Abstract
In this study, the structure of the purified extracellular eumelanin pigment isolated from Streptomyces spp. was elucidated by detailed analysis via two different spectroscopic techniques (FT-IR and NMR). In vitro antiproliferative effects of eumelanin were evaluated on HeLa cell line. These experiments were carried out with the evaluation of the parameters including cell viability, cell index, and mitotic index. With the cell viability and cell index, IC50 concentration of eumelanin was determined as 10 μM. This result showed that the IC50 concentration of eumelanin decreased the values of cell viability, cell index and mitotic index. These changes are statistically significant (p < 0.01). The ability of the dissolved eumelanin (250 μg mL−1) to scavenge free radicals was determined via DPPH and ABTS and was shown to be about 87.73% and 75.2%, respectively, compared with standard antioxidants. It was observed that dry weights of eumelanin yield among the selected strains ranged from 160 to 240 mg L−1. The strain with the highest production potential was selected for 16S rDNA sequence analysis and, accordingly, the selected strain BSB49 was identified as Streptomyces parvus and the sequence analysis results were deposited in NCBI under accession number MK894155.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pigments are substances that give color to living and/or non-living objects as the result of their selective color absorption. These colored objects are divided into two main groups as natural and synthetic pigments. In recent years, there has been an increasing demand for natural pigments over synthetic counterparts because of their nontoxic, nonpolluting, non-carcinogenic, nonpoisonous, and environmentally friendly nature. Additionally, many synthetic pigments have been banned or being banned by WHO (World Health Organization), FDA (U.S. Food and Drug Administration) and EFSA (the European Food Standards Authority) due to their toxic, allergic, carcinogenic properties besides other adverse effects (Tuli et al. 2015).
To date, a large number of studies have been carried out to produce natural pigments instead of synthetic ones. For this purpose, plants, animals and microorganisms have been used; however, many different studies showed that microorganisms are much more suitable and versatile compared to other living organisms (Tuli et al. 2015). Microbial pigments have key production advantages based on their features such as reproducibility and manageability, as well as being independent of geographic conditions and season (Joshi et al. 2003; Geng et al. 2010; Venil et al. 2013).
Microorganisms are able to produce versatile pigments such as prodigiosin, zeaxanthin, astaxanthin, lycopene, β-carotene, and melanin. These colored compounds have been utilized for many different industrial areas such as food, textiles, cosmetics, and pharmaceuticals. Among all these microbial pigments, melanin has recently aroused the interest of scientists because of its chemical, physical, and biological properties (Eisenman and Casadevall 2012; Venil et al. 2013; Kumar et al. 2015).
Melanins are complex natural pigments, which are widely distributed in nature from microorganisms to humans. These ubiquitous heteropolymer pigments are available in a range of colors like black, brown, and red. These pigments are classified as eumelanins, allomelanin, pheomelanin, pyomelanin, and neuromelanin and biosynthesized from different sources through different biochemical pathways. While eumelanin is produced by oxidation and polymerization of tyrosine amino acid with tyrosinase enzyme, allomelanin is produced from catechol in the presence of polyphenol oxidase. These dark colored and negatively charged pigments also have a high molecular weight and hydrophobic structure (Banerjee et al. 2014).
Melanin pigment gives advantage to microorganisms under various adverse environmental conditions and thus increase the chances of survival of microorganisms. Melanins play an important role in protecting microorganisms from various environmental stresses such as UV radiation, heavy metals, desiccation, temperature fluctuations, and hydrolytic enzymes and digestion (Rehnstrom and Free 1996; Paolo et al. 2006).
These complex macromolecules are widely used in the fields of medicine and pharmacology, since it possesses crucial biological activities such as antioxidant, antivenin, antiinflammatory, antimicrobial, antiviral, antitumor, and liver protective activity (Montefiori and Zhou 1991; Avramidis et al. 1998; Sava et al. 2003; Hung et al. 2004; El-Obeid et al. 2006; Sivaperumal et al. 2015; Srisuk et al. 2016; El-Naggar and El-Ewasy 2017). Another important application area for these biopolymers is sunscreen in the cosmetic industry with their unique UV absorption properties (Kalka et al. 2000; Joshi et al. 2003). In addition, melanin has also been known for amorphous organic semiconductor properties for decades (Mostert et al. 2012).
Recently, the interest on the melanin pigment has focused on its drug delivery properties. In a study reported by Araújo et al. (2014), metronidazole was loaded onto the melanin nanoparticles using supercritical CO2 (scCO2) technology (Araújo et al. 2014). This study showed that melanin nanoparticles are promising biocompatible drug nanocarrier candidates for the treatment of intestinal diseases such as amebiasis, giardiasis, and Crohn’s disease.
In another study, Zhang et al. (2015) used melanin nanoparticles as an efficient drug-delivery system for imaging-guided chemotherapy. Melanin nanoparticles with their biocompatibility and biodegradability properties have been utilized as an alternative to inorganic nanosystems (quantum dots, metal, and metal oxide) which exhibit toxic effects (Zhang et al. 2015).
Herein, Streptomyces strains isolated from different soil samples were qualitatively evaluated in terms of eumelanin pigment production potential. Eumelanin pigment-producing strain were transferred to a general purpose liquid media (nutrient broth = NB) and the optimal conditions for this strain to produce pigments were determined. The obtained eumelanin pigments as monomers and/or oligomers in liquid medium were polymerized and purified. The strain with the highest eumelanin production potential was selected for 16S rDNA sequence analysis. According to the 16S rDNA sequence analysis result, the strain BSB49 has been identified as Streptomyces parvus and the sequence result of the purified 16s rDNA was deposited in the NCBI GenBank with the accession number MK894155. The in vitro antiproliferative effect of the purified eumelanin pigment was assessed using the HeLa cell line and these experiments were carried out with the evaluation of the parameters including cell viability, cell index, and mitotic index. Next, the structure of the purified eumelanin pigment was elucidated via Fourier-transform infrared spectroscopy (FT-IR) and nuclear magnetic resonance (NMR) spectroscopy studies. At the final stage, the antioxidant properties of the purified eumelanin pigment were determined using two different methods (ABTS and DPPH).
Materials and methods
Pretreatments in isolation of Streptomyces strains
Streptomyces strains have been isolated from different soil samples from Bayburt province (Turkey). During the isolation processes made from soil, the air-dried soil samples were first mixed with CaCO3 in a ratio of 10:1 w/w and incubated at 37 °C for 4–7 days in a humid environment (Korn-Wendisch and Kutzner 1991). Dilutions prepared from pre-treated soil samples were seeded into ISP 2 medium and incubated at 26 °C for 2 weeks. Different colonies observed on Petri plates on the 3rd, 5th, 7th, and 14th days of incubation were isolated by transferring them to new media (ISP 2). The isolated pure Streptomyces strains have been stored at −80 °C in 1/2 nutrient broth containing 20% glycerol until further use (Cacchio et al. 2003; Şahin and Uğur 2003; Anansiriwattana et al. 2006).
Determination of eumelanin pigment producing strains
In this study, ISP2 agar and ISP4 agar were used to determine the eumelanin pigment production properties of Streptomyces strains. For this purpose, previously isolated Streptomyces strains were seeded (about 1 m2) to Petri dishes containing ISP2 and ISP4 medium and incubated 30 °C for 96–120 h (El-Naggar and El-Ewasy 2017). At the end of the incubation period, colonies that have dark colored extracellular pigment around it were considered to be positive for eumelanin pigment production (See Supplementary Figure 1).
Total genomic DNA extraction and PCR amplification of 16S rDNA
DNA extraction and PCR amplification process were performed with small modification based on the method reported by Dahal et al. (2017). Genomic DNA was extracted from pure cultures using the QiaAmp DNA Mini Kit (Qiagen) and stored at −20 °C until use. 16S rDNA gene was amplified by PCR using universal primers 27F and 1492R. The PCR was performed in 70 μL reactions consisting of 0.7 μL 5 unit μL−1 Taq DNA polymerase, 7 μL 10 × PCR buffer, 1.4 μL dNTP mix, 0.7 μL 50 μM primer 27F (forward 5′-AGA GTT TGA TCC TGG CTC AG-3′), 0.7 μL 50 μM primer 1492R (reverse 5′-CGG TTA CCT TGT TAC GAC TT-3′), 4.2 μL (25 mM) MgCl2, and 51 μL sterile distilled water. Finally, 1.5 μL extracted genomic DNA was added to the mixture and the total volume was completed to 70 μL. The PCR conditions were: initial denaturation at 95 °C for 3 min, 36 cycles 94 °C for 1 min, 53 °C for 1 min and 72 °C for 2 min and a final elongation at 72 °C for 5 min (Dahal et al. 2017).
Purification of eumelanin pigment
Streptomyces strains capable of producing eumelanin pigment in solid media were transferred to sterile liquid media (nutrient broth) and incubated in orbital shaker set at 35 °C, 200 rpm for 1 week (See Supplementary Figure 1). At the end of the 1-week incubation period, pigment-producing samples were centrifuged at 10,000 rpm for 10 min and this process was repeated twice. After the centrifugation, pH of the cell-free supernatant was adjusted to 2.0 with 6 M HCl and was allowed to stand at room temperature for 24 h. The obtained suspension was again centrifuged at 10,000 rpm for 10 min and precipitates were carefully separated from the supernatant. The eumelanin pigment separated from the supernatant was washed with distilled water three times and centrifuged at 10,000 rpm for 10 min. After this process, the purified eumelanin pigment was lyophilized and stored at −20 °C until use (Tarangini and Mishra 2014; El-Naggar and El-Ewasy 2017).
Dissolution of eumelanin pigment
Eumelanin is insoluble in acidic solutions and in common organic solvents. Therefore, to record the UV–VIS absorption spectrum (200–800 nm) and to study the radical-scavenging acitivities, the purified eumelanin pigments were dissolved in an ultrasonic water bath (Kudos) at 35 kHz with dimethylsulfoxide. Eumelanin was kept in an ultrasonic water bath at 60 °C for 30 min.
UV–VIS absorption spectroscopy of the purified eumelanin
The purified eumelanin powder was first dissolved in DMSO and then scanned in a UV–visible spectrophotometer (Epoch 2, Biotek Instruments) at UV, visible, and near-infrared wavelengths (200–800 nm). The blank control was conducted with DMSO.
Cell culture
HeLa cells used as human cervical cancer model were grown in Dulbecco’s modified Eagle’s medium (DMEM, high glucose) (Gibco: Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 2 mM l-glutamine and 10% fetal bovine serum (FBS; Gibco: Thermo Fisher Scientific, Inc.) plus antibiotics in a humidified atmosphere with 5% CO2 in air. The pH of the medium was adjusted to 7.40 with NaHCO3. The HeLa cell line used in this experiment was obtained from European cell culture collection (CCL) (Çeti̇n and Topçul 2019).
Eumelanin concentrations
5 μM, 10 μM, and 20 μM concentrations were prepared for HeLa cell line. These concentrations were prepared by dilution of the 1 mM stock solution with DMEM medium.
Cell viability assay
Cell viability was examined using the MTT (thiazolyl blue tetrazolium bromide, Sigma, Missouri, USA) colorimetric assay. HeLa cells (3 × 104 cells well−1) were dispensed into 96-well plate. After 24 h incubation, eumelanin concentrations were added to each well. At the end of the experimental period, the medium in each well was removed and 40 μL fresh MTT solution (5 mg mL−1 in PBS) was added into each well and cells were incubated at 37 °C for 4 h. After incubation, 160 μL of DMSO (solubilizing reagent) was added to each well and shaken thoroughly for 1 h on a shaker. Then, the absorbance of the samples was measured against a background control as a blank using an Elisa reader (μQuant, Bio-Tek Instruments Inc Vermont, USA) at 450–690 nm (Cetin and Topcul 2017).
Cell index (CI)
Real-time cell proliferation monitoring HeLa cells were seeded at a density of 6000 cells well−1 into an E-plate 16 (ACEA Biosciences, San Diego, CA) containing 100 μL medium per well and monitored on the xCELLigence real-time cell analyzer dual plate (RTCA DP) instrument (ACEA Biosciences). When the cells entered log phase, the different eumelanin concentrations were added to final concentrations of 5 μM, 10 μM and 20 μM. The cells were treated with eumelanin for 72 h and incubated at 37 °C in a 5% CO2 atmosphere. To calculate the proliferation of the cells, RTCA software v. 1.2.1 was used (Topçul et al. 2018).
Mitotic index (MI)
After fixation with Carnoy Fixative, HeLa cells were hydrolyzed by Feulgen method and stained with Giemsa and MI was scored using the following formula: MI = (n/C) × 100. The number of cells in the mitotic phases (including the late prophase, metaphase, anaphase and telophase; n) per total cells was determined (3000–3500; C) (Topcul and Cetin 2016).
Determination of antioxidant activity
ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) radical-scavenging activity
The ABTS reagent prepared at 7 mM concentration was dissolved in water. The ABTS radical cation was reacted with 2.45 mM potassium persulfate in water and left in the dark at room temperature for 12–16 h before use. For the spectrophotometric analysis of samples, the ABTS solution was diluted with methanol to an absorbance of 0.70 at 734 nm and equilibrated at 30 °C.
The extracts were diluted with 1 mL of sample and 1 mL of ABTS solution, and then diluted with methanol to a total volume of 5 mL. After the tubes were kept closed at room temperature for 6 min, the absorbances of the samples were read at 734 nm on a 96-well plate spectrophotometer (Biotek Instruments). Butylated hydroxytoluene (BHT) was used as a positive control. The scavenging activity (%) values of the eumelanin were calculated by using Eq. 1.1 for the ABTS method, where AC is the absorbance of the control and AS is the absorbance of eumelanin (Re et al. 1999). All tests were performed in triplicate and the values were shown as mean ± SD.
Scavenging effect (%) \(=\left[\frac{Ac-As}{Ac}\right]\times 100.\)
Assay of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity
The antioxidant activity of purified eumelanin was investigated based on the free radical scavenging effect of 1,1-diphenyl-2-picrylhydrazulin (DPPH) with some modifications in a previously described method by Moon and Terao (1998). Diluted working solutions of test compounds were prepared in methanol. One milliliter of DPPH (0.02%) was mixed with 1 mL of the eumelanin in dimethylsulfoxide (DMSO) diluted with methanol to a total volume of 5 mL. The mixture was shaken vigorously and allowed to stand in the dark for 30 min. The absorbance of the resulting solution was measured at 517 nm on a 96-well plate spectrophotometer (Biotek Instruments). BHT was used as a positive control. DPPH radical-scavenging activity was calculated using following Eq. 1.1 for DPPH method. The concentrations of the test compound were plotted as standard curves (Moon and Terao 1998). All tests were performed in triplicate and the values were shown as mean ± SD.
1H NMR spectroscopy
The 1H NMR spectrum was obtained by Bruker AVANCE III 400 MHz NMR Spectrometer at 25 °C (9 T). Chemical shifts δ were reported in ppm downfield from tetramethylsilane using the residual deuterated solvent signals as an internal reference [(CD3)2SO: δH = 2.50 ppm].
Fourier transform-infrared (FT-IR) spectroscopy
Infrared (IR) spectra were recorded on a Perkin-Elmer FT-IR spectrometer and reported as wavenumbers Ṽ (cm–1) with band intensities indicated as s (strong), m (medium), w (weak), b (broad).
Results and discussion
Eumelanin production and structure elucidation
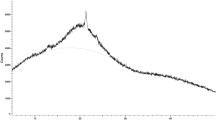
In this study, eumelanin pigment production potentials of Streptomyces strains isolated from different soil samples collected from Bayburt, Turkey, were evaluated in some general purpose liquid media (See Supplementary Figure 1). The purified pigment was analyzed with nuclear magnetic resonance (NMR) spectra (Fig. 1) and Fourier-transform infrared spectroscopy (FT-IR) (Fig. 2) and it has been observed that our spectroscopic findings are compatible with the results of the El-Naggar and El-Ewasy (2017). UV–visible spectra of purified pigment resembled the spectrum results of Ye et al. (2011) and obtained results from UV–vis, NMR, FT-IR, supporting that the purified pigment is eumelanin.
The 1H NMR spectrum of the extracted eumelanin pigment in DMSO-d6 was measured on a Bruker AVANCE III 400 MHz NMR Spectrometer at 25 °C (9 T). The extracted eumelanin polymer is sparingly soluble in DMSO-d6. Its spectroscopic data are in agreement with those previously reported (El-Naggar and El-Ewasy 2017). Chemical shifts (δ) are reported in ppm downfield from SiMe4, with the residual solvent signal as an internal reference. The overall pattern shows that signals between 6.00 and 8.00 ppm belong to the aromatic groups (indole/pyrrole repeating units). The line broadening in the aromatic region is probably due to irregular structural nature of the eumelanin polymers (Katritzky et al. 2002). On the other hand, resonance signals at the higher field of the diagram (0.50–2.30 ppm) represents residual protein peaks. Earlier literature report misassigned signals between 3.20–4.30 ppm and the protons attached to the N and O atoms (El-Naggar and El-Ewasy 2017). We would like to highlight that these regions were covered by the residual solvent (DMSO and H2O) signals (2.50 ppm and 3.33 ppm, respectively). Acidic OH/NH signals may overlap with the residual solvent peaks or may not be seen due to fast deuterium–proton exchange in DMSO-d6.
We subsequently used IR spectroscopy to further identify the structure of the extracted eumelanin pigments. The results obtained show strong correlations with the previous studies. A strong, broad band at around 3265 cm–1 indicates the presence of –OH groups. A weak signal at 2923 cm–1 can be assigned as the C–H stretching band (Coates 2006). The broad IR absorption in the region between 2500 cm–1 and 3600 cm–1 represents overlapped IR bands of amine, amide, carboxylic acid functional groups present in the indole and pyrrole core structures. The IR band near 1632 cm–1 in Fig. 2 is attributed to the NH bending. Additionally, the band at around 1528 cm–1 (N–H bending) indicates the presence of indole structure in the eumelanin polymer. Another characteristic IR band of eumelanin is observed near 1450 cm–1 (CH2–CH3 bending). The peak near 1210 cm–1 may be attributed to phenolic COH stretching. Based on the close similarities between literature and our experimental results, we propose that the extracted melanin pigment resembles the eumelanin structure (El-Naggar and El-Ewasy 2017).
Cell viability
The absorbance values of eumelanin on HeLa cell line at a concentration of 5 μM, 10 μM, and 20 μM for 24 h were decreased from 350 × 10−3 to 340 × 10−3; 170 × 10−3; 50 × 10−3, respectively 5 μM, 10 μM and 20 μM (Fig. 3). A statistically significant decrease in absorbance values especially at 10 μM and 20 μM concentrations was observed as a result of eumelanin application at 24 h (p < 0.01).
The results indicated that 24 h after the administration of eumelanin concentrations to HeLa cells, viability values were 97.14% for 5 μM; 48.57% for 10 μM and 14.28% for 20 μM compared to the control group which was considered as 100% (See Supplementary Figure 2). When the % viability values were examined, the IC50 concentration that caused the death of half of the cells in culture was determined to be 10 μM for HeLa cells. All other experiments were performed using IC50 values of the cells.
When the cell viability of HeLa cells for 72 h was examined, it was seen that the absorbance values decreased from 352 × 10–3 to 172 × 10–3 for 24 h; from 423 × 10–3 to 88 × 10–3 for 48 h and from 596 × 10–3 to 71 × 10–3 for 72 h (See Supplementary Figure 3).
Cell index
Cell index values obtained from xCELLigence RTCA system demonstrated that eumelanin had significant antiproliferative effects on HeLa cell line. Curves belonging to graphics suggest that 10 μM baicalein has DNA damaging effect, while 20 μM eumelanin has cytostatic effect on HeLa cells (see Supplementary Figure 4).
Mitotic index
As a result of 10 μM eumelanin application to HeLa cell line, mitotic index values decreased from 4.64 to 2.96 at 24 h, from 5.08 to 2.35 at 48 h and from 5.25 to 1.07 at 72 h (Fig. 4). The mitotic index values obtained as a result of the eumelanin application in the IC50 concentration to HeLa cells for 72 h showed a statistically significant decrease (p < 0.01) in a time-dependent manner.
Antioxidant activity
The pure eumelanin purified from Streptomyces strains were compared with the standard antioxidant BHT by DPPH radical-scavenging activity method. The results show that eumelanin has a high scavenging activity of 100 μg mL−1 (64.73%). Positive control BHT showed a radical-scavenging activity of 86.66%. ABTS radicals also quickly and effectively reacted with eumelanin pigment. Percent scavenging activity was 45.93% and 75.92% for eumelanin of 100 μg mL−1 and 250 μg mL−1, respectively (Fig. 5). However, the scavenging activity of BHT, a known antioxidant, which was used as a positive control, was determined 88.20% (100 μg mL−1). The radical-scavenging activity of pure melanin isolated from Klebsiella was compared to that of standard antioxidants (Sajjan et al. 2013). The results suggest that melanin with DPPH method has 74% scavenging activity (50 μg mL−1). In the ABTS method, the percent scavenging activity was 98% and 48.5% for eumelanin of 50 μg mL−1 and 25 μg mL−1, respectively.
In another study, the purified eumelanin pigment of the Streptomyces glaucescens strain NEAE-H has been reported to possess a good antioxidant activity. The results indicated that 100 μg mL−1 of eumelanin showed 57.2% radical-scavenging activity (El-Naggar and El-Ewasy 2017). Eumelanin showed better results with DPPH radical than ABTS radical. Eumelanin pigment shows strong scavenging activity against both DPPH and ABTS radicals, indicating that it may be a useful therapeutic agent for the treatment of radical-related pathological damage. The eumelanin pigment acts as an antioxidant that is readily available due to the presence of unconjugated electrons in its structure by interacting with free radicals and other reactive species, and recommending it to be used as a raw cosmetic material to minimize toxin-induced tissue destruction.
Spectrophotometric measurements
Eumelanin is a polyphenolic polymer with an irregular structure produced by the oxidation of polyhydric phenols. In general, natural eumelanin is only soluble in alkaline solutions, but not in water, acid solutions, and organic solvents. In addition, it can be oxidized by oxidants, is resistant to chelate metal ions and is capable of absorbing UV–VIS light. The ultraviolet (UV) invisible light absorption spectrum of the purified eumelanin of Streptomyces strains showed the absorption peak at 230 nm (See Supplementary Figure 5). The eumelanin pigment is strongly absorbed in the UV region and gradually decreases as the wavelength increases. This is due to the presence of many complex conjugated structures in the eumelanin molecule (Cockell and Knowland 1999). The decrease in absorption with increasing wavelength is almost linear to melanins. Therefore, the slopes of linear parcels are often used to describe eumelanins (Ravishankar et al. 1995). The UV–visible absorbance (200–800 nm) spectrum of the purified pigment showed a strong absorbance in the UV region and a characteristic absorption peak at 230 nm was observed. At 250–280 nm, a spectrum similar to the synthetized eumelanin was obtained with a small shoulder (Madhusudhan et al. 2014), suggesting the presence of phenol groups.
Antiproliferative effects
In a study performed by Ganesh Kumar et al. (2013), it was demonstrated that eumelanin pigment possesses antiproliferative effect against two different tumor cell lines (human lung epithelial carcinoma and human epithelial cervical cancer). In this study, to determine cell viability, MTT colorimetric assay was used and tumor cells were exposed to various concentrations of eumelanin for 48 h. The greatest cell viability they observed was a concentration of 50 μg mL−1. In addition to this study, El-Naggar and El-Ewasy (2017) tested in vitro cytotoxicity and anticancer activities of purified melanin pigment from Streptomyces glaucescens strain NEAE-H, against skin cancer cell line (HFB4) using MTT (El-Naggar and El-Ewasy 2017). In this study, both safety and the anticancer activities were measured in vitro on both cancerous [skin cancer cell line (HFB4)] and non-cancerous cells [human lung fibroblast (WI-38) and human amnion (WISH)]. To determine the inhibitory effects of melanin pigment on cell growth, HFB4 (skin cancer cell line) was used. The obtained results in this study revealed that the IC50 value was 16.34 ± 1.31 μg mL−1 for melanin pigment and 8.8 ± 0.5 μg mL−1 for standard 5-fluorouracil that is an antineoplastic antimetabolite and at a concentration 50 μg mL−1 melanin pigment inhibited cell viability by 70.9%. Lastly, in a study, Arun et al. (2015) revealed that the in vitro inhibition of Hep 2 (human epidermoid larynx carcinoma cell line) proliferation correlated with concentration. The melanin pigment was shown to inhibit cell life by 53% at a concentration of 60 μg mL−1.
Conclusion
When the results obtained in this study were compared with the results obtained from the literature, it was observed that the NMR and FT-IR peaks were found to be consistent and compatible. Based on these results, it was concluded that the purified pigment was eumelanin. The close similarity in the NMR and FT-IR spectroscopy results between our study and current literature supports these results. In addition, in a limited number of in vitro studies, it was determined that eumelanin pigment has an antiproliferative effect against different cell lines and these findings are in accordance with the result of this presented research. However, to support these results and to determine the anticancer activity of this organic biopolymer, further in vitro and in vivo studies needs to be performed. Additionally, it was observed that purified eumelanin pigments from S. parvus BSB49 have radical scavenging and photoprotective properties. Based on these properties, it has been concluded that this eumelanin pigment has the potential to be used in pharmaceutic and cosmetic industries.
References
Anansiriwattana W, Tanasupawat S, Amnuoypol S, Suwanborirux K (2006) Identification and antimicrobial activities of actinomycetes from soils in samed island, and geldanamycin from strain Pc4-3. Thai J Pharm Sci 30(2006):49–56
Araújo M, Viveiros R, Correia TR, Correia IJ, Correia VD, Bonifácio T, Casimiro A, Aguiar-Ricardo A (2014) Natural melanin: a potential pH-responsive drug release device. Int J Pharm 469(1):140–145. https://doi.org/10.1016/j.ijpharm.2014.04.051
Arun G, Eyini M, Gunasekaran P (2015) Characterization and biological activities of extracellular melanin produced by Schizophyllum commune. (Fries). Indian J Exp Biol 53:380–387
Avramidis N, Kourounakis A, Hadjipetrou L, Senchuk V (1998) Anti-inflammatory and immunomodulating properties of grape melanin. Inhibitory effects on paw edema and adjuvant induced disease. Arzneim Forsch 48(7):764–771
Banerjee A, Supakar S, Banerjee R (2014) Melanin from the nitrogen-fixing bacterium Azotobacter chroococcum: a spectroscopic characterization. PLoS ONE 9(1):e84574. https://doi.org/10.1371/journal.pone.0084574
Cacchio P, Ercole C, Cappuccio G, Lepidi A (2003) Calcium carbonate precipitation by bacterial strains isolated from a limestone cave and from a loamy soil. Geomicrobiol J 20(2):85–98. https://doi.org/10.1080/01490450303883
Cetin I, Topcul MR (2017) İn vitro antiproliferative effects of nab-paclitaxel with liposomal cisplatin on MDA-MB-231 and MCF-7 breast cancer cell lines. J BUON 22:347–354
Çetin I, Topçul MR (2019) Evaluation of the cytotoxic effect of Ly2109761 on HeLa cells using the xCELLigence RTCA system. Oncol Lett 17:683–687. https://doi.org/10.3892/ol.2018.9556
Coates J (2006) Interpretation of infrared spectra, a practical approach. Encyclopedia of analytical chemistry: applications, theory and instrumentation. doi: https://doi.org/10.1002/9780470027318.a5606
Cockell CS, Knowland J (1999) Ultraviolet radiation screening compounds. Biol Rev 74(3):311–345. https://doi.org/10.1017/S0006323199005356
Dahal B, Nandakafle G, Perkins L, Brözel VS (2017) Diversity of free-Living nitrogen fixing Streptomyces in soils of the badlands of South Dakota. Microbiol Res 195(2017):31–39. https://doi.org/10.1016/j.micres.2016.11.004
Eisenman HC, Casadevall A (2012) Synthesis and assembly of fungal melanin. Appl Microbiol Biotechnol 93(3):931–940
El-Naggar N-A, El-Ewasy SM (2017) Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucescens NEAE-H. Sci Rep 7(2017):42129. https://doi.org/10.1038/srep42129
El-Obeid A, Al-Harbi S, Al-Jomah N, Hassib A (2006) Herbal melanin modulates tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6) and vascular endothelial growth factor (VEGF) production. Phytomedicine 13(5):324–333. https://doi.org/10.1016/j.phymed.2005.03.007
Ganesh Kumar C, Sahu N, Narender Reddy G, Prasad RBN, Nagesh N, Kamal A (2013) Production of melanin pigment from Pseudomonas stutzeri isolated from red seaweed Hypnea musciformis. Lett Appl Microbiol 57(4):295–302. https://doi.org/10.1111/lam.12111
Geng J, Yuan P, Shao C, Yu SB, Zhou B, Zhou P, Chen XD (2010) Bacterial melanin interacts with double-stranded DNA with high affinity and may inhibit cell metabolism in vivo. Arch Microbiol 192(5):321–329. https://doi.org/10.1007/s00203-010-0560-1
Hung Y-C, Sava V, Hong M-Y, Huang GS (2004) Inhibitory effects on phospholipase A2 and antivenin activity of melanin extracted from Thea sinensis Linn. Life Sci 74:2037–2047. https://doi.org/10.1016/j.lfs.2003.09.048
Joshi V, Attri D, Bala A, Bhushan S (2003) Microbial pigments. Indian J Biotechnol 2(2003):362–369
Kalka K, Mukhtar H, Turowski-Wanke A, Merk H (2000) Biomelanin antioxidants in cosmetics: assessment based on inhibition of lipid peroxidation. Skin Pharmacol Physiol 13(3–4):143–149. https://doi.org/10.1038/srep42129
Katritzky AR, Akhmedov NG, Denisenko N, Ov D (2002) 1H NMR spectroscopic characterization of solutions of Sepia melanin, Sepia melanin free acid and human hair melanin. Pigment Cell Res 15(2):93–97. https://doi.org/10.1034/j.1600-0749.2002.1o062.x
Korn-Wendisch F, Kutzner H (1991) The family Streptomycetaceae. The Prokaryotes. A handbook on the biology of bacteria: ecophysiology, isolation, identification, applications, Chap. 41. Springer, Berlin. doi: https://doi.org/10.1002/adma.201502201
Kumar A, Hs V, Singh J, Dwivedi S, Kumar M (2015) Microbial pigments: production and their applications in various industries. Int J Pharm Biol Sci 5(1):203–208
Madhusudhan D, Mazhari BBZ, Dastager SG, Agsar D (2014) Production and cytotoxicity of extracellular insoluble and droplets of soluble melanin by Streptomyces lusitanus DMZ-3. Biomed Res Int 2014:1–11. https://doi.org/10.1155/2014/306895
Montefiori DC, Zhou J (1991) Selective antiviral activity of synthetic soluble l-tyrosine and l-dopa melanins against human immunodeficiency virus in vitro. Antiviral Res 15(1):11–25
Moon JH, Terao J (1998) Antioxidant activity of caffeic acid and dihydrocaffeic acid in lard and human low-density lipoprotein. J Agric Food Chem 46(12):5062–5065. https://doi.org/10.1021/jf9805799
Mostert AB, Powell BJ, Pratt FL, Hanson GR, Sarna T, Gentle IR, Meredith P (2012) Role of semiconductivity and ion transport in the electrical conduction of melanin. Proc Natl Acad Sci 109(23):8943–8947. https://doi.org/10.1073/pnas.1119948109
Paolo WF, Dadachova E, Mandal P, Casadevall A, Szaniszlo PJ, Nosanchuk JD (2006) Effects of disrupting the polyketide synthase gene WdPKS1 in Wangiella [Exophiala] dermatitidis on melanin production and resistance to killing by antifungal compounds, enzymatic degradation, and extremes in temperature. BMC Microbiol 6(1):55. https://doi.org/10.1186/1471-2180-6-55
Ravishankar J, Muruganandam V, Suryanarayanan T (1995) Isolation and characterization of melanin from a marine fungus. Bot Mar 38(1–6):413–416. https://doi.org/10.1515/botm.1995.38.1-6.413
Rehnstrom AL, Free SJ (1996) The isolation and characterization of melanin-deficient mutants of Monilinia fructicola. Physiol Mol Plant P 49(5):321–330. https://doi.org/10.1006/pmpp.1996.0057
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(910):12311237. https://doi.org/10.1016/S0891-5849(98)00315-3
Şahin N, Uğur A (2003) Investigation of the antimicrobial activity of some Streptomyces isolates. Turk J Biol 27(2):79–84
Sajjan SS, Anjaneya O, Guruprasad BK, Anand SN, Suresh BM, Karegoudar T (2013) Properties and functions of melanin pigment from Klebsiella sp. GSK Korean J Microbiol Biotechnol 41(1):60–69. https://doi.org/10.4014/kjmb.1210.10002
Sava V, Hung Y, Blagodarsky V, Hong MY, Huang G (2003) The liver-protecting activity of melanin-like pigment derived from black tea. Food Res Int 36(5):505–511. https://doi.org/10.1016/S0963-9969(02)00199-0
Sivaperumal P, Kamala K, Rajaram R (2015) Bioactive DOPA melanin isolated and characterised from a marine actinobacterium Streptomyces sp. MVCS6 from Versova coast. Nat Prod Res 29(22):2117–2121. https://doi.org/10.1080/14786419.2014.988712
Srisuk P, Correlo VM, Leonor IB, Palladino P, Reis RL (2016) Redox activity of melanin from the ink sac of Sepia officinalis by means of colorimetric oxidative assay. Nat Prod Res 30(8):982–986
Tarangini K, Mishra S (2014) Production of melanin by soil microbial isolate on fruit waste extract: two step optimization of key parameters. Biotechnol Rep 4(2014):139–146. https://doi.org/10.1016/j.btre.2014.10.001
Topçul M, Çeti̇n I, ÖzbaşKolusayin TSOMÖ (2018) In vitro cytotoxic effect of PARP inhibitor alone and in combination with nab-paclitaxel on triple-negative and luminal A breast cancer cells. Oncol Rep 40(1):527–535. https://doi.org/10.3892/or.2018.6364
Topcul MR, Cetin I (2016) In vitro cytotoxic effect of tyrosine kinase inhibitor sunitinib malate alone and in combination with hyperthermia on breast adenocarcinoma MCF-7 cells. J BUON 21(3):556–563
Tuli HS, Chaudhary P, Beniwal V, Sharma AK (2015) Microbial pigments as natural color sources: current trends and future perspectives. J Food Sci Technol 52(8):4669–4678. https://doi.org/10.1007/s13197-014-1601-6
Venil CK, Zakaria ZA, Ahmad WA (2013) Bacterial pigments and their applications. Process Biochem 48(7):1065–1079. https://doi.org/10.1016/j.procbio.2013.06.006
Ye M, Wang Y, Qian M, Chen X, Hu X (2011) Preparation and properties of the melanin from Lachnum singerianum. IJBAS-IJENS 11(3):51–58
Zhang R, Fan Q, Yang M, Cheng K, Lu X, Zhang L, Huang W, Cheng Z (2015) Engineering melanin nanoparticles as an efficient drug–delivery system for imaging-guided chemotherapy. Adv Mater 27(34):5063–5069. https://doi.org/10.1002/adma.201502201
Acknowledgements
This study was financially supported by Scientific Research Projects Coordination Unit of Bayburt University (project number: 2019/01-69001-04). We would like to thank Dr. Nesrin ECEM BAYRAM for her precious help in determining the antioxidant activity of eumelanin pigment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bayram, S., Dengiz, C., Gerçek, Y.C. et al. Bioproduction, structure elucidation and in vitro antiproliferative effect of eumelanin pigment from Streptomyces parvus BSB49. Arch Microbiol 202, 2401–2409 (2020). https://doi.org/10.1007/s00203-020-01956-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-01956-2