Abstract
Endophytic bacteria have been reported to have symbiotic, mutualistic, commensalistic or trophobiotic relationships with various plant parts. As part of its adaptation, many endophytic organisms are known to exhibit properties with multiple beneficial effects to the plant system. Even though many bacterial genera have been identified to have endophytic association, isolation of those which were previously demonstrated well for human association is quite interesting. In the study, endophytic bacteria Ceb1 isolated from the rhizome of Curcuma longa was identified by 16S rDNA sequencing as Staphylococcus sp. Further, Ceb1 was observed to have the ability to tolerate drought stress. While screening for the plant growth-promoting traits, Ceb1 was found to be positive for IAA production both under drought-stressed and normal conditions as confirmed by HPLC. The Ceb1 priming with Vigna unguiculata was observed to enhance the growth parameters of the plant. Analysis of Ceb1-treated plants by ICP-MS further showed modulation of both macro- and micronutrients. Upon drought stress induction in Vigna unguiculata, Ceb1 was found to provide synergistic plant growth-promoting effect to the plant along with the supplemented silicate sources. Under the changing agroclimatic conditions, exploring the plant stress-alleviating effects of endophytes is highly significant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
By living within the host plant, endophytic bacteria are sheltered from environmental stress and microbial competition (Santoyo et al. 2016). Their association with plants can generally be symbiotic and mutualistic. Within the plant system, endophytes have already been described to influence the physiological and biochemical mechanisms (Lata et al. 2018). They promote the plant growth by facilitating the acquisition of nutrients or modulating the level of hormones. This makes the consideration of endophytic association to be a universal phenomenon in plants. Indole-3-acetic acid (IAA) synthesized by endophytic bacteria has been reported to influence the cell division, differentiation, tuber germination, xylem and root development. It is also involved in vegetative growth, lateral and adventitious root formation, responses to light, gravity and photosynthesis. Endophytic bacteria solubilize the inorganic phosphate through acidification, secretion of organic acids or chelation and exchange based mechanisms (Adnan et al. 2017). 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase produced by them could degrade the precursor of ethylene and thereby repress the adverse effects of elevated ethylene level (Jaemsaeng and Jantasuriyarat 2018). They also assist iron acquisition in plants through siderophore production (Etminani and Harighi 2018). Performance of endophytic diazotrophs over rhizobacteria is also remarkable due to their effective N2 fixation (Yan et al. 2018).
Soil microbial community acts as the immediate source for the recruitment of endophytic bacteria and hence many soil bacterial genera like Pseudomonas, Burkholderia and Bacillus have been reported as endophytes from many plants (Ryan et al. 2008). Many others like Stenotrophomonas, Micrococcus, Pantoea, Microbacterium, etc. have also been isolated as common endophytes. However, only limited reports are there on characterization of endophytic Staphylococcus sp. with plant growth promotion promises. Plant beneficial features of these organisms with well-known human association can be demonstrated only by its isolation and characterization from diverse plants. Hence, the Staphylococcus sp. Ceb1 isolated endophytically in the study was subjected to detailed analysis.
Along with the microbiome, the composition of elements can also significantly influence the response of plants towards stress conditions like drought. The elements such as silicon (Si) have already been reported to be effective to enhance the drought tolerance of plants. Mechanistically, silicate is known to favor the maintenance of the water potential of leaf, photosynthetic activity, stomatal conductance and strength of stem (Shi et al. 2016). Hence, the synergistic functions of both microbiome and elements can expect to make the plant more competent to manage environmental stress. So, the study was mainly focused on plant beneficial features of a newly isolated endophytic Staphylococcus sp. and its synergistic effects with silicate on plant stress management. The observed results indicate the possibilities to broaden the functioning and applications of endophytic bacteria.
Materials and methods
Isolation and identification of endophytic bacteria
Endophytic bacterial isolation was carried out from the rhizome of Curcuma longa. The rhizome collected from the experimental garden was washed with tap water, Teepol and surface sterilized with 70% ethanol and 1% sodium hypochlorite (Jayakumar et al. 2018). The rhizomes were finally washed several times with sterilized distilled water and from this last wash was spread plated onto nutrient agar as control. Then the rhizome pieces were macerated and serially diluted up to 10–7. Then 0.1 mL from the dilution was plated onto nutrient agar followed by incubation at room temperature (Zinniel et al. 2002). Different bacterial morphotypes obtained were further subjected to the isolation of genomic DNA. Then 16S rDNA PCR amplification-based identification was carried out using the primers 16S F(5′-GAG TTT GAT CCT GGC TCA G-3′) and 16S R (5′-GAT ATT ACC GCG GCG CCT G-3′). The PCR product formed was sequenced and identified by BLAST analysis (Jasim et al. 2013).
Screening of drought tolerance potential of Ceb1
The isolate Ceb1 was further selected and screened for drought tolerance potential by culturing in trypticase soya broth (TSB) supplemented with different concentrations of polyethylene glycol (PEG) (− 0.25 MPa, − 0.5 MPa, − 0.75 MPa, − 1.0 MPa, − 1.25 MPa and − 1.5 MPa) followed by measurement of optical density (OD) at 600 nm (Sandhya et al. 2017).
Screening of selected endophyte for plant growth-promoting properties under stressed and nonstressed conditions
The bacterial isolate Ceb1 was screened for IAA production under non-stress and drought-induced stress by inoculating into 20 mL of nutrient broth supplemented with 0.2% (v/v) of L-tryptophan in the presence or absence of induced water potential of − 0.5 MPa, − 1.0 MPa and − 1.5 MPa. The experiment was conducted in triplicates and incubated for 10 days at 28ºC. After this, culture supernatant was collected by centrifugation at 3000 rpm for 20 min (Gutierrez et al. 2009). Methanolic extract from this was prepared and analyzed by C18 reverse-phase column of analytical HPLC (Thermo Scientific) to confirm the IAA production. Here, the elution was performed using 6:4 ratio of H2O and methanol, each containing 0.5% acetic acid. The elution was monitored at 280 nm and was carried out at a flow rate of 1 mL/min. The ACC deaminase production by Ceb1 was screened using DF salt minimal medium amended with 2 g/L ammonium sulphate. For stress induction, medium was supplemented with different concentrations of PEG (− 0.5 MPa, − 1.0 MPa, and − 1.5 MPa). After incubation for 7 days at 28 °C, bacterial growth under stress was estimated by measuring the OD at 600 nm. The nitrogen-fixing ability of Ceb1 was also screened under stress and non-stress conditions using Jensen’s media as per previous reports with slight modification (Bag et al. 2017). Here, the Jensen’s medium supplemented with PEG as described above was used to provide the stressed condition. Phosphate solubilization property of Ceb1 was also analyzed using Pikovskaya medium (Sandhya et al. 2010a).
Plant growth-promoting effect of Ceb1 on Vigna unguiculata
For this, seeds of V. unguiculata were surface sterilized using 1% sodium hypochlorite for 10 min and were washed several times with sterile distilled water and allowed to germinate. The seedlings were further primed with overnight-grown culture of Ceb1 for 1 h. For pot experiments, sterilized soil with ten seedlings were used with triplicates in each set. After 7 days of growth, the plant growth parameters such as shoot length, root length and root numbers were measured and the results were statistically analyzed (Ahmad et al. 2008).
ICP-MS analysis of Ceb1-treated plants
For this, Ceb1-treated and control (distilled water and nutrient broth) V. unguiculata plants were collected after 7 days of growth and were dried in an oven at 65 °C. These were further finely powdered and 0.1 g from each was then digested at 85 °C by microwave digestion using 8 mL of supra pure (65%) HNO3. The processed samples were made up to 25 mL with ultra pure water and used for ICP-MS (Thermo Fischer iCAP Q). The obtained concentration of each element in ppb was further converted to µg/mg and the result was statistically analyzed (Mihaylova et al. 2013).
Plant growth-promoting potential of Ceb1 in the presence of silicate and drought stress
Here, the surface-sterilized V. unguiculata seeds were allowed to germinate and the seedlings were treated with Ceb1. After 3 days of growth, sodium silicate and potassium silicate were added to separate sets of Ceb1 primed and control plants. Then drought was induced by terminating the watering of the seedlings for 3 weeks. After this, watering was resumed for 1 day and the plant growth parameters such as shoot length, root length and root numbers were determined. In the study, each set containing ten seedlings in triplicate was used (Chen et al. 2017).
Results
Isolation and identification of endophytic bacteria
The bacterial isolate Ceb1 obtained from the surface-sterilized rhizome of C. longa was confirmed as endophyte due to the absence of any microbial growth in the control plate. By analysis of 16S rDNA sequence, Ceb1 was confirmed to have 100% identity with Staphylococcus sp. and the sequence was submitted to NCBI (accession number MN420821). The phylogenetic analysis showed clustering of Ceb1 with Staphylococcus sp. (Fig. 1).
Screening of the drought tolerance potential of Ceb1
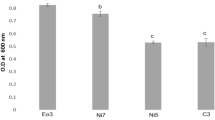
Ceb1 was found to tolerate the water potential up to − 1.5 MPa due to its growth in PEG (− 0.25 MPa, − 0.5 MPa, − 0.75 MPa, − 1.0 MPa, − 1.25 MPa and − 1.5 MPa)-containing medium. The drought tolerance ability of Ceb1 was further indicated by measuring its optical density analysis of control samples at 600 nm. Here the control sample provided a value of 0.2 and that of Ceb1 treated with PEG (− 1.5 MPa) was 0.18.
Screening of Ceb1 for plant growth-promoting properties
Staphylococcus sp. Ceb1 was found to produce IAA both under normal and drought conditions. HPLC analysis confirmed the IAA production by Ceb1 due to the presence of peak at 15.4 min which was comparable to the standard IAA (Fig. 2). Under water potential of − 0.5 MPa, − 1.0 MPa and − 1.5 MPa Ceb1 produced 70, 65 and 60 µg/mL of IAA, respectively, and the same for control was 80 µg/mL. However, Ceb1 was found to be negative for nitrogen fixation, phosphate solubilisation, ACC deaminase and ammonia production.
Plant growth-promoting effect of Ceb1 on Vigna unguiculata
Ceb1-treated V. unguiculata seedlings showed significant enhancement in shoot length, root length and root numbers when compared to the control. In Ceb1-treated plants, the shoot, length root length and root numbers were 17.11905 ± 1.74195 cm, 7.42857 ± 1.39728 cm and 15 ± 3.09377 respectively. The same for distilled water control were 11.00 ± 1.07 cm, 2.38 ± 0.93 cm, and 8 ± 1.50, and for nutrient broth control, it were 11.93 ± 1.58 cm, 2.52 ± 0.62 cm and 10 ± 1.22 (Fig. 3).
ICP-MS analysis of Ceb1-treated plants
The elemental analysis of plants treated with Ceb1 showed increase in phosphorous, magnesium, sodium, manganese, nickel, copper, neodymium, lanthanum, barium and thorium when compared to the distilled water and nutrient broth control. Aluminum, iron, chromium, gallium, vanadium, rubidium, strontium, cerium, lead, lithium, arsenic, cadmium, zinc, praseodymium, gadolinium, samarium, beryllium, indium, caesium, europium, scandium, terbium, bismuth, uranium, holmium, erbium, thulium, ytterbium, lutetium, thallium and dysprosium concentration in Ceb1-treated plants were lower than control (Fig. S1).
Plant growth-promoting potential of Ceb1 in the presence of silicate and drought stress
After 21 days of water deprivation, the control plants were severely wilted and desiccated. The plants treated with Ceb1 were not severely affected but slight curling of leaf could be observed. The sodium silicate and potassium silicate alone treated control plants were also completely wilted and desiccated. However, synergistic effect could be observed for Ceb1 and silicate treated plants as evidenced by the increased shoot length and increased leaf number (Fig. 4). When comparing the synergistic action of Ceb1–sodium silicate with Ceb1 potassium silicate, the latter was found to favor better growth with more greenish leaves.
Discussion
Drought is one of the harsh environmental conditions that causes the reduced crop production worldwide. Mitigating the negative effects of drought by plant-associated microbes hence has great significance when compared with the existing techniques. Among the plant microbiome, endophytic bacteria have significant role in the plant growth, development, fitness, and protection from various environmental conditions. However, only limited reports are there on endophytic bacterial support to plant to manage abiotic stress conditions. In the study, endophytic Staphylococcus sp. Ceb1 was isolated and characterized for plant growth-promoting traits under drought stressed conditions. Even though endophytic Staphylococcus spp. have previously been reported from Zingiber officinale (Jasim et al. 2013), Sorghum bicolor (Mareque et al. 2014), Solanum melongena (Achari and Ramesh 2014), Anacardium occidentale (Milca et al. 2014), Ammodendron bifolium (Zhu and She 2018) and Corchorus olitorius (Haidar et al. 2018), their functional significance in plant physiology is not known.
The endophytic Ceb1 isolated in the study was found to tolerate up to a water potential of − 1.5 MPa, which indicated its drought tolerance. Bacterial cells are already reported to have the ability to accumulate osmolytes to promote cell growth under drought conditions (Sandhya et al. 2010a, b).
The IAA production by Ceb1 under drought stress further showed its ability to support plant growth under harsh environmental conditions. Because, microbial IAA together with the endogenous IAA of plant is expected to synergistically execute regulatory effects on many physiological processes in plant (Penrose et al. 2001). Under the stressed condition, the microbial contribution can have a determining role to keep the plant functions.
The impact of Ceb1 on V. unguiculata was evident due to the observed increase in shoot length, root length and root numbers. Microbially produced IAA is considered to trigger shoot and root elongation and there by the maximal nutrient absorption in plants. In a previous study, Staphylococcus pasteuri isolated from Corchorus olitorius was demonstrated to cause increased root and shoot length by phosphate solubilization, ACC deaminase production, nitrogen fixation and siderophore production (Haidar et al. 2018). Staphylococcus arlettae inoculation in Triticum aestivum has also demonstrated to increase the germination, root and shoot length as well as dry and wet weight (Sagar et al. 2012). As the isolate Ceb1 was positive only for IAA, the observed morphological changes of V. unguiculata can be mostly related to the IAA production.
During the analysis on the elemental modulatory effect of Ceb1 in V. unguiculata, enhancement was observed for phosphorous, magnesium, sodium and manganese. In the study, biopriming might have caused an increased uptake of nutrients, required in higher concentration for plant growth (Tripathi et al. 2014). The microelements such as copper and nickel were also found to get enhanced. Nickel is already known to be essential for the functioning of enzymes (urease, glycosylase-I, etc.) which are involved in plant metabolic process (Fabiano et al. 2015). Copper is also required for enzymatic functioning and it supports plant growth through various mechanisms (Dreyer and Schippers 2019). Hence, the observed enhancement of macro- and micronutrients can have significant impact on plant growth and health. Several studies have already reported the inoculation of microorganisms to result in the reduction of arsenic content of plants and there by the promotion of plant growth (Das et al. 2014; Shagol et al. 2014; Lampis et al. 2015). Hence, the observed decrease in arsenic for the bioprimed plants can be beneficial. Pseudomonas sp. from tobacco plants have recently been reported to reduce the lead contents in Nicotiana tabacum (Li et al. 2019). Inoculation of S. maritima with metal-resistant endophytes has been reported to decrease the metal accumulation in plant tissues (Mesa et al. 2015). Endophytic isolates from plants grown in cadmium-contaminated soils were reported to reduce the cadmium content of roots and shoots (Begum et al. 2018). Endophytes from rice plants were also reported to reduce the nickel and cadmium toxicity in tomato plants (Madhaiyan et al. 2007). Hence, the elemental modulation observed in the study demands more detailed investigation. Inoculation with Bacillus and Pseudomonas spp. has previously been reported to increase the plant nutrient contents such as P, Fe, Zn, K and Mg along with enhanced growth (Esitken et al. 2010). Pratiwi et al (2016) have reported the inoculation of P. fluorescens to result in increased availability of Fe by 34.75% when compared to the control plants. Praveen et al. (2012) have also previously reported the seed bacterization of sorghum with Pseudomonas spp. to result in enhanced uptake of essential macro and micro nutrients and there by the enhanced growth of plant. These indicate the observed elemental changes in the study to be due to the functioning of Ceb1.
The plant growth-promoting effect of Ceb1 under drought-stressed condition is of great significance due to its field application potential. In a previous study, Pantoea alhagi isolated from leaves of Alhagi sparsifolia has described to improve the growth and drought tolerance in wheat (Chen et al. 2017). Generally, endophytic bacteria from dry environment can be considered to have stimulatory effect on plant growth under drought due to its naturally acquired adaptations. In the study, the performance of Ceb1 under drought stress was found to get improved with silicate supplementation. The synergistic action of Ceb1 and silicate here suggests the promises of development of microbial formulation with supplemented silicate to improve its field performance. As the silicate and drought-tolerant bacteria can protect the plant from drought stress, its combined application in the field may reduce the need for irrigation which makes the study important.
Many Staphylococcal species are demonstrated to have plant association with significant growth enhancement effects. However, limited results have been reported on mechanistic basis of the same. In a recent study, genome-wide analysis was carried out to identify the adaptive changes of endophytic bacteria which were previously demonstrated well for human association (Chaudhry and Patil 2016a). Analysis of S. epidermidis genome appraised 97% average nucleotide identity between Staphylococci from human and plant sources. Analysis of a cluster of three unique ORFs in rice endophytic S. epidermidis also showed its function in stress tolerance and survival of the plant (Conlan et al. 2012; Chaudhry and Patil 2016b). Likewise, the enzymes like peptidase are also considered to function for the plant entry and subsequent colonization by endophytic organisms. The human pathogen Pseudomonas aeruginosa has also been demonstrated as a plant beneficial endophyte from diverse plants. Significant differences in genome, genome expression profile, virulence activity and antibiotic resistance have been reported for diverse strains of Pseudomonas through genome-based analysis (Cabot et al. 2016; Hwang and Yoon 2019). This indicates the need for a detailed and periodic analysis to identify the genomic trend of organisms with association in both humans and plants. However, a key insight into the adaptation and its phenotypic expression can have significant promises to be explored in agriculture.
Endophytes are organisms associated within plants as obligate or facultative partner. The current study resulted in the isolation of an endophytic Staphylococcus sp. (Ceb1) from rhizome of Curcuma longa with drought tolerance and IAA-producing features. The screening for plant growth-promoting traits under stressed and non-stressed conditions showed the ability of the isolate to enhance the plant growth possibly through the IAA production. By the synergistic action with silicate, Ceb1 support enhanced the plant growth under drought stress. Hence, this combination can be used to develop biofertilizer formulations for enhanced crop productivity under harsh environmental conditions.
References
Achari GA, Ramesh R (2014) Diversity, biocontrol, and plant growth promoting abilities of xylem residing bacteria from solanaceous crops. Int J Microbiol 2014:1–14. https://doi.org/10.1155/2014/296521
Adnan M et al (2017) Phosphate-solubilizing bacteria nullify the antagonistic effect of soil calcification on bioavailability of phosphorus in alkaline soils. Sci Rep. https://doi.org/10.1038/s41598-017-16537-5
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181. https://doi.org/10.1016/j.micres.2006.04.001
Bag PB, Bappa Paramanik PP, Ashok Choudhury BM (2017) Atmospheric nitrogen fixing capacity of azotobacter isolate from Cooch Behar and Jalpaiguri Districts soil of West Bengal, India. Int J Curr Microbiol Appl Sci 6:1775–1788. https://doi.org/10.20546/ijcmas.2017.603.204
Begum N, Afzal S, Zhao H, Lou L, Cai Q (2018) Shoot endophytic plant growth-promoting bacteria reduce cadmium toxicity and enhance switchgrass (Panicum virgatum L.) biomass. Acta Physiol Plantarum 5:5. https://doi.org/10.1007/s11738-018-2737-1
Cabot G et al (2016) Evolution of pseudomonas aeruginosa antimicrobial resistance and fitness under low and high mutation rates. Antimicrob Agents Chemother 60:1767–1778. https://doi.org/10.1128/aac.02676-15
Chaudhry V, Patil PB (2016a) Genomic investigation reveals evolution and lifestyle adaptation of endophytic Staphylococcusepidermidis. Sci Rep 6:19263. https://doi.org/10.1038/srep19263
Chaudhry V, Patil PB (2016b) Genomic investigation reveals evolution and lifestyle adaptation of endophytic Staphylococcusepidermidis. Sci Rep. https://doi.org/10.1038/srep19263
Chen C et al (2017) Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci Rep 7:41564. https://doi.org/10.1038/srep41564
Conlan S et al (2012) Staphylococcus epidermidis pan-genome sequence analysis reveals diversity of skin commensal and hospital infection-associated isolates. Genome Biol 13:R64. https://doi.org/10.1186/gb-2012-13-7-r64
Das S, Jean J-S, Kar S, Chou M-L, Chen C-Y (2014) Screening of plant growth-promoting traits in arsenic-resistant bacteria isolated from agricultural soil and their potential implication for arsenic bioremediation. J Hazard Mater 272:112–120. https://doi.org/10.1016/j.jhazmat.2014.03.012
Dreyer BH, Schippers JHM (2019) Copper-zinc superoxide dismutases in plants: evolution enzymatic properties, and beyond. Annu Plant Rev. https://doi.org/10.1002/9781119312994.apr0705
Esitken A, Yildiz HE, Ercisli S, Figen Donmez M, Turan M, Gunes A (2010) Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown strawberry. Sci Hortic 124:62–66. https://doi.org/10.1016/j.scienta.2009.12.012
Etminani F, Harighi B (2018) Isolation and identification of endophytic bacteria with plant growth promoting activity and biocontrol potential from wild pistachio trees. Plant Pathol J 34:208–217. https://doi.org/10.5423/PPJ.OA.07.2017.0158
Fabiano CC, Tezotto T, Favarin JL, Polacco JC, Mazzafera P (2015) Essentiality of nickel in plants: a role in plant stresses. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00754
Gutierrez CK, Matsui GY, Lincoln DE, Lovell CR (2009) Production of the phytohormone indole-3-acetic acid by estuarine species of the genus vibrio. Appl Environ Microbiol 75:2253–2258. https://doi.org/10.1128/aem.02072-08
Haidar B, Ferdous M, Fatema B, Ferdous AS, Islam MR, Khan H (2018) Population diversity of bacterial endophytes from jute (Corchorus olitorius) and evaluation of their potential role as bioinoculants. Microbiol Res 208:43–53. https://doi.org/10.1016/j.micres.2018.01.008
Hwang W, Yoon SS (2019) Virulence characteristics and an action mode of antibiotic resistance in multidrug-resistant Pseudomonas aeruginosa. Sci Rep. https://doi.org/10.1038/s41598-018-37422-9
Jaemsaeng R, Jantasuriyarat C, Thamchaipenet A (2018) Molecular interaction of 1-aminocyclopropane-1-carboxylate deaminase (ACCD)-producing endophytic Streptomyces sp. GMKU 336 towards salt-stress resistance of Oryza sativa L. cv. KDML105. Sci Rep. https://doi.org/10.1038/s41598-018-19799-9
Jasim B, Joseph AA, John CJ, Mathew J, Radhakrishnan EK (2013) Isolation and characterization of plant growth promoting endophytic bacteria from the rhizome of Zingiber officinale. 3 Biotech 4:197–204. https://doi.org/10.1007/s13205-013-0143-3
Jayakumar A, Krishna A, Mohan M, Nair IC, Radhakrishnan EK (2018) Plant growth enhancement, disease resistance, and elemental modulatory effects of plant probiotic endophytic Bacillus sp. Fcl1. Probiotics Antimicrob Proteins 11:526–534. https://doi.org/10.1007/s12602-018-9417-8
Lampis S, Santi C, Ciurli A, Andreolli M, Vallini G (2015) Promotion of arsenic phytoextraction efficiency in the fern Pteris vittata by the inoculation of As-resistant bacteria: a soil bioremediation perspective. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00080
Lata R, Chowdhury S, Gond SK, White JF (2018) Induction of abiotic stress tolerance in plants by endophytic microbes. Lett Appl Microbiol 66:268–276. https://doi.org/10.1111/lam.12855
Li J et al (2019) Pseudomonas species isolated from tobacco seed promote root growth and reduce lead contents in Nicotiana tabacum K326. Can J Microbiol 65:214–223. https://doi.org/10.1139/cjm-2018-0434
Madhaiyan M, Poonguzhali S, Sa T (2007) Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 69:220–228. https://doi.org/10.1016/j.chemosphere.2007.04.017
Mareque C, Taulé C, Beracochea M, Battistoni F (2014) Isolation, characterization and plant growth promotion effects of putative bacterial endophytes associated with sweet sorghum (Sorghum bicolor (L.) Moench). Ann Microbiol 65:1057–1067. https://doi.org/10.1007/s13213-014-0951-7
Mesa J, Mateos-Naranjo E, Caviedes MA, Redondo-Gómez S, Pajuelo E, Rodríguez-Llorente ID (2015) Endophytic cultivable bacteria of the metal bioaccumulator Spartina maritima improve plant growth but not metal uptake in polluted marshes soils. Front Microbiol. https://doi.org/10.3389/fmicb.2015.01450
Mihaylova V, Lyubomirova V, Djingova R (2013) Optimization of sample preparation and ICP-MS analysis for determination of 60 elements for characterization of the plant ionome. Int J Environ Anal Chem 93:1441–1456. https://doi.org/10.1080/03067319.2012.736978
Milca RdCRL et al (2014) Plant growth promoting potential of endophytic bacteria isolated from cashew leaves. Afr J Biotech 13:3360–3365. https://doi.org/10.5897/ajb2014.13835
Pratiwi H, Aini N, Soelistyono R (2016) Effects of Pseudomonas fluorescens and sulfur on nutrients uptake, growth and yield of ground nut in an alkaline soil. J Degrad Min Lands Manag 3:507–516
Praveen Kumar G, Desai S, Leo Daniel Amalraj E, Mir Hassan Ahmed SK, Reddy G (2012) Plant growth promoting Pseudomonas spp. from diverse agro-ecosystems of India for Sorghum bicolor L. J Biofert Biopest S. https://doi.org/10.4172/2155-6202.s7-001
Penrose DM, Moffatt BA, Glick BR (2001) Determination of 1-aminocycopropane-1-carboxylic acid (ACC) to assess the effects of ACC deaminase-containing bacteria on roots of canola seedlings. Can J Microbiol 47:77–80
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 278:1–9. https://doi.org/10.1111/j.1574-6968.2007.00918.x
Sagar S, Dwivedi A, Yadav S, Tripathi M, Kaistha SD (2012) Hexavalent chromium reduction and plant growth promotion by Staphylococcus arlettae Strain Cr11. Chemosphere 86:847–852. https://doi.org/10.1016/j.chemosphere.2011.11.031
Sandhya V, Ali SZ, Grover M, Reddy G, Venkateswarlu B (2010a) Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul 62:21–30. https://doi.org/10.1007/s10725-010-9479-4
Sandhya V, Ali SZ, Venkateswarlu B, Reddy G, Grover M (2010b) Effect of osmotic stress on plant growth promoting Pseudomonas spp. Arch Microbiol 192:867–876. https://doi.org/10.1007/s00203-010-0613-5
Sandhya V, Shrivastava M, Ali SZ, Prasad VSSK (2017) Endophytes from maize with plant growth promotion and biocontrol activity under drought stress. Russ Agric Sci 43:22–34. https://doi.org/10.3103/s1068367417010165
Santoyo G, Moreno-Hagelsieb G, del Carmen O-M, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microbiol Res 183:92–99. https://doi.org/10.1016/j.micres.2015.11.008
Shagol CC, Krishnamoorthy R, Kim K, Sundaram S, Sa T (2014) Arsenic-tolerant plant-growth-promoting bacteria isolated from arsenic-polluted soils in South Korea. Environ Sci Pollut Res 21:9356–9365. https://doi.org/10.1007/s11356-014-2852-5
Shi Y et al (2016) Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front Plant Sci 7:196. https://doi.org/10.3389/fpls.2016.00196
Tripathi DK, Singh VP, Chauhan DK, Prasad SM, Dubey NK (2014) Role of macronutrients in plant growth and acclimation: recent advances and future prospective, pp 197–216. https://doi.org/10.1007/978-1-4614-8824-8_8
Yan X et al (2018) Isolation, diversity, and growth-promoting activities of endophytic bacteria from tea cultivars of Zijuan and Yunkang-10. Front Microbiol. https://doi.org/10.3389/fmicb.2018.01848
Zhu Y, She X (2018) Evaluation of the plant-growth-promoting abilities of endophytic bacteria from the psammophyte Ammodendron bifolium. Can J Microbiol 64:253–264. https://doi.org/10.1139/cjm-2017-0529
Zinniel DK et al (2002) Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Appl Environ Microbiol 68:2198–2208. https://doi.org/10.1128/aem.68.5.2198-2208.2002
Acknowledgements
The authors acknowledge Dr. Mahesh Mohan, School of Environmental Sciences, Mahatma Gandhi University for instrumental facility. And also thank KSCSTE—SRS, DST PURSE and KSCSTE KBC YIPB for the support provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
During the study, we have not used any animal models and thus do not require ethical committee clearance.
Human and animal rights statement
The manuscript does not contain human studies.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jayakumar, A., Krishna, A., Nair, I.C. et al. Drought-tolerant and plant growth-promoting endophytic Staphylococcus sp. having synergistic effect with silicate supplementation. Arch Microbiol 202, 1899–1906 (2020). https://doi.org/10.1007/s00203-020-01911-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-01911-1