that pasteurization at 80 °C causes decline in the amount of vitamin C content). 200 ml of tomato juice from the package was transferred into sterile glass bottles under sterile conditions. Samples of fresh juices have been taken for antioxidant analysis.
Incubation of juices
Each type of juice had three replication of 200 ml in glass bottles for three strains of bacteria Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus delbrueckii. 5 ml of inoculum of all the strains from saline solution was added to each type of juice sample. Samples with Lactobacillus plantarum were incubated for 24 h and 48 h at 30 °C whereas samples with Lactobacillus acidophilus and Lactobacillus delbrueckii were incubated at 37 °C for 24 h and 48 h. Juice samples were taken after 24 h and 48 h for bacterial count and chemical analysis of antioxidants.
Koch’s method for total viable bacterial cells counting for juices
The juice samples which were taken after incubation have been serial diluted in 0.85% NaCl solution in 1:9 ratio. The saline solution were taken in tubes and sterilised before use. All the equipment like pipette, tubes which were used for experiments were sterilised. MRS agar has been weighed and prepared in 500 ml bottle in 70% of the total volume of the bottle. MRS agar has been sterilised for 15 min and was left for cooling. Petri plates have been named according to the samples. After serial dilution 1 ml of sample has been poured on the plate using pipette and MRS agar has been added. The plates were left to cool for some time then they were incubated for 48 h. Lactobacillus plantarum has been incubated at 30 °C while Lactobacillus acidophilus and Lactobacillus delbrueckii have been incubated at 37 °C for 48 h. After 48 h the colony forming unit has been calculated. The entire experiment has been carried out in a completely sterile condition.
Preparation of HPLC samples for vitamin C
10 ml of sample has been taken in the tubes (three replications) and 25 ml 1% metaphosphoric acid has been added to it. The sample has been homogenized for 1 min; after homogenization it has been centrifuged for 15 min at 1545×g. The extract has been transferred to 50 ml volumetric flask and made up to the volume by 1% metaphosphoric acid. 5 ml of the prepared extract has been transferred to 10 ml volumetric flask, 5% 1 ml dithiothreitol has been added and made up the volume by 1% metaphosphoric acid. The prepared sample has been filled in the HPLC vials using filter (Kurilich et al. 2002).
Preparation of HPLC samples for carotenoids and lycopene
10 g of sample has been weighed into a centrifuge tube (three replications). 20 ml of acetone has been added to each of the three tubes and homogenized for 5 min. After homogenization it has been centrifuged for 15 min at 1545×g. The supernatant has been filtered through a filter paper using buchner funnel and transferred into the round bottom flask and evaporated. After the evaporation the extract was obtained using acetone for HPLC. The extract has been quantitatively transferred to 10 ml volumetric flask and made up to the volume by acetone for HPLC. The extract has been filtered by 0.45 µm PTFE filter and filled in HPLC vials (de Sá and Rodriguez-Amaya 2004).
Determination of changes in the content of metabolites and sugars using high-performance liquid chromatography (HPLC)
Preparation of the samples for analysis
The juice samples to measure the metabolites were diluted 10 times while the vegetable juice samples which were brought directly from the market were diluted 20 times. The samples were filtered using 0. 45 μm diameter filters (Millipore, USA).
Parameters of the liquid chromatograph
-
eluent: 0.005 M H2SO4,
-
column temperature: 50 °C,
-
flow: 0.5 ml/min,
-
Phenomenex USA column for acid and sugar analysis—Rezex Polymer Based Column.
Analysis of antioxidant activity
ABTS solution has been prepared by dissolving ABTS in water to a 7-Mm concentration. ABTS radical cation has been prepared by reacting ABTS stock solution with 2.45 Mm potassium persulfate. The mixture has been allowed to stand in a dark room for 12–16 h (Re et al. 1999). Trolox mixture was prepared by 1 mg/ml concentration in methanol. Different dilutions of 100 µg, 150 µg, 200 µg, 250 µg, 300 µg and 400 µg of Trolox mixture were prepared. ABTS was diluted with methanol by 1:40 ratio. Initially the absorbance of ABTS was measured to be in the range of 0.7. Samples have been prepared from each dilution by taking 3 ml of ABTS in tubes and adding 30 µl of each dilution of Trolox. The samples have been allowed to stand in the dark for 6 min. Absorbance has been measured immediately at 734 nm.
1 N HCl has been prepared to extract antioxidants in beetroot juice. 1 N HCl has been added to the juice samples to acidify to pH 3.0 and was left at 4 °C overnight. The weight of the samples was measured before addition. The samples have been centrifuged at 12000×g for 20 min at 4 °C. 3 ml of ABTS solution has been taken in a tube and 30 µl of supernatant from the extracted beetroot juice has been added to the tube. The solution has been allowed to stand in the dark for 6 min. Absorbance has been measured immediately at 480 and 535 nm for betaxanthins and betacyanin, respectively.
For measuring the antioxidant capacity of tomato and carrot juice samples of tomato and carrot juice have been weighed and have been centrifuged at 1188×g. for 15 min. At first ABTS analysis has been carried out without extraction by taking 3 ml of ABTS solution in a tube and adding 30 µl of supernatant from each sample. Absorbance has been measured at 734 nm. For extraction 70% methanol has been prepared. Juice samples have been centrifuged and supernatant from the carrot juice has been discarded only the pellet was retained while tomato juice samples were used as it is. Samples have been prepared my adding 70% methanol twice the amount of the juice sample w/v and have been left for overnight extraction for 12 h on rolling shaker. ABTS analysis has been carried out by taking 3 ml of ABTS and adding 30 µl of juice sample and were kept in dark for 6 min. Absorbance has been measured at 734 nm immediately.
Results and discussion
Analysis of bacteria growth during juices fermentation and storage in 4 °C
From Fig. 1 it can be seen that the bacterial growth is quite high after 24 h in carrot juice compared to other two juices. However bacterial growth decreases in carrot juice after 24 h while it increases in tomato and beetroot juices. As observed by Panghal et al. (2017) the decrease in the amount of lactic acid bacteria is mainly due to the decrease in the amount of sugar level, as sugar level decreases due to fermentation. Table 1 represents the colony-forming unit of Lactobacillus plantarum and Lactobacillus delbrueckii, respectively, in three types of juices including tomato juice, carrot juice and beetroot juice. The results for Lactobacillus acidophilus could not be obtained as bacterial growth could not be observed on the culture plates. Figure 1 represents the growth of Lactobacillus delbrueckii in tomato, carrot and beetroot juices at the start of fermentation, 24 h, 48 h and storage at 4 °C. As it is observed in tomato juice the bacterial growth gradually increases after 24 h and is quite constant even after refrigeration at 4 °C so it can be said that tomato juice is optimum for lactic acid fermentation of Lactobacillus delbrueckii. Similar to tomato juice beetroot juice also showed considerable amount of growth though after 48 h it decreased as in beetroot juice the sugar levels decreased which didn’t provide appropriate environment for lactic acid fermentation. In carrot juice the amount of bacteria increased and was stable up to 48 h however it decreased due to contamination of juice.
Comparison of different bacterial strains in juices
Figure 1 shows the activity of different strains of bacteria in carrot juice and it can be seen that the strain of L. plantarum has a steady growth till 24 h and it declines after 48 h and is stable at 4 °C, while L. delbreuckii shows a good growth up to 48 h. The decrease in the amount of L. delbreuckii at 4 °C can be due to contamination of the juice. Overall, on comparing both the bacterial strains it can be said that both the strains are suitable for fermentation of carrot juice if there is no contamination. From Fig. 1 and Table 1 it can be seen that L. plantarum shows positive growth up to 48 h, whereas L. delbreuckii showed good growth only up to 24 h and decreased after 48 h which says that for beetroot juice L. plantarum is more suitable for fermentation of beetroot juice than L. delbreuckii. Both the juices show decline after cold storage so similar to tomato juice, beetroot juice also cannot be stored for long at 4 °C to maintain the viability of the bacteria.
Analysis of bioactive components in juices
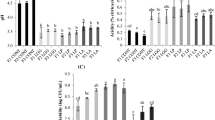
Figure 2 describes the carotenoid amount in carrot juice as it is seen the amount of β—carotene is highest in fresh carrot juice as it is without treatment; however, the amount decreases to half. The important reason for the loss could be because of the high temperature of pasteurization. Because the juice containing L. acidophilus and L. delbrueckii pasteurized at 70 °C shows stable activity compared to L. plantarum. So the optimum temperature for pasteurization of carrot juice is 70 °C.
Comparison of carotenoids activity in carrot juice during 48 h fermentation (1. Fresh juice 2. Juice fermented with Lactobacillus plantarum for 24 h 3. Juice fermented with Lactobacillus plantarum for 48 h 4. Fresh Juice 5. Juice fermented with Lactobacillus acidophillus for 24 h 6. Juice fermented with Lactobacillus acidophillus for 48 h 7. Fresh juice 8. Juice fermented with Lactobacillus delbreuckii for 24 h, 9. Fresh juice 8. Juice fermented with Lactobacillus delbreuckii for 48 h)
On the other hand, lutein and α—carotene show decrease in the amount after 24 h and 48 h, whereas the juice containing L. delbrueckii show an increase in the amount of α—carotene after 48 h also juice with L. delbrueckii and L. acidophilus show stable activity of carotenoids. This shows that L. delbrueckii and L. acidophilus can be used as probiotics strains in carrot juice.
On comparing the vitamin C amount in carrot juice, it has been observed that in Lactobacillus plantarum the amount of vitamin C is gradually decreased after 24 h of fermentation whereas after 24 h it is stable. The carrot juice with Lactobacillus plantarum was pasteurized at 80 °C, so one of the reasons for the decrease in the amount of vitamin C could be the fact that pasteurization at high temperature causes decline in the amount of vitamin C content (Figs. 3 and 4).
Comparison of Vitamin C in carrot juice during 48 h fermentation with different strains of lactic acid bacteria (1. Fresh juice 2. Juice fermented with Lactobacillus plantarum for 24 h 3. Juice fermented with Lactobacillus plantarum for 48 h 4. Fresh Juice 5. Juice fermented with Lactobacillus acidophillus for 24 h 6. Juice fermented with Lactobacillus acidophillus for 48 h 7. Fresh juice 8. Juice fermented with Lactobacillus delbreuckii for 24 h 9. Fresh juice 8. Juice fermented with Lactobacillus delbreuckii for 48 h)
Comparison of the most abundantly found components lutein and lycopene in tomato juice during 48 h fermentation with different strains of lactic acid bacteria (1. Fresh tomato juice to be fermented with Lactobacillus acidophillus 2. Tomato juice fermented with Lactobacillus acidophillus for 24 h 3. Tomato juice fermented with Lactobacillus acidophillus for 48 h 4. Fresh tomato juice to be fermented with Lactobacillus delbreuckii 5. Tomato juice fermented with Lactobacillus delbreuckii for 24 h 6. Tomato juice fermented with Lactobacillus delbreuckii for 48 h
The juice containing Lactobacillus acidophilus shows increase in the amount of vitamin C in carrot juice after 48 h compared to fresh juice. This is an indication that Lactobacillus acidophilus is the appropriate lactic acid bacteria for fermentation of carrot juice. On the other hand, juices containing Lactobacillus delbrueckii showed a good amount of increase in juice after 24 h but the amount decreased after 48 h. Another reason for the decrease in the amount of vitamin C could be that vitamin C is a water-soluble vitamin and water-soluble vitamin tend to degrade over a period of time if kept in a water medium like juices.
Overall it can be said that Lactobacillus acidophilus gives the best results for fermentation of juice containing vitamin C and also Lactobacillus delbrueckii if it is fermented only up to 24 h. If we consider Lactobacillus plantarum the amount of vitamin C is stable after 24 h of fermentation i. e. if we pasteurize the juice at a lower temperature like 70 °C like the other two juices it shall give good results as well.
Vitamin C is the major water-soluble antioxidant and acts as a radical scavenger. The intake of fresh vegetables, containing vitamin C along with many other vitamins and micronutrients, can reduce incidence of various cancers (Chambial et al. 2013).Vitamin C increases the absorption of iron, boost the immune system, and participates in many physiological functions. Vitamin C as well as B vitamins can be easily leached or destroyed during cooking and food processing. Therefore, the introduction of fermented, non heat-treated products in a diet can have substantial advantages. Such fermented products are a valuable source of vitamin C, in which its strongly acidic medium protects the products from oxidation. Content of vitamin C in various foods is relatively high (10–100 mg/100 g), and in some cases, it reaches units of grams per 100 g. This is possibly related to the fact that vitamin C is formed from sugars, which are common compounds in different organisms. Peñas et al. (2010) state that the content of vitamin C in fermented cabbage (sauerkraut) is even higher than in most fresh vegetables (Korus et al. 2021). Vitamin C has antioxidant activity, and the content of vitamin C at the end of fermentation is equivalent to twice that of the original juice. After fermentation, the antioxidant activity of the juice increases as it has been reported in fermented apple juice (Li et al. 2021).
In tomato juice only the juice containing Lactobacillus acidophilus and Lactobacillus delbrueckii was observed due to the lack of amount of sample of Lactobacillus plantarum it cannot be tested (Tables 2, 3, 4 and 5).
Tomato is the main source of lycopene among all the other fruits and vegetables. Fermentation does affect the amount of lycopene in the juices. As it is seen tomato juice fermented with lactic acid bacteria Lactobacillus acidophilus and Lactobacillus delbrueckii, the amount of lycopene is decreased after a period of fermentation. The fermentation is carried out between the temperature range 36–37 °C and lycopene is proven to be inhibited at the temperatures above 32 °C. However, comparatively the juice containing Lactobacillus delbrueckii showed better stability of lycopene than Lactobacillus acidophilus.
Lutein is the second most commonly found component in tomato juice; however, the quantity of lutein is very less as compared to lycopene. Lutein showed a varied activity in both the types of juices. It is observed that the amount of lutein increased after 24 h in both the juices and later decreased after 48 h.
Analysis of sugars and acids in juices
If all the above juices are taken into consideration it can be seen that only juice containing beetroot and mixd vegetable juice contain sugars. Other juices do not show any traces of sugar content but only acids. According to Priecina and Karklina (2015) the acids present in the vegetable juices play a very important role when it comes to processing of the juices. These acids reduce the processing time of the vegetable and vegetable-based products. They also play a vital role in inhibiting the growth of sprouts that can survive temperature based treatments. The presence of acids in vegetable decreases the sterilization time as microorganisms have lower heat resistance at high acidity. Acids in combination with sugars have a higher potential during food processing and sterility.
On comparing the metabolite activity in tomato juice it was observed that the major change was in the content of glucose. In fresh tomato juice the amount of glucose was considerably high but after fermentation with lactic acid bacteria the amount was gradually decreased, whereas the amount of lactic acid showed an increase. According to El-Sayed et al. (2018) bacterial growth and organic acid production result in the changes in reducing sugars. These changes occur during 30 h and 48 h of fermentation. The selected strains of L. acidophilus use the sugars like fructose and glucose as a carbon source for acid production due to this reason a significant decrease in the amount of sugars is observed (Tables 6 and 7).
The results for metabolite activity in carrot juice show that fresh carrot juice and pasteurized carrot juice show little amount of lactic acid with fermentation with lactic acid bacteria or there is a possibility that since citric acid and lactic acid have similar peak values the determination couldn’t been accurate (Tables 6 and 7). Also it is observed that the amount of citric acid and lactic acid is depleted after fermentation whereas the amount of sugars increases. According to Kun et al. (2008) who worked with fermentation of carrot juice with Bifidobacterium which show that the amount of sucrose and glucose were utilized by the bacteria as carbon source for growth while the other sugars did not show any change. Similarly in juices fermented with L. plantarum, L. acidophilus and L. delbreuckii it can be seen that the amount of fructose is decreased while other sugars is increased so it can be said that fructose is a good source of carbon for these three strains of bacteria.
The results for fermented beetroot show similar changes: the sugar level in the beetroot juice show decline in amount after fermentation (Tables 6 and 7). According to Panghal et al. (2017) the strains of Lactobacillus rhamnnosus, Lactobacillus plantarum and Lactobacillus delbreuckii, which were used to ferment beetroot juice showed a significant decline in the sugar levels as the sugars were being used as a carbon source by the bacteria.
Fermentation of the commercial fruits and vegetable juice promoted considerable changes such as: a decrease in sugar content, and increase in acidity, total phenols, carbon dioxide and organic acids (lactic acid, acetic acid, and succinic acid). The content of reducing sugars declines because probiotics need sugar metabolism for energy to maintain their own growth and reproduction during fermentation, and reducing sugars are continuously consumed as fermentation substrates.
The antimicrobial effect of juices was mainly related to production of lactic acid and other organic acids such as acetic acid, propionic acid, phenyllactic acid, formic acid, and succinic acid by lactic acid bacteria. The mode of action of organic acids is the reduction of pH in the environment, causing inhibition of several microorganisms. Moreover, the antimicrobial effects of organic acids are associated with their undissociated form. Kimchi can be recommended for consumption as a source of dietary fiber, ash, vitamin C and B, and high amounts of phenolic content. Therefore, consumption of such products with a substantial amount of health-promoting compounds can contribute to the improvement of well-being, condition, and overall health (Wang et al. 2021).
Results for ABTS analysis
Figure S13 gives the information about the antioxidants amount of betaxanthins and betacyanins in fermented beetroot juice. According to Panghal et al. (2017) the fermented beetroot juice showed an increase in the antioxidant amount by 3% due to microbial hydrolysis reaction which lead to an increase in phenols and flavonoids. In the figure S13 the amount of betaxanthin does not show positive results as the is a decrease in amount after fermentation. We can say that fermentation is not favorable for this compound. On the contrary if figure S13 is observed which shows the activity for betacyanin, the activity is increased after fermentation. This indicates that fermentation is good for this component. Also according to Panghal et al. (2017) fermentation results in the structural disintegration of cell wall which results in the synthesis of different antioxidants. Antioxidants possess free radical scavenging capacity and they have good health benefits, so this probiotic drink is beneficial (Tables 8, 9, 10, 11 and 12).
The figures S14 show the antioxidant activity in carrot juice before and after extraction a rise in the antioxidant activity can be seen after fermentation. As discussed earlier, the antioxidant activity increases after fermentation. However, after pasteurization process the activity decreases, which might be due to heat processing which affects the antioxidant activity; however, it increases after fermentation.
The figure S14 show the antioxidant activity in tomato juice before and after extraction and the results show that the antioxidant activity is better in the juice without extraction. There is a loss of antioxidants after extraction process, which can be due to the chemicals used for extraction which caused the loss. Because the antioxidant activity in the figure S14 increase after fermentation which is positive.
The advantages of food fermentation are as follows: renders foods resistant to microbial spoilage and the development of food toxins makes foods less likely to transfer pathogenic microorganisms, generally preserves foods between the time of harvest and consumption, modifies the flavour of the original ingredients and often improves nutritional value. Many studies have linked consumption of fruits and vegetables with a reduction of the risk for several chronic diseases, such as cancer, cardiovascular diseases, cataracts, or immune dysfunction. These natural protective effects have been attributed to the antioxidant potential of several components, such as carotenoids, betalains, vitamins, polyphenols, and other phytochemicals (Parades et al. 2022).
Conclusions
The three bacterial strains Lactobacillus plantarum, Lactobacillus acidophilus and Lactobacillus delbrueckii used in three types of juices including tomato juice, carrot juice and beetroot juice showed good growth except Lactobacillus acidophilus due to reasons like insufficient nutrients and contamination. Among the other two strains Lactobacillus plantarum and Lactobacillus delbrueckii, Lactobacillus plantarum showed consistent positive growth in all the three types of juices. So the most suitable bacterial strain for fermenting vegetable juices was Lactobacillus plantarum.
The amount of carotenoids in carrot juice has decreased after fermentation which is not positive but they are not depleted completely and show satisfying amount. On the other hand, there is an increase in the amount of vitamin C which is a good indicator as vitamin C acts as an immunity booster.
The amount of sugars and acids of the three juices indicated that the fermentation process takes place at a good and satisfying rate. As the consumption of sugars shows a positive activity in the juices. Similarly for antioxidant activity, the increase in amount of antioxidant in the juices shows that along with the benefits provided by the lactic acid bacteria antioxidant also plays an important role in providing health benefits.
As an overall conclusion it can be said that like traditionally used probiotic dairy products, the nondairy products with probiotic properties have tremendous potential of highly nutritious health drink providing additional health benefits which cannot be found in some dairy products. This product can be used by all the groups, especially who are lactose intolerant.
References
Anjum N, Maqsood S, Masud T, Ahmad A, Momin A (2014) Lactobacillus acidophilus: Characterization of the species and application in food production. Crit Rev Food Sci Nutr 54(9):1241–1251. https://doi.org/10.1080/10408398.2011.621169
Aween MM, Hassan Z, Muhialdin BJ, Noor HM, Eljamel YA (2012) Evaluation on antibacterial activity of Lactobacillus acidophilus strains isolated from honey. Am J Appl Sci 9(6):807–817. https://doi.org/10.3844/ajassp.2012.807.817
Butel MJ (2013) Probiotics, gut microbiota and health. Med Mal Inf 44(1):1–8. https://doi.org/10.1016/j.medmal.2013.10.002
Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P (2013) Vitamin C in disease prevention and cure: an overview. Ind J Clin Biochem 28(4):314–328. https://doi.org/10.1007/s12291-013-0375-3
de Sá MC, Rodriguez-Amaya DB (2004) Optimization of HPLC Quantification of carotenoids in cooked green vegetables—comparison of analytical and calculated data. J Food Compos Anal 17:37–51. https://doi.org/10.1016/S0889-1575(03)00100-5
de Vries MC, Vaughan EE, Kleerebezem M, de Vos WM (2006) Lactobacillus plantarum—survival, functional and potential probiotic properties in the human intestinal tract. Int Dairy J 16:1018–1028. https://doi.org/10.1016/j.idairyj.2005.09.003
El-Sayed AA, Rabie MA, El-Maaty SAM, El-Nemr SEA (2018) Fermented tomato juice (Lycopersicon esculentum Mill.) produced via lactic acid bacteria during cold storage. Carpathian J Food Sci Technol 10(1):5–18. https://doi.org/10.13140/RG.2.2.28590.41287
Fernandes Pereira AL, Rodrigues S (2018) Chapter 15 - turning fruit juice into probiotic beverages. In: Rajauria G, Tiwari BK (eds) Fruit juices. Academic Press, pp 279–287. https://doi.org/10.1016/B978-0-12-802230-6.00015-1
Georgieva R, Iliev I, Haertlé T, Chobert JM, Ivanova I, Danova S (2009) Technological properties of candidate probiotic Lactobacillus plantarum strains. Int Dairy J 19:696–702. https://doi.org/10.1016/j.idairyj.2009.06.006
Guo X, Zhao J, Wang R, Zhang H, Xing B, Naeem M, Yao T, Li R, Xu R, Zhang Z, Wu J (2021) Effects of graphene oxide on tomato growth in different stages. Plant Physiol Biochem 162:447–455. https://doi.org/10.1016/j.plaphy.2021.03.013
Hamid AA, Aiyelaagbe OO, Usman LA, Ameen OM, Lawal A (2010) Antioxidants: Its medicinal and pharmacological applications. Afr J Pure Appl Chem 4(8):142–151. https://doi.org/10.5897/AJPAC.9000020
Horáčková Š, Rokytová K, Bialasová K, Klojdová I, Sluková M (2018) Fruit juices with probiotics—new type of functional foods. Czech J Food Sci 36(4):284–288. https://doi.org/10.17221/39/2018-CJFS
Khan S, Ud-Din A, Ali GM, Khan SI, Arif I, Riaz MN, Ghazanfar S (2020a) Screening of lactic acid bacteria for their use as buffalo probiotic. J Anim Plant Sci 30(6):1357–1365. https://doi.org/10.36899/JAPS.2020.6.0155
Khan M, Anjum AA, Nawaz M, Awan AR (2020b) In vitro characterization of probiotic properties and anti Campylobacter activity of Lactobacillus spp. Isolated from poultry, fermented foods and human faeces. J Anim Plant Sci 30(2):336–344. https://doi.org/10.36899/JAPS.2020.2.0053
Korus A, Bernaś E, Korus J (2021) Health-promoting constituents and selected quality parameters of different types of kimchi: fermented plant products. Int J Food Sci. https://doi.org/10.1155/2021/9925344 (Article ID 9925344)
Kun S, Rezessy-Szabo JM, Nguyen QD, Hoschke A (2008) Changes of microbial population and some components in carrot juice during fermentation with selected Bifidobacterium strains. Process Bioch 43(8):818–820. https://doi.org/10.1016/j.procbio.2008.03.008
Kurilich AC, Jeffery EH, Juvik JA, Wallig MA, Klein BP (2002) Antioxidant capacity of different broccoli (Brassica oleracea) genotypes using the oxygen radical absorbance capacity (ORAC) assay. J Agric Food Chem 50:5053–5057. https://doi.org/10.1021/jf025535l
Li H, Huang J, Wang Y, Wang X, Ren Y, Yue T, Wang Z, Gao Z (2021) Study on the nutritional characteristics and antioxidant activity of dealcoholized sequentially fermented apple juice with Saccharomyces cerevisiae and Lactobacillus plantarum fermentation. Food Chem 363:130351. https://doi.org/10.1016/j.foodchem.2021.130351
Mousavi ZE, Mousavi SM, Razavi SH, Hadinejad M, Emam-Djomeh Z, Mirzapour M (2013) Effect of fermentation of pomegranate juice by Lactobacillus plantarum and Lactobacillus acidophilus on the antioxidant activity and metabolism of sugars, organic acids and phenolic compounds. Food Biot 27:1–13. https://doi.org/10.1080/08905436.2012.724037
Naeem M, Muqarab R, Waseem M (2019) The Solanum melongena COP1 delays fruit ripening and influences ethylene signaling in tomato. J Plant Physiol 240:152997. https://doi.org/10.1016/j.jplph.2019.152997
Nguyen TDT, Kang JH, Lee MS (2007) Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Inter J Food Microbiol 113:358–361. https://doi.org/10.1016/j.ijfoodmicro.2006.08.015
Panghal A, Virkar K, Kumar V, Dhull SB, Gat Y, Chhikara N (2017) Development of probiotic beetroot drink. Cur Res Nutr Food Sci 5(3):257–262. https://doi.org/10.12944/CRNFSJ.5.3.10
Paredes JL, Escudero-Gilete ML, Vicario IM (2022) A new functional kefir fermented beverage obtained from fruit and vegetable juice: development and characterization. LWT Food Sci Technol 154:112728. https://doi.org/10.1016/j.lwt.2021.112728
Peñas E, Frias J, Sidro B, Vidal-Valverde C (2010) Chemical evaluation and sensory quality of Sauerkrauts obtained by natural and induced fermentations at different NaCl levels from Brassica oleracea Var. capitata Cv. Bronco grown in Eastern Spain. Effect of storage. J Agric Food Chem 58(6):3549–3557. https://doi.org/10.1021/jf903739a
Priecina L, Karklina D (2015) Composition of major organic acid in vegetable and spices. CBU Int Conf Proc 3(0):447–454. https://doi.org/10.12955/cbup.v3.637
Ranadheera CS, Vidanarachchi JK, Rocha RS, Cruz AG, Ajlouni S (2017) Probiotic delivery through fermentation: dairy vs. non-dairy beverages. Fermentation 3(4):67–84. https://doi.org/10.3390/fermentation3040067
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9–10):1231–1237. https://doi.org/10.1016/s0891-5849(98)00315-3
Saqib S, Zaman W, Ayaz A, Habib S, Bahadur S, Hussain S, Muhammad S, Ullah F (2020) Postharvest disease inhibition in fruit by synthesis and characterization of chitosan iron oxide nanoparticles. Biocatal Agri Biotechnol 28:101729. https://doi.org/10.1016/j.bcab.2020.101729
Sheehan VM, Ross P, Fitzgerald GF (2007) Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innov Food Sci Emerg Technol 8:279–284. https://doi.org/10.1016/j.ifset.2007.01.007
Wang C-Y, Ng C-C, Su H, Tzeng W-S, Shyu Y-T (2009) Probiotic potential of noni juice fermented with lactic acid bacteria and bifidobacteria. Int J Food Sci Nutr 60(suppl6):98–106. https://doi.org/10.1080/09637480902755095
Wang Y, Wu J, Lv M, Shao Z, Hungwe M, Wang J, Bai X, Xie J, Wang Y, Geng W (2021) Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2021.612285
Wootton-Beard PC, Ryan L (2011) A beetroot juice shot is a significant and convenient source of bioaccessible antioxidants. J Funct Foods 3:329–334. https://doi.org/10.1016/j.jff.2011.05.007
Yoon KY, Woodams EE, Hang YD (2006) Production of probiotic cabbage juice by lactic acid bacteria. Biores Technol 97:1427–1430. https://doi.org/10.1016/j.biortech.2005.06.018
Funding
The authors received no financial support for the research, authorship and publication of this article.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [KG], [KD] and [ER-K]. The first draft of the manuscript was written by [KG] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Author (Kamila Goderska) declares that she has no conflict of interest. Author (Kanan Dombhare) declares that she has no conflict of interest. Author (Elżbieta Radziejewska-Kubzdela) declares that she has no conflict of interest.
Ethics approval
This article does not contain any studies with human participants or animals performed by the author.
Consent to participate
Informed consent not applicable.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goderska, K., Dombhare, K. & Radziejewska-Kubzdela, E. Evaluation of probiotics in vegetable juices: tomato (Solanum lycopersicum), carrot (Daucus carota subsp. sativus) and beetroot juice (Beta vulgaris). Arch Microbiol 204, 300 (2022). https://doi.org/10.1007/s00203-022-02820-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02820-1