Abstract
The present study investigated the biofilm organisms growing on selected monuments of the Champaner Pavagadh complex (Gujarat, India), which is a UNESCO World Heritage Site. The cyanobacteria and microalgae were isolated from biofilms collected through non-destructive methods. The identification of these biological organisms was done using micro-morphological characters and confirmed by 16S rRNA gene sequencing. The exopolysaccharide of each of the isolated strains was extracted, hydrolysed and analysed by the HPTLC. Six isolated strains representing five cyanobacteria and one microalga belong to the genera Desmonostoc, Nostoc, Leptolyngbya, Chroococcidiopsis and Asterarcys. The relationships between substrates’ specificity of these isolated biofilm organisms and those identified globally were evaluated using maximum parsimony analysis to generate a consensus phylogenetic tree. The five strains of cyanobacteria isolated were closely clustered with cyanobacteria belonging to a tropical region. At the generic level, no relationship between the species and substratum specificity was recorded. The exopolysaccharide analysis of the isolated strains revealed the presence of seven monosaccharides. While glucose was present in all the analysed species, the concentration of either fucose or arabinose was high. The current study presents a novel HPTLC-based method for determination of monosaccharides composition from the extracellular polymeric substances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monuments are witnesses of the past and guardian pillars of the tangible cultural heritage of the area where they are found (Hassan 2014). India is known for its culture, ancient buildings, forts, temples and palaces, and these monuments are inspiration for future generations. (Dhotre and Joshi 2019). The state of Gujarat has a rich diversity of monuments which represent its glorious historical past. Three hundred and seventeen of these monuments have been protected as per the records of the Archaeological Survey of India, (ASI 2020). Due to a lack of active maintenance, several of these monuments show the presence of different stages of greenish and blackish biofilms. These biofilms cause damage to the monument wall structure and this process is termed as biodeterioration (Allsopp et al. 2004). While many biological organisms such as cyanobacteria, green algae, bryophyte, lichen, fungi and some allied vascular plants are responsible for biodeterioration, the cyanobacteria and the green algae are major pioneer organisms (Ortega Calvo et al. 1991; Tiano et al. 1995; Gaylarde and Gaylarde 2000; Crispim and Gaylarde 2005; Samad and Adhikary 2008). At the global level, the presence of cyanobacteria and microalgae biofilms has been reported from diverse monuments. These include monuments and buildings in South Eastern Spain (Uher et al. 2005), diverse substrate building facades in France (Barberousse et al. 2006), archaeological structures at El Palacio of Palenque, Mexico (Ramírez et al. 2010) and the Stucco Mask of North Acropolic, Tikal, Guatemala (Ortega-Morales et al. 2013). Similar studies have been conducted on a diversity of monuments like temples, caves and statues across India. The main temple at Thanjavur, the Sivan temple and the Venkatasaperumal temple all located at Thanjavur District, South India (Bhavani et al. 2013), the Parasurameswar temple and Khandagiri cave from Bhubaneswar, Orrisa, East India (Rossi et al. 2012) and several sculptures from Shantiniketan, West Bengal, East India (Keshari and Adhikary 2013) have been studied in the past.
Pioneering organisms secrete polysaccharides into the external environment which are known as exopolysaccharides or extracellular polymeric substances (EPS) (Sureshkumar et al. 2007; Nwodo et al. 2012; Rossi and Philippis 2015). Being mucilaginous in nature, the surface of the exopolysaccharide facilitates the attachment of propagules of other organisms which leads to further colonization on it. During colonization, phototrophic organisms may either grow on the rock surface as epilithic phototrophs or may occupy large pores of the rock surface as endolithic phototrophs. Due to enlargement of pores, such organisms contribute to the breakdown of the rock crystalline structures such as sandstones, limestones, basalt, bricks, granite etc. (Crispim and Gaylarde 2005). EPS protect the organisms against environmental stresses like drought and UV irradiation (Rossi and De Philippis 2015). EPS has ability to enhance the aggregation of soil particles, maintain the moisture of the environment and trap nutrients (Costa et al. 2018). A polysaccharide sheath has the ability to regulate the humidity and temperature of their internal body of a cell. Consequently, it protects the biofilm on the stone surface and invites other biota on it. These exopolysaccharides are composed of several different sugars in different combinations (Sutherland 1999; Nishanth et al. 2020). The present study focuses on the documentation of cyanobacteria and algae found on selected monuments and characterization of the principal sugars in their extracellular polysaccharide matrix. This study was carried out using the HPTLC method as this technique gives very precise results and it has not yet been used in studies employing EPS characterization.
Materials and methods
Study site
The Champaner Pavagadh complex, which is a UNESCO World Heritage Site since 2004, is located in central Gujarat (Western India). This world heritage site comprises various monuments at Champaner and the adjoining Pavagadh hills (Sinha et al. 2004; Modi 2008). Champaner was the regional capital city of Gujarat for some time after it was built in the fifteenth century (Sinha et al. 2004). The Pavagadh hills have several monuments which date back to the ninth century. A total of six monuments were selected for the study. Three monuments namely Makai kothar (22° 27′ 54.40″ N, 73° 31′ 20.30″ E), Navlakha kothar (22° 28′ 02.19″ N, 73° 30′ 40.79″ E) and Jain temple (22° 28′ 00.8″ N, 73° 30′ 54.7″ E) are located on the Pavagadh hills. Two of the other three monuments, Saher ki Masjid and Mandavi are located next to each other within the Champaner fort (22° 29′ 0.94″ N, 73° 31′ 53.60″ E) while Amir manzil (not protected by the ASI but under supervision of the ASI 22° 29′ 25.08″ N, 73° 31′ 44.17″ E) is located outside the fort ramparts (Supplementary Fig. 1).
Sample collection, culturing and identification
The biofilm sample collection was carried out using a non-destructive method with adhesive tape (La Cono and Urzi 2003; Keshari and Adhikary 2013, 2014) in August 2017 and April 2018. The month of August falls within the rainy season during which conditions are most favourable for biofilm growth, while the month of April falls within the dry summer season where conditions restrict the growth of biofilms. Samples were thus collected during these two different times. Permission to collect samples was taken from the Archaeology Survey of India, Vadodara Circle. At the laboratory, the collected biofilm clumps were transferred to conical flasks containing BG 11 media (Rippka et al. 1979) and incubated at 27–30 °C temperature in 2500 lux white light with 16-h light and 8-h dark photoperiod. During the light period, the samples were shaken at 100 RPM on a rotatory shaker for 2 h. Repeated streaking from broth onto agar media was carried out to obtain pure cultures. These pure cultured samples were confirmed microscopically (Leica DM 2000 microscope) and identified using micro morphological characters referred to from Desikachary (1959), Komarek and Anagnostidis (1989, 1999) and Anagnostidis and Komarek (1990). Optimization of nutrient media for further growth was done using response surface method of the Design Expert 7.0.2 (state-ease, USA) (Wang et al. 2007). Two of the media components K2HPO4 and NaNO3 were optimized for increasing the in vitro biofilm growth of pure culture (Mehta et al. 2020).
Genomic DNA extraction, amplification of 16S rRNA gene and phylogenetic analysis
DNA was extracted using the xanthogenate-SDS method (Tillett and Neilan 2000) with minor modifications. In addition to XS buffer, 10 µl RNase A was added and mixed using a pipette. As the incubation time is dependent on the thickness of the capsular polysaccharides sheath, the optimum time was standardized separately for each of the species after several attempts. The eppendorf tubes were incubated at 70 °C for 30 min (Asterarcys), 60 min (Chroococcidiopsis) and 90 min (Desmonostoc, Nostoc and Leptolyngbya). After incubation, the tube was vortexed for 10 s and then kept on ice for 60 min. Cell debris was removed by centrifugation at 14,000 rpm for 7–10 min. DNA was precipitated by adding cold ethanol and pelleted by centrifuging at 12,000 rpm for 10 min. It was rinsed with 70% ethanol and air dried. A TE buffer was used to dissolve the pellet and then stored at − 20 °C until further analysis.
The amplification of DNA was attempted using 16S rRNA universal primers like 359F, 106F and 781R (Nübel et al. 1997; Keshari and Adhikary 2013, 2014). However, the standard amplicon size for species level identification could not be obtained. Hence, new primers were designed using NCBI database sequences and the appropriate 16S rRNA region was selected for both cyanobacteria and microalgae for species confirmation (Burja et al. 2001; Alonso et al. 2012; Mohamed et al. 2014; Kong et al. 2017). PCR amplification process was carried out by designed primers pairs DNM1 F “GAAAGCCTGACGGAGCAATA”/DNM1 R “CGGGACTTAACCCAACATCT”; DNM2 F “GCGGTGAAATGCGTAGAGAT”/DNM2 R “GTAAGGGGCATGCTGACTTG” and DNM3 F “AGAGGATGAGCAGCCACACT”/DNM3 R “GGGATGTCAAGCCTTGGTAA”. PCR reaction mixture (20 µl) was prepared by mixing 10 µl Dream Taq master mix, 0.5 µl forward and reverse primers from 1:10 stock, 1 µl template DNA and 8 µl MilliQ water. 35 PCR cycles were performed consisting of initial denaturation at 94 °C for 4 min, denaturation at 94 °C for 30 s, elongation at 55 °C for 45 s, extension at 72 °C for 2 min and final extension 7 min at 72 °C. DNA purification was carried out by GeneJET Gel Extraction (Thermo scientific). The purified amplicon quality and quantity was assessed using nanophotometer (IMPLEN NP80) and it was then subjected to Sanger sequencing. The sequence obtained from this process were compared with the 16S rRNA partial gene sequences of cyanobacterial biofilm on monuments (Keshari and Adhikary 2013; Adhikary et al. 2015) as well as other global 16S rRNA cyanobacterial sequences retrieved from GenBank through BLAST analysis. All selected sequences and the sequences obtained in the current study were used for the construction of a consensus phylogenetic tree by maximum parsimony analysis using Mega X software (Kumar et al. 2018). Bootstrap value was selected as 1000 replicates.
Production and extraction of exopolysaccharide
The production of exopolysaccharide was first confirmed by Alcian Blue staining (Tamaru et al. 2005). Extraction of EPS was done using minor modification of method followed by Sureshkumar et al., (2007) and Khattar et al., (2010). 500 ml of culture was centrifuged at 15,000 rpm for 15 min and cell pellets were separated. Cold acetone equal to two or three volumes of the supernatant was added and this solution was kept at 4 °C for 24 h. The precipitated exopolysaccharide was centrifuged at 8000 rpm for 20 min at 4 °C and the pellet was washed with acetone. Impurity like exo DNA and protein (Nwodo et al. 2012) in the EPS pellets could hinder further analysis. Hence, purity of carbohydrate was checked by the Anthrone test (Delattre et al. 2016). The EPS pellet was purified by 2–3 repeated washes with chilled acetone and re-centrifuged at 8000 rpm for 20 min at 4 °C. The pure pellet was stored at 4 °C for further study.
Acid hydrolysis and separation of monosaccharides from EPS by HPTLC method
The digestion of EPS was carried out using the acid hydrolysis method of Khattar et al. 2010 with a few modifications. A X mg exopolysaccharide was hydrolysed by 3X ml 4 N TFA (Trifluoroacetic acid) at 100 °C for 60 min. The TFA was removed by evaporation along with drop by drop addition of an equal volume of methanol. The remaining TFA residue in this neutralized sample was evaporated using a rotary vacuum evaporator (Heidolph Laborota 4000 efficient). This sample was used in to HPTLC analysis to separate the monosaccharide present in the exopolysaccharide. The HPTLC method was carried out using the CAMAG HPTLC system setup with Linomat 3 as a sample applicator, Scanner 3 for scanning the derivatized plate and winCATS planar chromatography manager software version 1.2.0. The standards and samples were applied using a 100 µl CAMAG Linomat syringe. 20 × 10 cm TLC plates (60 F254 Merck, India) were used to show the separation through a chromatogram. The plate was saturated with 0.3 N NaH2PO4 solution and air dried. The saturated plate was laid out with the first band positioned 20 mm away from the edge on X axis and 8 mm away from the edge on Y axis. The length of each band was 6 mm. The plate was developed using 4:5:1 ratio of n butanol:acetone:0.3 N NaH2PO4 as the mobile phase. For development of the TLC plate, a CAMAG twin-trough glass chamber was used. The developed plate was derivatized by ADP (aniline diphenylamine phosphoric acid). The derivatized plate was heated at 110 °C for 10–15 min in an oven. The plate was scanned by the CAMAG TLC Scanner 3 at a wavelength of 366 nm.
Results and discussion
In current study, a total of six different strains were isolated as pure cultures from the biofilms of selected study locations. Identification of all these strains was confirmed by 16S rRNA gene sequence. Exopolysaccharide of all six strains was extracted and their separation was done by HPTLC technique.
Biofilms strain confirmation
Sanger sequencing data were obtained from purified amplicon of each strains of biofilm. The BLAST analysis of the acquired sequence showed 96–100% similarity, which helped to reveal the identity of the isolated strains. Therefore, both microscopic morphological features and 16S rRNA gene sequence data were considered during the identification of the strain. The six species belonging to five different genera were identified as Leptolyngbya foveolarum and Leptolyngbya crispata (Synechococcales), Desmonostoc muscorum and Nostoc punctiforme (Nostocales), Chroococcidiopsis cubana (Chroococcidiopsidales) as well as Asterarcys quadricellulare (Chlorococcales) (Supplementary Fig. 2). All sequence data of these strains were deposited in the NCBI GenBank database and their accession numbers were obtained (Table 1).
Biofilm organisms on different substrata
In the current study, the maximum number of species (four species) were recorded on mortar followed by sandstone and lime coating substratum (Table 1). Earlier studies (Barberousse 2006; Keshari and Adhikary 2013; Adhikary et al. 2015) have reported cyanobacterial biofilm from sandstone and mortar substratum. Mortar, sandstone and limestone have a porous surface which allows organisms to settle and accumulate photosynthetic biomass. Phototropic organisms release carbohydrate and other organic nutrients facilitating the growth of complex microbiota which leads to initiation of biodeterioration activity (Crispim and Gaylarde 2005). In the current study, members of Synechococcales and Nostocales were observed to be dominant on biofilms. Likewise, researchers in India have also documented a higher species diversity of Synechococcales and Nostocales on different monuments (Samad and Adhikary 2008; Keshari and Adhikary 2013; Adhikary et al. 2015). Members of these two orders have also been recorded as prominent members of biofilms on monuments from France and Spain (Uher et al. 2005; Barberousse 2006). To understand the relationships between different species from different substratum for biofilm growth, a consensus phylogenetic tree showing the relationship between the isolated cyanobacterial 16S rRNA partial gene sequences obtained in the present study and those reported from other parts of the world was constructed (Fig. 1).
Analysis of the phylogenetic tree revealed that Leptolyngbya was the most common genera isolated from cyanobacterial biofilm on monuments followed by Chroococcidiopsis and Nostoc. This analysis revealed differences in the taxa based on their substratum as well as their location in either tropical or temperate region of the world which are similar to the findings of Keshari and Adhikary (2013). The sequences of the organisms isolated in the current study are closely clustered with sequences of organisms reported from India and the tropical regions. Nostoc punctiforme, Leptolyngbya crispata and Leptolyngbya foveolarum were closely clustered with Tolypothrix scytonemoides and Leptolyngbya sp. isolated from India while Desmonostoc muscurorum was closely clustered with Tolypothrix distorta var. penicillata and formed a group with other Nostoc and Hassallia isolated from monuments in India. Chroococcidiopsis cubana was closely clustered with Chroococcidiopsis sp. that has been reported from Cambodia, a tropical region. Leptolyngbya has been reported from a variety of substrates like sandstone (India, Cambodia), mortar (India), marble, concrete and catacombs (Italy) while Chroococcidiopsis has been reported from sandstone (Cambodia, Antarctica, Israel), granite (Egypt & Chile) and lime mortar (India). Hence, at the generic level, there seems to be no specificity of taxa with any substratum.
Exopolysaccharides and its characterization by HPTLC fingerprint profile
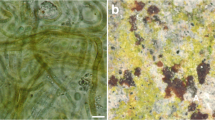
Extracellular polysaccharide attaches to the cell surface sheath (capsular polysaccharide) and also secretes into medium as slime or released polysaccharide (RPS). The slime was visually confirmed by Alcian Blue staining (Fig. 2). EPS is mainly composed of heteropolysaccharides or homopolysaccharides (Sutherland 1999; Nishanth et al. 2020).
The characterisation of EPS of the isolated cyanobacteria and microalga from the Champaner Pavagadh complex by HPTLC method revealed the presence of heteropolyscharides. This method provided a fingerprint profile chromatogram by densitometric evaluation. The obtained chromatogram was viewed at two different wavelengths of 580 and 366 nm which has been depicted at Fig. 3a, b, respectively. This method allowed the generation of three sets of Rf values for each compound which facilitated the separation of compounds in which max Rf values were similar. Comparison with standard Rf values (Table 2) and colour of the fingerprints after derivatization indicated the presence of specific monosaccharides in the EPS which were confirmed based on their Rf values and peaks of spectra obtained at 366 nm. The obtained spectra of all tracks are shown in Fig. 4.
HPTLC fingerprints of exopolysaccharides of Champaner Pavagadh complex biofoulants, A. Chromatogram at 580 nm and B. Chromatogram at 366 nm. 1—Xylose, 2—arabinose, 3—mannose, 4—glucose, 5—galactose, 6—fucose, 7—galacturonic acid, S1—Desmonostoc muscorum, S2—Nostoc punctiforme, S3—Leptolyngbya crispata, S4—Leptolyngbya foveolarum, S5—Chroococcidiopsis cubana, S6—Asterarcys quadricellulare
This analysis revealed the presence of six neutral sugars (fucose, xylose, mannose, glucose, arabinose, and galactose) and one acidic sugar (uronic acid). Each strain had the presence of three to five monosaccharides. Table 3 depicts the amount of different sugars isolated from the EPS of the isolated strains. Xylose was visible at 580 nm only and hence it has not been included in Table 3 which indicates the area covered values, observed at 366 nm. The spectra revealed that concentration of fucose and arabinose was high in comparison to other monosaccharides. However, in terms of dominance, glucose was the dominant monosaccharide except Asterarcys quadricellulare which is a microalga. Glucose was present in all the studied strains like Leptolyngbya sp., Gloeocapsosis cf. crepidinum, Plectonema sp., Gloeocapsa kuetzingigiana (Rossi et al. 2012) and Limnothrix redekei (Khattar et al. 2010). In current study, we observed the seven monosaccharides from the EPS of cyanobacteria and microalga. While earlier study reported that presence of a minimum of two and up to a maximum of nine monosaccharides from the EPS of several microalgae and bacterial groups like Bacillariophyta (6–8 monosaccharides), Chlorophyta (6 monosaccharides), Haptophyta (2–6 monosaccharides) and Cyanobacteria (3–9 monosaccharides). (De Philippis et al. 1998; Hu et al. 2003; Kielme et al. 2007; Pereira et al. 2009; Pignolet et al. 2013; Raposo et al. 2015).
Monosaccharides from the EPS of different bacterial and microalga strains have been separated using various methods such as ion exchange chromatography, GC/MS, HPLC with refractive index detection and LC-high resolution mass spectroscopy (LC-HRMS) (Khattar et al. 2010; Rossi et al. 2012; Baldev et al. 2015; Delattre et al. 2016). In these methods, the separation of sugars (e.g. glucose and mannose) with a similar retention time was difficult to identify and they had to be subsequently confirmed using TLC (Khattar et al. 2010; Baldev et al. 2015). The HPTLC method used in the current study proved to be more accurate and rapid as it provided results in both form spectra as well as chromatogram with different Rf values.
Conclusion
It can be concluded that the results obtained through molecular study of biofilm organisms which led to the deterioration of the monuments of the Champaner Pavagadh area were composed of five cyanobacteria and one microalga. The chemical characterization study of isolated exopolysaccharide suggests that HPTLC fingerprint analysis could be a reliable tool for monosaccharides profiling of exopolysaccharide from biofilm forming microorganisms. The method standardised in this investigation could be useful for various applications in industrial, pharmaceutical and biodeterioration studies.
Data availability
National Centre of Biotechnology Information (NCBI).
Code availability
GenBank Accession No. MN950976, MN950971, MN950972, MN950974, MN950974, MN969628, MN955451.
References
Adhikary SP, Keshari N, Urzì C, De Philippis R (2015) Cyanobacteria in biofilms on stone temples of Bhubaneswar, Eastern India. Algol Stud 147:67–93
Allsopp D, Seal KJ, Gaylarde CC (2004) Introduction to biodeterioration. Cambridge University Press
Alonso M, Lago FC, Vieites JM, Espiñeira M (2012) Molecular characterization of microalgae used in aquaculture with biotechnology potential. Aquac Int 20(5):847–857
Anagnostidis K, Komárek J (1990) Modern approach to the classification system of Cyanophytes 5-Stigonematales. Archiv für Hydrobiologie Supplementband Monographische Beiträge 86:1–73
ASI (2020) Protected monuments of Gujarat. Archaeological Survey of India. https://asi.nic.in/protected-monuments-in-gujarat/. Accessed 20 July 2020
Baldev E, Mubarakali D, Shriraman R, Pandiaraj D, Alharbi NS, Thajuddin N (2015) Extraction and partial characterization of exopolysaccharides from marine cyanobacteria and their flocculation property. Res J Environ Sci 9:28–38
Barberousse H, Tell G, Yéprémian C, Couté A (2006) Diversity of algae and cyanobacteria growing on building facades in France. Algol Stud 120:81–105
Bhavani BA, Manoharan CH, Vijayakumar SU (2013) Studies on diversity of cyanobacteria from temples and monuments in India. Int J Environ Ecol Fam Urban Stud 3(1):21–32
Burja AM, Tamagnini P, Bustard MT, Wright PC (2001) Identification of the green alga, Chlorella vulgaris (SDC1) using cyanobacteria derived 16S rDNA primers: targeting the chloroplast. FEMS Microbiol lett 202(2):195–203
Costa OY, Raaijmakers JM, Kuramae EE (2018) Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front Microbiol 9:1636
Crispim CA, Gaylarde CC (2005) Cyanobacteria and biodeterioration of cultural heritage: a review. Microb Ecol 49:1–9
De Philippis R, Margheri MC, Materassi R, Vincenzini M (1998) Potential of unicellular cyanobacteria from saline environments as exopolysaccharide producers. Appl Environ Microbiol 64(3):1130–1132
Delattre C, Pierre G, Laroche C, Michaud P (2016) Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol Adv 34:1159–1179
Desikachary TV (1959) Cyanophyta, vol 2. Indian Council of Agricultural Research, New Delhi
Dhotre R, Joshi DA (2019) Conservation of ancient monuments. Int J Adv Eng Res Dev 6:1–4
Gaylarde PM, Gaylarde CC (2000) Algae and cyanobacteria on painted buildings in Latin America. Int Biodeter Biodegr 46:93–97
Hassan F (2014) Tangible heritage in archaeology. In: Smith C (ed) Encyclopedia of global archaeology. Springer, New York, pp 7213–7215
Hu C, Liu Y, Paulsen BS, Petersen D, Klaveness D (2003) Extracellular carbohydrate polymers from five desert soil algae with different cohesion in the stabilization of fine sand grain. Carbohydr Polym 54(1):33–42
Keshari N, Adhikary SP (2013) Characterization of cyanobacteria isolated from biofilms on stone monuments at Santiniketan, India. Biofouling 29:525–536
Keshari N, Adhikary SP (2014) Diversity of cyanobacteria on stone monuments and building facades of India and their phylogenetic analysis. Int Biodeter Biodegr 90:45–51
Khattar JIS, Singh DP, Jindal N, Kaur N, Singh Y, Rahi P, Gulati A (2010) Isolation and characterization of exopolysaccharides produced by the cyanobacterium Limnothrix redekei PUPCCC 116. App Biochem Biotechnol 162:1327–1338
Kielme SN, Domozych DS, Gretz MR (2007) The extracellular polymeric substances of desmids (Conjugatophyceae, Streptophyta): chemistry, structural analyses and implications in wetland biofilms. Phycologia 46(6):617–627
Komárek J, Anagnostidis K (1989) Modern approach to the classification system of cyanophytes. 4. Nostocales Arch Hydrobiol Suppl Algol Stud 56:247–345
Komárek J, Anagnostidis K (1999) Cyanoprokaryota, volume 19/ 1, I. Series: Chroococcales [Cyanoprokaryota, volume 19/1, I. Series: Chroococcales]. In: Ettl H, Gärtner G, Heynig H, Mollenhauer D (eds) SüßWasserflora von Mitteleuropa [Freshwater flora of Central Europe]. Gustav Fisher Verlag, Jena
Kong P, Richardson P, Hong C (2017) Diversity and community structure of cyanobacteria and other microbes in recycling irrigation reservoirs. PLoS ONE 12(3):e0173903
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Bio Evol 35:1547–1549
La Cono V, Urzı̀ C (2003) Fluorescent in situ hybridization applied on samples taken with adhesive tape strips. J Microbiol methods 55:65–71
Mehta D, Sivan P, Shah D (2020) Statistical in vitro model for upscaling biofilm of Chroococcidiopsis cubana by media optimization and its protocol for DNA extraction. Biosci Biotechnol Res Commun 13(4):1962–1966
Modi SM (2008) Champaner Pavagadh—Managing conflicts—A conservation challenge. Structural analysis of historic construction: preserving safety and significance: proceedings of the VI international conference on structural analysis of historic construction, SAHC08, 2-4 July 2008, vol 1. CRC Press, Bath, UK, p 175
Mohamed MS, Tan JS, Kadkhodaei S, Mohamad R, Mokhtar MN, Ariff AB (2014) Kinetics and modelling of microalga Tetraselmis sp. FTC 209 growth with respect to its adaptation toward different trophic conditions. Biochem Eng J 88:30–41
Nishanth S, Bharti A, Gupta H, Gupta K, Gulia U, Prasanna R (2020) Cyanobacterial extracellular polymeric substances (EPS): biosynthesis and their potential applications. Microbial and natural macromolecules. Academic Press, pp 349–369
Nübel U, Garcia-Pichel F, Muyzer G (1997) PCR primers to amplify 16S rRNA genes from cyanobacteria. App Environ Microbiol 63:3327–3332
Nwodo UU, Green E, Okoh AI (2012) Bacterial exopolysaccharides: functionality and prospects. Int J Mol Sci 13:14002–14015
Ortega-Calvo JJ, Hernandez-Marine M, Sáiz-Jiménez C (1991) Biodeterioration of building materials by cyanobacteria and algae. Int Biodeter 28:165–185
Ortega-Morales BO, Nakamura S, Montejano-Zurita G, Camacho-Chab JC, Quintana P, De la Rosa SDC (2013) Implications of colonizing biofilms and microclimate on west stucco masks at North Acropolis, Tikal. Guatemala Herit Sci 1(1):32
Pereira S, Zille A, Micheletti E, Moradas-Ferreira P, De Philippis R, Tamagnini P (2009) Complexity of cyanobacterial exopolysaccharides: composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol Rev 33(5):917–941
Pignolet O, Jubeau S, Vaca-Garcia C, Michaud P (2013) Highly valuable microalgae: biochemical and topological aspects. J Ind Microbiol Biot 40(8):781–796
Ramírez M, Hernandez-Marine M, Novelo E, Roldán M (2010) Cyanobacteria-containing biofilms from a Mayan monument in Palenque. Mexico Biofouling 26(4):399–409
Raposo MF, De Morais AMB, De Morais RMSC (2015) Marine polysaccharides from algae with potential biomedical applications. Mar Drugs 13(5):2967–3028
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111:1–61
Rossi F, De Philippis R (2015) Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 5:1218–1238
Rossi F, Micheletti E, Bruno L, Adhikary SP, Albertano P, De Philippis R (2012) Characteristics and role of the exocellular polysaccharides produced by five cyanobacteria isolated from phototrophic biofilms growing on stone monuments. Biofouling 28:215–224
Samad LK, Adhikary SP (2008) Diversity of micro-algae and cyanobacteria on building facades and monuments in India. Algae 23:91–114
Sinha A, Kesler G, Ruggles DF, Wescoat J Jr (2004) Champaner Pavagadh, Gujarat, India: challenges and responses in cultural heritage planning and design. Tour Recreat Res 29:75–78
Sureshkumar A, Mody K, Jha B (2007) Bacterial exopolysaccharides–a perception. J Basic Microbiol 47:103–117
Sutherland IW (1999) Polysaccharases for microbial exopolysaccharides. Carbohydr Polym 38:319–328
Tamaru Y, Takani Y, Yoshida T, Sakamoto T (2005) Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. App Environ Microbiol 71:7327–7333
Tiano P, Accolla P, Tomaselli L (1995) Phototrophic biodeteriogens on lithoid surfaces: an ecological study. Microb Ecol 29:299–309
Tillett D, Neilan BA (2000) Xanthogenate nucleic acid isolation from cultured and environmental cyanobacteria. J Phycol 36:251–258
Uher B, Aboal M, Kovacik L (2005) Epilithic and chasmoendolithic phycoflora of monuments and buildings in South-Eastern Spain. Cryptogam Algol 26:275–308
Wang JP, Chen YZ, Ge XW, Yu HQ (2007) Optimization of coagulation–flocculation process for a paper-recycling wastewater treatment using response surface methodology. Colloids Surf A Physicochem Eng Asp 302:204–210
Acknowledgements
Authors acknowledge to Research and Consultancy Cell (RCC) of The M. S. University of the Baroda for research grant (project sanction no. RCC/Dir./2018/56/7). They are thankful to Dr. K. S. Rajput (Plant Anatomy Lab) for providing facilities to carry out the culturing, molecular study and Prof. K. C. Patel, Head of the Bioscience department (S. P. University) for permission to use the HPTLC instrument facility. Authors also acknowledge the Archaeological Survey of India (ASI) for giving the permission (letter no.: 36/13/MIS/T&A-2016-17-3771 and 36/13/MIS/T&A-2018-19-2701) to collect the samples. DM is grateful to the Anchrom Pvt. Ltd. for giving hands on training on HPTLC method and the M. S. University of Baroda for providing university research scholarship. The authors acknowledge the constructive comments of the anonymous reviewer which have immensely improved the manuscript.
Funding
Sanction no. RCC/Dir./2018/56/7 (RCC, The M. S. University of Baroda).
Author information
Authors and Affiliations
Contributions
Authors conceptualized and agreed the study. DM designed, conducted all the experiments and prepared the manuscript. DS oversaw the study, sourced the funding and improved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflicts of interest.
Consent for publication
On acceptance of the manuscript, the copyright from the author will be transferred to the journal.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mehta, D., Shah, D. Cyanobacteria and microalgae growing on monuments of UNESCO World Heritage site Champaner Pavagadh, India: biofilms and their exopolysaccharide composition. Arch Microbiol 203, 3425–3433 (2021). https://doi.org/10.1007/s00203-021-02334-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-021-02334-2