Abstract
The golden chanterelle represents one of the commonly found, edible mushrooms that is highly valued in various cuisines. The present study focused on assessing the requirements of Cantharellus cibarius such as pH, temperature, as well as the carbon and nitrogen sources for mycelial growth. Optimization of the growth parameters was carried out by one-factor-at-a-time method. The optimal pH and temperature were determined to be 6.0 and 22.5 °C, respectively. Among the various carbon sources studied, sucrose at a concentration of 2% gave maximum mycelial growth and proved to be the most suitable one. Amongst the nitrogen sources studied, peptone, ammonium sulphate, and sodium nitrate, gave the maximum mycelial growth at an optimized concentration of 0.5%. In the presence of beef extract and yeast extract, a change in colony pigmentation from yellow to dark grey was observed. Finally, the carbon to nitrogen ratio of 2:0.5 proved to be optimal for mycelial growth. This study is the first report on the optimisation of in vitro growth requirements of C. cibarius.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wild mushrooms have been valued for their nutritious and appetising qualities for several decades in various regions of the world (Kozarski et al. 2015). Cantharellus cibarius, also known as the golden chanterelle is a commonly found, wild, edible, ectomycorrhizal mushroom (Pilz et al. 2003). It belongs to the phylum Basidiomycota and the family Cantharellaceae (Nyman et al. 2016). It is well-recognised as a delicious food from Scandinavia to the Mediterranean in Europe (Kozarski et al. 2015). It ranks among the top five selling mushrooms in Europe that is harvested from the wild for commercial purpose (Nowacka-Jechalke et al. 2018). The production of Chanterelle is estimated to be 1,50,000 to 2,00,000 tons per year; the marketplace value of which is around $1.7 billion (Nowacka-Jechalke et al. 2018). C. cibarius is considered as the most desirable amongst the edible wild mushrooms and is recognized as a delicious food. The color of C. cibarius ranges from orange to yellow and it gives a fruity, apricot-like aroma which is highly appreciated in cooking throughout Europe (Kozarski et al. 2015).
Owing to the significance of the fruiting bodies of mushroom in agriculture, ecology, and medicine, mushroom-based functional foods have started gaining the attention of researchers (Wang et al. 2020). For over 2000 years, several species of mushrooms have been used for medicinal purposes in the Asian countries (Nyman et al. 2016). Today, several of them are used to treat diseases associated with the immune system, and also as supplementary medicines in the conventional treatment of cancer. These activities have been attributed to the presence of bioactives such as polysaccharides (Nyman et al. 2016). Similar to these medicinal mushrooms, C. cibarius displays various promising biological activities such as antioxidant, neuroprotective (Lemieszek et al. 2018), and antimicrobial (Kolundžić et al. 2017) activities. Furthermore, C. cibarius has also been reported for its anticancer, immunomodulatory, anti-inflammatory, and antiaging activities (Lemieszek et al. 2018). Chanterelle species have a rich macronutrient profile with high amounts of carbohydrates and proteins, while featuring only low amounts of fats (Kumari et al. 2011; Kozarski et al. 2015). It contains 53.7% crude protein, 31.9% carbohydrates and 2.9% lipids of dry matter (Barros et al. 2008). With its high content of protein, carbohydrates, and dietary fibre, C. cibarius shows a substantial nutritive value. Furthermore, C. cibarius is also rich in minerals such as calcium, phosphorous, and potassium as well as vitamin D (Nowacka-Jechalke et al. 2018). Chanterelles exhibit good antioxidant and free radical scavenging activity (Ebrahimzadeh et al. 2010) owing to the presence of phenolic acids, specifically caffeic acid and catechins (Palacios et al. 2011). Owing to all the benefits that Chanterelles offer, studying their growth in controlled conditions to obtain maximum production becomes crucial. There has been a considerable interest in pigment production by edible mushrooms. In case of C. cibarius, the characteristic color is attributed mainly to the presence of carotenoid pigments. The pigment mixture consists of β-carotene as a major constituent along with lycopene, α-carotene, and the xanthophyll canthaxanthin (Haxo 1950; Sandmann and Misawa 2002).
Although C. cibarius species grow in a variety of soils, the best growth is observed in well-drained forest soils (pH 4–5.5) with low nitrogen content. Despite the wide-spread occurrence of C. cibarius, cultivating it has been a difficult task, the reason behind which has been speculated to be the presence of bacteria and other foreign microbes within the sporocarp tissues (Kozarski et al. 2015). The complex symbiotic association between chanterelle mushrooms and its host trees makes its natural cultivation nearly impossible (Kozarski et al. 2015).
Presently, the information on the utilization of various carbon and nitrogen sources by C. cibarius mycelium is scarce. Furthermore, the effect of nutritional components on pigment production by the mycelium has so far caught little attention and needs to be investigated.
The present investigation aims to determine the optimal mycelial growth conditions (pH, temperature, carbon and nitrogen source) of C. cibarius under laboratory conditions. To the best of our knowledge, this is the first report on investigation of nutritional requirements of C. cibarius for its growth as well as its effect on the pigmentation.
Materials and methods
Materials
Murashige and Skoog (MS) growth media, carbon (glucose, fructose, galactose, sucrose, and xylose) and nitrogen (beef extract, yeast extract, peptone, ammonium nitrate, ammonium sulphate, ammonium citrate, sodium nitrate, and potassium nitrate) sources were purchased from Sigma Aldrich, USA. All the other chemicals used were of analytical reagent grade.
Collection of C. cibarius and preparation of pure culture
The fruiting bodies of C. cibarius were collected from forests in Juva, Finland. Pure culture of the mycelia was obtained using Murashige and Skoog (MS) media (Pamire and Parawira 2020) (Table S1, supplementary data) and were incubated in the dark at 22 °C for 2 weeks.
Optimization of the nutritional requirements/growth parameters
The optimal nutritional requirements for the growth of the mycelia were determined using agar plate technique (Nasim et al. 2001) by one-factor-at-a-time (OFAT) method. From the advancing (actively growing) margins of the subcultures, mycelial discs of 5 mm diameter were cut with a cork borer and carefully placed at the centre of new Petri plates (9 cm diameter) filled with sterile MS media. The inoculated Petri plates were sealed with a parafilm and incubated at room temperature (22 °C) for 2 weeks in the dark. The parameter showing the highest growth of the mycelia (in terms of diameter) was selected as optimum and kept constant for the rest of the study. Mycelial colony diameter (mm) was determined by measuring along two axes with a meter scale and an average was calculated.
Optimum pH requirement of the media was determined by growing the mycelium in the pH range of 5 to 8. The pH adjustments were made using 1 M NaOH and 1 M HCl. The optimisation of incubation temperature was carried out in the range of 15–30 °C.
In order to investigate the effect of nitrogen sources on the mycelial growth, various nitrogen sources such as beef extract, yeast extract, peptone, ammonium nitrate, ammonium sulphate, ammonium citrate, sodium nitrate, and potassium nitrate were added to MS media at a concentration of 1% (w/v). After selecting the optimal nitrogen source, its optimal concentration was studied in the range of 0.5–5% (w/v). For the optimization of carbon source, MS media was supplemented with 5 different sugars viz. glucose, fructose, galactose, sucrose, and xylose. The sugars were filter-sterilized and then aseptically added at a concentration of 2% (w/v) to the MS medium. The medium without any carbon source served to be the control. After selecting the optimal carbon source, its optimal concentration was studied in the range of 0.5–5% (w/v). Finally, the effect of carbon to nitrogen ratio was evaluated by varying the range from 2:0.25 to 2:2.
Growth characteristics such as colony diameter, and colony pigmentation were observed for each varying parameter. The colony diameter was a direct measure of the suitability of the respective parameter.
Statistical analysis
All the experiments were performed in triplicates, unless stated otherwise. The statistical analysis of the data obtained was performed using IBM® SPSS® version 20. The data was subjected to Tukey’s HSD test (p < 0.05).
Results and discussion
Effect of temperature on mycelial growth of C. cibarius
Factors such as temperature and pH play a significant role in mycelial growth. Several studies have reported the effect of temperature on the growth of edible mushrooms (Zervakis et al. 2001; Hoa and Wang 2015). Thus, it was crucial to take into consideration the effect of temperature on mycelial growth of C. cibarius. The colony growth of C. cibarius was studied at a varying temperature range (15–30 °C) (Fig. S1, supplementary data). Significantly low mycelial growth was observed at 15 °C, which may be attributed to lower metabolic activities of the fungus that are responsible for the absorption and assimilation of essential nutrients required for growth (Mensah-Attipoe and Toyinbo 2019). The colony diameter was observed to increase with an increase in the temperature from 15 to 22.5 °C. The growth was significantly highest at an incubation temperature of 22.5 °C. At a temperature of 30 °C no mycelial growth was detected, which may be attributed to denaturation of some essential enzymes catalysing the metabolic processes.
Kalyoncu et al. (Kalyoncu et al. 2009) studied the effect of temperature on the mycelial growth of the edible fungi of Morchella species. The authors reported similar results where the highest growth was observed in the temperature range of 20–25 °C, which dropped significantly with a further increase up to 30 °C. A few other edible fungi such as Pleurotus eryngii and Auricularia auricula-judae have also shown a temperature optima ranging around 25 °C, which was influenced by the nutrient medium used (Zervakis et al. 2001). However, the temperature dependence of mycelial growth is species-specific where some species might show optimal growth at temperature of 30–35 °C. For instance, mycelia of the edible fungi Volvariella volvacea grew faster at temperature as high as 35 °C (Zervakis et al. 2001).
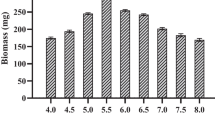
Effect of pH on mycelial growth of C. cibarius
The pH of the culture media also plays a significant role in the growth of the mycelia of basidiomycetes. Although fungi are capable of growing over a wide range of pH (Neto et al. 2009), the optimum pH value of the growth media is crucial to obtain maximum mycelial growth. The pH of the medium was found to substantially affect the mycelial growth and pigment production of C. cibarius. The mycelial growth significantly (p < 0.05) increased with an increase in the pH from 5 to 6. The maximum growth was observed at a pH of 6. An increase in the pH beyond this, lead to a significant decrease in the mycelial growth. The lowest mycelial growth was observed at pH 8 (Fig. S2, supplementary data). These results are in accordance with a previous report on the pH optima for the mycelial growth of C. cibarius, where the highest growth was observed at a pH of 5.5 and 6, whereas the growth dropped at lower or higher pH values (Straatsma and van Griensven 1986). Similar study was performed by Kalyoncu et al. (2009) on the growth of various species of the edible fungi of the genus Morchella. Alike the results obtained in this study, the authors reported a pH optima of 6 for the mycelial growth of M. intermedia, and the lowest growth was observed at a pH of 8.
Effect of carbon source and concentration on mycelial growth
The substrates that are used to cultivate mushrooms greatly affect their chemical, sensorial, and functional characteristics (Bellettini et al. 2019). Considering this, it becomes crucial to study the effect of the carbon and nitrogen sources on the growth of mushrooms. The effect of carbon sources such as glucose, fructose, sucrose, galactose, and xylose was studied on the mycelial growth of C. cibarius (Fig. 1). The maximum growth was observed in case of sucrose as the carbon source, followed by fructose, glucose, xylose, and galactose, in that order. Sucrose showed growth twice as much as the control. In case of glucose and xylose, the growth was statistically similar. Utilization of sucrose could be due to the activity of invertase enzyme leading to maximum mycelial growth.
On the basis of visual inspection, cultures grown on sucrose showed the most intense yellow pigment production, followed by fructose and glucose (Fig. 2). Thus, the pigmentation could be correlated with the mycelial growth which is in turn dependent on the carbon source and its utilisation. Chen and Johns (1994) studied the effect of carbon sources such as glucose and maltose on the pigment production by Monascus purpureus. The authors observed the highest pigmentation using maltose as the carbon source, especially with peptone as the nitrogen source. Thus, the variation in the carbon source affects not only the mycelial growth, but also the pigmentation.
After choosing sucrose as the optimal carbon source, its concentration in the nutrient medium was studied in the range of 0.5–5% (Fig. S3, supplementary data). Significantly low growth was observed with 0.5% sucrose, and increased with an increase in the concentration up to 2%. Further increase in the sucrose concentration up to 5% lead to a decrease in the mycelial growth. Such a drop in the mycelial growth with an increase in the concentration of carbon source has been reported (Yuan et al. 2012; Itoo and Reshi 2014). Hence, 2% was selected as the optimal sucrose concentration.
Effect of nitrogen source on mycelial growth and pigmentation
Apart from the carbon source, the growth and pigmentation of fungi is also dependent on the nitrogen source. In the present study, the mycelial growth of C. cibarius was evaluated for various nitrogen sources such as beef extract, yeast extract, peptone, ammonium nitrate, ammonium sulphate, ammonium citrate, sodium nitrate, and potassium nitrate. As shown in Fig. 3, the highest mycelial growth was seen and was statistically similar (p < 0.05) in case of ammonium sulphate, peptone, and sodium nitrate. The lowest growth was seen in case of yeast extract, followed by beef extract, ammonium citrate, ammonium nitrate, and potassium nitrate, in that order. Ammonium sulphate has been reported to show a positive effect (p ≤ 0.05) on the biomass and phenolic content gain (Schmidt and Furlong 2012). Ammonium sulphate has also been reported as a better supplement for primordial initiation in case of Pleurotus florida cultivation (Naraian et al. 2009). Considering this ammonium sulphate was chosen as the suitable nitrogen source.
Researchers have shown that pigment production by mushrooms is affected by factors such as the nature of nitrogen source and pH of the growth media (Chen and Johns 1993). In the present study, the nitrogen source affected not only the mycelial growth, but also the pigmentation. The pigmentation of the colonies did not vary with the use of peptone, ammonium nitrate, ammonium sulphate, ammonium citrate, sodium nitrate, and potassium nitrate as the nitrogen sources. However, in case of beef and yeast extracts, the colour of the colonies was observed to be dark grey (Table 1). The effect of beef extract and yeast extract on pigmentation has already been reported in case of the fungi Fusarium verticillioides (Boonyapranai et al. 2008). The change caused by complex nitrogen sources (beef extract and yeast extract) may be due to alteration in pH due to metabolites or bio-actives released by the mycelium (Fig. 4).
The effect of the concentration of ammonium sulphate was also studied on the mycelial growth of C. cibarius in the range of 0.5–5% (Fig. S4, supplementary data). The optimal mycelial growth was observed at the lowest tested concentration i.e. 0.5%. Further increase in the concentration lead to a significant decrease in the mycelial growth. Such decrease in the mycelial colony diameter with an increase in the concentration of nitrogen sources has been reported in case of Rhizoctonia solani (Koehler and Miller 2017).
Effect of carbon to nitrogen (C:N) ratio on the mycelial growth
Apart from the source and concentrations of carbon and nitrogen sources, the carbon:nitrogen (C:N) ratio in the growth medium also acts as a significant factor in mycelial growth in anexic cultivation (Mantovani et al. 2007). The C:N ratio has also been reported to affect the exopolysaccharide production (Kim et al. 2002). The presence of nitrogen in excess, or its lack leads to compromised fungal growth. Excessive nitrogen causes the reduction in the substrate compounds such as ammonium sulphate and urea, which are converted to ammonia (Mantovani et al. 2007). The toxicity of ammonia depends on its concentration, and hence excessive ammonia could substantially hinder the mycelial growth. On the other hand, excessive carbon is readily converted and stored as glycogen (Mantovani et al. 2007). This explains the higher proportion of carbon in culture media used for fungi.
In the present study, the C:N ratio was studied ranging from 2:0.25 to 2:2 (%) (Fig. 5). The optimal mycelial growth was observed for C:N ratios of 2:0.5 and 2:1. This is close to 20:1 which is the optimal C:N ratio for mycelial growth of most of the fungal species. For instance, for A. bisporous, the optimal C:N ratio ranges between 15:1 and 17:1 (Mantovani et al. 2007). In another study (Dong and Yao 2005) a C:N ratio of 12:1 was reported for the optimal growth of the medicinal fungi Cordyceps sinensis. A ratio of 12:1 has also been reported as optimal for the growth of the fungi Paecilomyces sinclairii (Kim et al. 2002). However, in case of certain fungi such as Pleurotus tuber-regium, an optimal C:N ratio as high as 24:1 has been reported (Wu et al. 2004). In the present study, increasing the C:N ratio from 2:1 to 2:2 lead to a significant (p < 0.05) decrease in the mycelial growth. This could be due to generation of ammonia from excessive ammonium sulphate, leading to a compromised growth.
Conclusion
Although extensive research has been carried out on various edible fungi, C. cibarius remains less studied for its growth requirements. The present study focused on assessing the growth requirements of the edible mushroom C. cibarius. The growth was significantly affected with variations in the parameters. The use of yeast and beef extracts as nitrogen sources showed substantial variations in the pigmentation of the colonies, where the colour changed from yellow/orange to dark grey. Further investigation is required for understanding the change in metabolism of such complex organic nitrogen sources. This study could be considered as a base to lead to additional insights into optimum growth requirements. We emphasize on the fact that C. cibarius is a popular edible mushroom and has great economic value. Knowledge of the nutritional requirements of C. cibarius would be helpful in improving its cultivation technology. Further investigations in liquid media with varying carbon and nitrogen sources needs to be carried out to study pH variations and release of metabolites.
References
Barros L, Venturini BA, Baptista P, Estevinho LM, Ferreira IC (2008) Chemical composition and biological properties of Portuguese wild mushrooms: a comprehensive study. J Agric Food Chem 56:3856–3862. https://doi.org/10.1021/jf8003114
Bellettini MB, Fiorda FA, Maieves HA, Teixeira GL, Ávila S, Hornung PS, Júnior AM, Ribani RH (2019) Factors affecting mushroom Pleurotus spp. Saudi J Biol Sci 26:633–646. https://doi.org/10.1016/j.sjbs.2016.12.005
Boonyapranai K, Tungpradit R, Lhieochaiphant S, Phutrakul S (2008) Optimization of submerged culture for the production of naphthoquinones pigment by Fusarium verticillioides. Chiang Mai J Sci 35:457–466
Chen MH, Johns MR (1994) Effect of carbon source on ethanol and pigment production by Monascus purpureus. Enzyme Microb Technol 16:584–590. https://doi.org/10.1016/0141-0229(94)90123-6
Chen MH, Johns MR (1993) Effect of pH and nitrogen source on pigment production by Monascus purpureus. Appl Microbiol Biotechnol 40:132–138. https://doi.org/10.1007/BF00170441
Dong CH, Yao YJ (2005) Nutritional requirements of mycelial growth of Cordyceps sinensis in submerged culture. J Appl Microbiol 99:483–492. https://doi.org/10.1111/j.1365-2672.2005.02640.x
Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Eslami S (2010) Antioxidant and free radical scavenging activities of culinary-medicinal mushrooms, golden chanterelle Cantharellus cibarius and Angel’s wings Pleurotus porrigens. Int J Med Mushrooms. https://doi.org/10.1615/IntJMedMushr.v12.i3.50
Haxo F (1950) Carotenoids of the mushroom Cantharellus cinnabarinus. Bot Gaz 112:228–232. https://doi.org/10.1086/335653
Hoa HT, Wang CL (2015) The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 43:14–23. https://doi.org/10.5941/MYCO.2015.43.1.14
Itoo ZA, Reshi ZA (2014) Effect of different nitrogen and carbon sources and concentrations on the mycelial growth of ectomycorrhizal fungi under in-vitro conditions. Scand J For Res 29:619–628. https://doi.org/10.1080/02827581.2014.964756
Kalyoncu F, Oskay M, Kalyoncu M (2009) The effects of some environmental parameters on mycelial growth of six Morchella species. J Pure Appl Microbiol 3:467–472
Kim SW, Hwang HJ, Xu CP, Na YS, Song SK, Yun JW (2002) Influence of nutritional conditions on the mycelial growth and exopolysaccharide production in Paecilomyces sinclairii. Lett Appl Microbiol 34:389–393.
Koehler JF, Miller GL (2017) Impact of nitrogen source and a ph buffer on the in vitro growth and morphology of Rhizoctonia Solani AG 2–2 LP. Int Turfgrass Soc Res J 13:198–202. https://doi.org/10.2134/itsrj2016.07.0592
Kolundžić M, Stanojković T, Radović J, Tačić A, Dodevska M, Milenković M, Sisto F, Masia C, Farronato G, Nikolić V, Kundaković T (2017) Cytotoxic and antimicrobial activities of Cantharellus cibarius Fr. (Cantarellaceae). J Med Food 20:790–796. https://doi.org/10.1089/jmf.2016.0176
Kozarski M, Klaus A, Vunduk J et al (2015) Nutraceutical properties of the methanolic extract of edible mushroom Cantharellus cibarius (Fries): primary mechanisms. Food Funct 6:1875–1886. https://doi.org/10.1039/c5fo00312a
Kumari D, Reddy MS, Upadhyay RC (2011) Antioxidant activity of three species of wild mushroom genus Cantharellus collected from North-Western Himalaya, India. Int J Agric Biol 13:415–418
Lemieszek MK, Nunes FM, Cardoso C, Marques G, Rzeski W (2018) Neuroprotective properties of Cantharellus cibarius polysaccharide fractions in different in vitro models of neurodegeneration. Carbohydr Polym 197:598–607. https://doi.org/10.1016/j.carbpol.2018.06.038
Mantovani TRD, Linde GA, Colauto NB (2007) Effect of the addition of nitrogen sources to cassava fiber and carbon-to-nitrogen ratios on Agaricus brasiliensis growth. Can J Microbiol 53:139–143. https://doi.org/10.1139/W06-112
Mensah-Attipoe J, Toyinbo O (2019) Fungal Growth and Aerosolization from Various Conditions and Materials. In: Loreto ÉS and Tondolo JSM (Eds) Fungal Infection, IntechOpen.
Naraian R, Sahu RK, Kumar S, Garg SK, Singh CS, Kanaujia RS (2009) Influence of different nitrogen rich supplements during cultivation of Pleurotus florida on corn cob substrate. Environmentalist 29:1. https://doi.org/10.1007/s10669-008-9174-4
Nasim G, Malik SH, Bajwa R, Afzal M, Mian SW (2001) Effect of three different culture media on mycelial growth of oyster and Chinese Mushrooms. J Biol Sci. https://doi.org/10.3923/jbs.2001.1130.1133
Neto SLM, Matheus DR, Machado KMG (2009) Influence of pH on the growth, laccase activity and RBBR decolorization by tropical basidiomycetes. Brazilian Arch Biol Technol 52:1075–1082. https://doi.org/10.1590/S1516-89132009000500003
Nowacka-Jechalke N, Nowak R, Juda M, Malm A, Lemieszek M, Rzeski W, Kaczyński Z (2018) New biological activity of the polysaccharide fraction from Cantharellus cibarius and its structural characterization. Food Chem 268:355–361. https://doi.org/10.1016/j.foodchem.2018.06.106
Nyman AAT, Aachmann FL, Rise F, Ballance S, Samuelsen ABC (2016) Structural characterization of a branched (1 → 6)-α-mannan and β-glucans isolated from the fruiting bodies of Cantharellus cibarius. Carbohydr Polym 146:197–207. https://doi.org/10.1016/j.carbpol.2016.03.052
Palacios I, Lozano M, Moro C, D’arrigo M, Rostagno MA, Martínez JA, García-Lafuente A, Guillamón E, Villares A, (2011) Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem 128:674–678. https://doi.org/10.1016/j.foodchem.2011.03.085
Pamire M, Parawira W (2020) Spore germination for three edible Zimbabwean mushrooms using biostimulants. Tanzania J Sci 46:129–136
Pilz D (2003) Ecology and management of commercially harvested chanterelle mushrooms. USDA For Serv - Gen Tech Rep PNW. https://doi.org/10.2737/PNW-GTR-576
Sandmann G, Misawa N (2002) Fungal Carotenoids. In: Osiewacz HD (ed) Industrial Applications. Springer, Berlin, Heidelberg, pp 247–262
Schmidt CG, Furlong EB (2012) Effect of particle size and ammonium sulfate concentration on rice bran fermentation with the fungus Rhizopus oryzae. Bioresour Technol 123:36–41. https://doi.org/10.1016/j.biortech.2012.07.081
Straatsma G, Van Griensven LJLD (1986) Growth requirements of mycelial cultures of the mycorrhizal mushroom Cantharellus cibarius. Trans Br Mycol Soc 87:135–141. https://doi.org/10.1016/s0007-1536(86)80013-4
Wang Z, Wang H, Kang Z, Wu Y, Xing Y, Yang Y (2020) Antioxidant and anti-tumour activity of triterpenoid compounds isolated from Morchella mycelium. Arch Microbiol 13:1–9. https://doi.org/10.1007/s00203-020-01876-1
Wu JZ, Cheung PCK, Wong KH, Huang NL (2004) Studies on submerged fermentation of Pleurotus tuber-regium (Fr.) Singer. Part 2: Effect of carbon-to-nitrogen ratio of the culture medium on the content and composition of the mycelial dietary fibre. Food Chem 85:101–105. https://doi.org/10.1016/j.foodchem.2003.06.009
Yuan B, Chi X, Zhang R (2012) Optimization of exopolysaccharides production from a novel strain of Ganoderma lucidum CAU5501 in submerged culture. Brazilian J Microbiol 43:490–497. https://doi.org/10.1590/S1517-83822012000200009
Zervakis G, Philippoussis A, Ioannldou S, Dlamantopoulou P (2001) Mycelium growth kinetics and optimal temperature conditions for the cultivation of edible mushroom species on lignocellulosic substrates. Folia Microbiol (Praha) 46:231. https://doi.org/10.1007/BF02818539
Acknowledgement
The authors wish to thank Mr. Antti Kinnunen for administrative service and Mrs. Heli Valtonen for her technical assistance. We appreciate and are grateful for the financial support of Regional Council of Southern Savo, Finland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Deshaware, S., Marathe, S.J., Bedade, D. et al. Investigation on mycelial growth requirements of Cantharellus cibarius under laboratory conditions. Arch Microbiol 203, 1539–1545 (2021). https://doi.org/10.1007/s00203-020-02142-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-02142-0