Abstract
Acinetobacter baumannii is the major nosocomial pathogen that causes serious infections such as ventilator-associated pneumonia and bacteremia due to its biofilms. Hence, this study investigated the antimicrobial and antibiofilm potentials of cell-free supernatants (CFS) obtained from Clostridium butyricum, as probiotic, against A. baumannii. Our results demonstrated that C. butyricum CFS inhibited A. baumannii cell growth in planktonic culture. Also, C. butyricum CFS not only inhibited the biofilm development and dispersed mature biofilms, but also suppressed the metabolic activity of biofilm cells, showing antibiofilm activity. The biofilm components reduced by C. butyricum CFS were observed via confocal laser scanning microscopy. In addition, C. butyricum CFS exhibited antivirulence effect by inhibiting the motility of A. baumannii. Furthermore, C. butyricum CFS significantly downregulated the expression of efflux pump-related genes including adeA, adeB and adeC in A. baumannii. Our data demonstrate that C. butyricum CFS showed antimicrobial and antibiofilm effects on A. baumannii. These effects are closely associated with suppression of motility and efflux pump-related genes in A. baumannii. The findings suggest that C. butyricum CFS can be used as a new therapeutic alternative against biofilm-associated infection caused by multidrug-resistant A. baumannii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acinetobacter baumannii, an opportunistic bacterial pathogen, causes a variety of nosocomial infections with high mortality rates including ventilator-associated pneumonia (VAP), bacteremia, wound and urinary tract infections in patients using contaminated catheters (Dijkshoorn et al. 2007). According to the Infectious Diseases Society of America (Boucher et al. 2009; Antunes et al. 2014), A. baumannii is one of the multidrug-resistant (MDR) ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species), associated with antimicrobial resistance. The excessive use of antibiotics has promoted the emergence of antibiotic-resistant A. baumannii, resulting in serious public health challenges worldwide (Komolafe 2003). It is extremely difficult to treat the nosocomial infection caused by biofilms due to antibiotic tolerance (Lewis 2001).
Biofilm is described as a microbial aggregate enclosed within self-released extracellular polymeric substances including proteins, polysaccharides and extracellular DNA (Hall-Stoodley et al. 2004). Biofilm is also formed in a variety of biotic or artificial abiotic surfaces and is very difficult to eradicate, resulting in severe chronic bacterial infections (Lewis 2001). Also, biofilm formation is strongly resistant to conventional antibiotics, the host immune system, and stressful environment compared with free-living cells (Gunn et al. 2016). In addition, in the case of A. baumannii, virulence factors such as the biofilm-associated protein (Bap), efflux system (AdeABC, AdeFGH and AdeIJK), quorum sensing system, motility by pili and porins are closely involved in biofilm formation and the strong pathogenicity of A. baumannii (Eze et al. 2018). Therefore, the development of new therapeutic alternatives is necessary to prevent and treat biofilm-associated infections attributed to multidrug-resistant A. baumannii.
Probiotics have attracted attention as safe and beneficial therapeutics (Reid et al. 2003). According to United Nations Food and Agricultural Organization (FAO)/World Health Organization (WHO) (FAO/WHO 2001), probiotics are indicated as live microorganisms that provide health benefits when administered in appropriate amounts. Previous studies demonstrated that probiotics regulate the host immune system by stimulating cytokine production and cellular activity, and inhibit the clustering of pathogens (Mendonca et al. 2012; Roy et al. 2014; Hager et al. 2019). Among the numerous probiotics, Clostridium butyricum, an anaerobic butyric-acid-forming bacterium, is usually found in the intestines of healthy humans or animals. C. butyricum is used to treat non-antimicrobial- or antimicrobial-associated diarrhea and is widely available commercially in Asia because of its probiotic properties (Cassir et al. 2016). C. butyricum is highly resistant to antibiotics and the lower pH of gastric acid by producing endospores (Courvalin 2006). C. butyricum also produces a large amount of short-chain fatty acids (SCFAs), mostly butyric acid, which promote regulatory T cell generation (Kashiwagi et al. 2015) and downregulate the expression levels of virulence genes in pathogenic bacteria (Gantois et al. 2006). Furthermore, C. butyricum shows antimicrobial activities against Helicobacter pylori, enterotoxigenic E. coli (ETEC), Vibrio spp. and Clostridium difficile (Kuroiwa et al. 1990; Takahashi et al. 2000, 2004).
Although various studies have investigated the probiotic effect of C. butyricum, the potential effects of C. butyricum on A. baumannii biofilm have yet to be reported. The aim of this study was to determine the antagonistic activity of metabolites derived from C. butyricum against planktonic cell growth, biofilm development and RND (resistance–nodulation–cell division)-type efflux pump-related genes of A. baumannii. Our findings advance the boundaries of knowledge on the potential effects of C. butyricum against biofilm-associated infection by multidrug-resistant A. baumannii.
Materials and methods
Bacterial strains, media and growth conditions
Acinetobacter baumannii ATCC 19606 and Clostridium butyricum ATCC 19398 (NCTC 7423, a non-toxigenic strain) were purchased from the American Type Culture Collection (ATCC). Multidrug-resistant A. baumannii clinical isolates P2713 (respiratory isolate) and P3000 (blood isolate) strains were obtained from the Gyeongsang National University Hospital Culture Collection. A. baumannii strains were cultured in trypticase soy broth (TSB; Difco, Becton–Dickinson and Company, USA) at 37 °C. C. butyricum ATCC 19398 was cultured in reinforced clostridial medium (RCM; Difco, Becton–Dickinson and Company, USA) at 37 °C under anaerobic conditions. All bacterial strains were preserved in a broth containing 20% (v/v) glycerol at − 80 °C until experimental use.
To prepare the cell-free supernatant (CFS), 1 mL of 1 × 108 CFU mL−1C. butyricum culture was added to the reinforced clostridial medium and incubated anaerobically at 37 °C for 24 h. The broth was centrifuged at 3,000 rpm for 15 min at 4 °C, and the supernatant was filtered through a 0.2-μm pore size syringe filter (Advantec, Tokyo, Japan). To neutralize the effect of organic acid on the antimicrobial activity, C. butyricum CFS was adjusted to pH 6.5 with 1 N NaOH as previously described (Wasfi et al. 2018).
Susceptibility testing of C. butyricum CFS on A. baumannii in planktonic culture
The antimicrobial activity of CFS extracted from C. butyricum was evaluated via the broth microdilution method described by the Clinical and Laboratory Standards Institute (CLSI) guidelines (Clinical and Laboratory Standards Institute 2018) with a few modifications. Briefly, the overnight culture of A. baumannii strains was prepared in TSB medium. A reference to A. baumannii strains was adjusted to 5 × 105 CFU mL−1 and dispensed into a 96-well microtiter plate (BD Falcon™, BD, NJ, USA) with the various concentrations of C. butyricum CFS or adjusted C. butyricum CFS. The C. butyricum CFS or adjusted C. butyricum CFS was serially diluted twofold in the TSB medium. A 96-well microtiter plate was incubated at 37 °C for 24 h, followed by the analysis of cell growth in planktonic culture at 600 nm (A600) wavelength through a Multiskan GO plate reader (Thermo Fisher Scientific, USA). Overnight-cultured A. baumannii strains served as the control.
Biofilm formation and C. butyricum CFS treatment
The biofilm formation assay and biofilm dispersal assay were performed in a 96-well microtiter plate as mentioned previously (Kaur et al. 2018; Kim et al. 2018), with some modifications. To determine the inhibitory effect of CFS derived from C. butyricum on biofilm formation, overnight-cultured bacterial suspensions were diluted to 5 × 105 CFU mL−1 in TSB medium. Diluted bacterial suspensions were inoculated with twofold serial dilutions of C. butyricum CFS into a 96-well microtiter plate, followed by incubation at 37 °C for 24 h.
To investigate the dispersal effect of C. butyricum CFS on mature biofilms, the bacterial suspensions were adjusted to 5 × 105 CFU mL−1, dispensed into a 96-well microtiter plate and incubated at 37 °C for 24 h. Non-adherent cells were removed by washing with phosphate-buffered saline (PBS). Next, fresh TSB medium containing the twofold serial dilutions of C. butyricum CFS was added to the mature biofilms and incubated at 37 °C for an additional 24 h. The control was used as described above. To evaluate quantitatively the antibiofilm activity of C. butyricum CFS, the crystal violet assay was conducted as described in the following.
Crystal violet assay
The crystal violet assay was conducted to investigate the total biomass of biofilm quantitatively as mentioned previously (Cady et al. 2012), with a few modifications. Briefly, biofilms formed in a 96-well microtiter plate were rinsed with PBS to remove planktonic cells. Biofilms were dried at 60 °C for 1 h and stained with 1% crystal violet for 5 min. To remove the remaining dye, the stained biofilms were washed with sterile distilled water and dried at 60 °C for 1 h. At the end of drying, the stained biofilms were dissolved by adding 33% acetic acid for 15 min to extract the crystal violet. The total biomass of biofilm was quantitatively analyzed at 570 nm (A570).
Quantitative analysis of biofilm metabolic activity via XTT reduction assay
The XTT [2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide] reduction assay was performed to evaluate the metabolic activity of A. baumannii biofilm cells as described previously (Pierce et al. 2008; Fischer et al. 2010), with minor modifications. Briefly, biofilms were established in a 96-well microtiter plate, as mentioned above. The twofold serial dilutions of C. butyricum CFS were inoculated into the well of a 96-well microtiter plate containing the established biofilms. Following incubation, A. baumannii biofilms were washed with PBS to remove weakly attached cells. The XTT Cell Proliferation Assay Kit (ATCC, Manassas, VA, USA) was employed to analyze the metabolic activity of A. baumannii biofilm cells quantitatively. The XTT and activation reagents were dispensed at a ratio of 50:1 (v/v) into the well of a 96-well microtiter plate and incubated at 37 °C in the dark for 3 h. To quantify the biofilm metabolic activity, the specific absorbances were measured at a test wavelength of 475 nm (A475) and a reference wavelength of 655 nm (A655), subtracting the mean background values from the data.
Confocal laser scanning microscopy (CLSM)
To perform confocal laser scanning microscopy (CLSM), the biofilm of A. baumannii strain was developed in the presence or absence of C. butyricum CFS on a tissue culture-treated 24-well glass bottom imaging plate (Eppendorf AG, Hamburg, Germany, cat. no.: 0030741021) as reported previously (Nosyk et al. 2008), with minor modifications. The biofilms were fixed using 3.7% (v/v) formaldehyde for 1 h. Subsequently, the amino groups, carbohydrates and extracellular nucleic acids were stained with fluorescein isothiocyanate isomer I (FITC, 10 μg µL− 1; Sigma-Aldrich, Germany), Concanavalin A-Alexa Fluor 594 conjugate (Con A, 0.1 μg µL− 1; C-11253, Molecular Probes, Eugene, OR, USA) and 4,6-diamidino-2-phenylindole dihydrochloride (DAPI, 1 mg L− 1; Molecular Probes, Eugene, OR, USA), respectively. After each staining, the biofilms were rinsed with PBS to remove the excess dye. All staining procedures were carried out in the dark room. The stained biofilms of A. baumannii strain were observed at laser beam wavelengths of 495, 590 and 358 nm to detect FITC, Con A and DAPI, respectively. Zeiss LSM-710 confocal laser microscope (Carl Zeiss, Thornwood, NY, USA) and ZEN software (Carl Zeiss, Thornwood, NY, USA) were utilized to visualize and image the biofilms of A. baumannii strain.
Motility assay
A motility assay was performed as described previously (Nait Chabane et al. 2014; Raorane et al. 2019), with a few modifications. A. baumannii strain was inoculated onto modified LB broth (tryptone 10 g L− 1; NaCl 5 g L− 1; yeast extract 5 g L− 1) containing 0.2% agar supplemented with or without twofold serial dilutions of C. butyricum CFS. Following inoculation, polystyrene petri dishes (SPL 10060, Pocheon, Korea, 60 × 15 mm) were incubated at 37 °C overnight. Motility was determined by measuring the diameters of turbid zone formed by bacterial cells traveling across agar plates.
Quantitative polymerase chain reaction (qPCR)
To isolate the total RNA, A. baumannii strains were incubated at a density of 5 × 105 CFU mL− 1 at 37 °C for 8 h. At the end of incubation, the twofold serial dilutions of C. butyricum CFS were inoculated into the bacterial culture and incubated at 37 °C for additional 10 h. Each bacterial culture was centrifuged at 25,000 × g for 90 s at 4 °C to collect the A. baumannii cells. NucleoSpin RNA Mini Kit (Macherey–Nagel, Düren, Germany) was used to extract and purify the total RNA of A. baumannii strains. The redundant DNA in RNA samples was treated with RNase-free DNase to prevent DNA contamination. The concentration and quality of isolated total RNA were identified through BioDrop µLITE (BioDrop Ltd., Cambridge, UK). Total RNA (1 μg) was reverse-transcribed into cDNA, using ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo, Japan) in compliance with manufacturer’s recommendations. To evaluate the expression of RND-type efflux pump-related genes in A. baumannii, qPCR was performed using a StepOnePlus™ Real-Time PCR System (Applied Biosystems, USA). The qPCR amplification products were detected by Power SYBR® Green PCR Master Mix (Applied Biosystems, USA). The primers used this study are listed in Table 1 (Peleg et al. 2007; Coyne et al. 2010). The 16S rRNA gene, a housekeeping gene, was used for normalization. The qPCR data were analyzed using the 2−ΔΔCT method.
Statistical analysis
All results are presented as means ± standard deviations (SD) of triplicate from three independent experiments. To compare the significant differences between the treated groups and the untreated control group, statistical analyses were carried out via one-way analysis of variance (ANOVA) with Dunnett’s test using the GraphPad Prism, version 5 (GraphPad Software, CA, USA). The Student’s t test was used to analyze the qPCR data. A value of *p < 0.05, **p < 0.01 and ***p < 0.001 was considered statistically significant.
Results
Antimicrobial activity of C. butyricum CFS on planktonic cell growth of A. baumannii
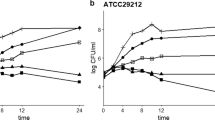
The antimicrobial activity of C. butyricum CFS against A. baumannii strains was evaluated at 600 nm (A600). In the presence of 50% C. butyricum CFS, the planktonic cell growth of A. baumannii ATCC 19606, clinical isolates P2713 and P3000 strains was drastically inhibited by 98.51%, 99.22% and 98.6%, respectively (Fig. 1a–c). Although 12.5% and 25% C. butyricum CFS were less effective, these concentrations showed a statistically significant reduction in planktonic cell growth of A. baumannii strains. Cassir et al. reported that a bacteriocin-like inhibitory substance produced by C. butyricum exhibited antimicrobial effects against several organisms (Cassir et al. 2016). Thus, our study investigated the antimicrobial effect of bacteriocin-like inhibitory substance on A. baumannii. According to a previous study, neutralized probiotic CFS had a similar effect to bacteriocin-like inhibitory substance (Kim and Kang 2019). Based on previous study, C. butyricum CFS was neutralized to pH 6.5 to eliminate the action of organic acids. Unexpectedly, the treated C. butyricum CFS did not show any significant effects against planktonic cell growth of A. baumannii strains (Fig. 1d–f). The data showed that the neutralized C. butyricum CFS exhibited less antimicrobial activity than C. butyricum CFS against A. baumannii (Fig. 1), indicating that the bacteriocin-like inhibitory substance was not involved in significant effects against planktonic cell growth of A. baumannii.
Antimicrobial activity of C. butyricum CFS against the growth of A. baumannii in planktonic culture. A. baumannii ATCC 19606 (a, d), clinical isolates P2713 (b, e) and P3000 (c, f) strains were incubated following treatment with C. butyricum CFS (a–c) or adjusted C. butyricum CFS to pH 6.5 (d–f) at 37 °C for 24 h. The growth of A. baumannii in planktonic culture was measured at A600 using microplate spectrophotometers. The results are expressed as means ± standard deviations (SD). ** and *** denote significant differences at p < 0.01 and p < 0.001, respectively
Inhibitory effect of C. butyricum CFS on A. baumannii biofilm formation
The inhibitory effect of C. butyricum CFS on biofilm development of A. baumannii strains was quantitatively determined using the crystal violet assay. As shown in Fig. 2a, ATCC 19606 strain was suppressed by 33.97%, 43.17% and 99.65% following treatment with 12.5%, 25% and 50% C. butyricum CFS, respectively. Biofilm formation of clinical isolate P2713 strain was inhibited 24.43%, 28.29% and 93.69% (Fig. 2b) and that of P3000 strain was also suppressed by 30.92%, 36.45% and 97.06% (Fig. 2c) in the presence of 12.5%, 25% and 50% C. butyricum CFS, respectively. The results showed that C. butyricum CFS significantly inhibited the biofilm development of all A. baumannii strains used in this study in a dose-dependent manner (Fig. 2).
Inhibitory effect of C. butyricum CFS on biofilm development of A. baumannii. A. baumannii ATCC 19606 (a) and clinical isolates P2713 (b) and P3000 (c) strains were incubated with C. butyricum CFS at 37 °C for 24 h. Biofilms of A. baumannii strains were stained with 1% crystal violet and analyzed by measuring the absorbance at A570. The results are expressed as means ± standard deviations (SD). *** denotes significant differences at p < 0.001
Dispersal effect of C. butyricum CFS on mature biofilms of A. baumannii
The dispersal of mature biofilm is an important strategy for controlling biofilms. The C. butyricum CFS had the potential to disperse mature biofilms as well as to inhibit biofilm development of A. baumannii, as described above. The C. butyricum CFS (12.5–25%) disrupted the mature biofilms of A. baumannii ATCC 19606, clinical isolates P2713 and P3000 strains by 33.57–66.5%, 24.02–28.44% and 63.27–84%, respectively. Notably, the mature biofilms of ATCC 19606, clinical isolates P2713 and P3000 strains were significantly eradicated by 82.47%, 80.52% and 92.66% following exposure to 50% C. butyricum CFS, respectively (Fig. 3).
Dispersal effect of C. butyricum CFS on mature biofilms of A. baumannii. Following exposure to C. butyricum CFS, the mature biofilms of A. baumannii ATCC 19606 (a) and clinical isolates P2713 (b) and P3000 (c) strains were incubated at 37 °C for 24 h. The mature biofilms of A. baumannii strains were stained with 1% crystal violet and assessed by measuring at A570. The results are expressed as means ± standard deviations (SD). *** denotes significant differences at p < 0.001
Effect of C. butyricum CFS on the metabolic activity of A. baumannii biofilm cells
To demonstrate the antibiofilm effect of C. butyricum CFS, the metabolic activity of A. baumannii biofilm cells was analyzed via XTT reduction assay. The specific absorbance values were calculated using the results of testing samples and the background blank (Huyck et al. 2012). In the presence of 25% C. butyricum CFS, the metabolic activity of the clinical isolate P2713 was inhibited by only 47%, less than that of the other strains. However, 50% C. butyricum CFS decreased the metabolic activity of all A. baumannii strains by 92.93–100% (Fig. 4). As illustrated in Fig. 4, the data suggested that the C. butyricum CFS effectively inhibited the metabolic activity of A. baumannii biofilm cells as well as suppressed and dispersed the biofilm by A. baumannii.
Effect of C. butyricum CFS on the metabolic activity of A. baumannii biofilm cells. The established biofilms of A. baumannii ATCC 19606 (a) and clinical isolates P2713 (b) and P3000 (c) strains were incubated in the presence of C. butyricum CFS at 37 °C for 24 h. The metabolic activity of A. baumannii biofilm cells was analyzed via XTT reduction assay. Specific absorbance was expressed as A475 (Test)—A475 (Blank)—A655 (Test). The results are expressed as means ± standard deviations (SD). *** describes significant differences at p < 0.001
Confocal laser scanning microscopy
To evaluate the antibiofilm activity of C. butyricum CFS against A. baumannii, the most abundant biofilm of the clinical isolate P2713 was analyzed using CLSM. As shown in Fig. 5a, the untreated control showed noticeable bacterial cell aggregates and massive amounts of extracellular matrix in A. baumannii biofilms. In the presence of 12.5% and 25% C. butyricum CFS, A. baumannii biofilms dose-dependently reduced the biomass concentration and thickness and showed a poorly developed architecture (Fig. 5b–c). As shown in Fig. 5, a significant reduction in biofilm integrity, especially carbohydrates and proteins, was observed upon treatment with C. butyricum CFS when compared with the untreated group. This result suggests that CFS collected from C. butyricum showed a reduction in biomass and thickness, and structural disintegration of A. baumannii.
Confocal laser scanning micrographs of A. baumannii biofilms. a Non-treated biofilms; (b) biofilms treated with 12.5% C. butyricum CFS; (c) biofilms treated with 25% C. butyricum CFS. Proteins, carbohydrates and nucleic acids in A. baumannii biofilm were visualized by staining with FITC (green fluorescent), Con A (red fluorescent), and DAPI (blue fluorescent), respectively. A. baumannii biofilms were observed at × 40 magnification. The scale bar indicates 50 μm
Inhibition of motility by C. butyricum CFS treatment
The antivirulence effect of C. butyricum CFS on the motility of A. baumannii was determined using a semisolid agar. The clinical isolate P2713 strain formed the most abundant biofilm used in the motility assay. The clinical isolate P2713 was active and motile with a mean diameter of 5.45 ± 0.05 cm in the turbid zone for non-treated groups. Following exposure to 12.5% and 25% C. butyricum CFS, the diameters of turbid zone were 4.05 ± 0.15 cm and 1.95 ± 0.35 cm, respectively. In the presence of 50% C. butyricum CFS, the motility of A. baumannii was completely inhibited (Fig. 6). Results showed that C. butyricum CFS significantly suppressed bacterial migration, in a dose-dependent manner.
Antivirulence effect of C. butyricum CFS on A. baumannii motility. a Mean diameters of twitch colonies (cm); b twitch colonies of A. baumannii cells. A. baumannii was inoculated onto LB plates containing 0.2% agar and C. butyricum CFS. The results are expressed as means ± standard deviations (SD). * and *** denote significant differences at p < 0.05 and p < 0.001, respectively
Effect of C. butyricum CFS on the expression of RND-type efflux pump-related genes in A. baumannii
qPCR was used to evaluate the changes in transcriptional levels of RND-type efflux pump-related genes in A. baumannii. In the presence of 50% C. butyricum CFS, the expression of adeA gene in A. baumannii ATCC 19606, clinical isolates P2713 and P3000 strains was decreased by 106.99-fold, 37.89-fold and 9.08-fold, respectively (Fig. 7a). Also, treatment with 50% C. butyricum CFS downregulated the expression of adeB gene in ATCC 19606, clinical isolates P2713 and P3000 strains by 173.67-fold, 88.39-fold and 16.66-fold, respectively (Fig. 7b). Furthermore, the expression of adeC gene in ATCC 19606, clinical isolates P2713 and P3000 strains was suppressed by 286.95-fold, 98.82-fold and 11.22-fold, respectively (Fig. 7c). As shown in Fig. 7, the expression of RND-type efflux pump-related genes in all strains used in the study was significantly downregulated by treatment with C. butyricum CFS in a dose-dependent manner. The result was consistent with the results obtained above.
qPCR analysis of RND-type efflux pump-related gene adeA (a), adeB (b) and adeC (c) in A. baumannii. Gene expression of A. baumannii ATCC 19606 (black bar) and clinical isolates P2713 (gray bar) and P3000 (white bar) strains. The samples were normalized to compare the relative expression levels using housekeeping gene, 16S rRNA. The data are expressed as fold changes and analyzed by Student’s t-test to compare the gene expression between treated and non-treated groups. The results are expressed as means ± standard deviations (SD). * and ** denote significant differences at p < 0.05 and p < 0.01, respectively
Discussion
C. butyricum secretes a variety of antimicrobial substances, such as bacteriocins called butyricin, as well as organic acids, mainly butyric acid (Cassir et al. 2016). Bacteriocins are described as antibacterial peptides that possess killing or inhibiting action against the growth of closely related bacteria (Silva et al. 2018). Bacteriocins released by C. butyricum are bactericidal against a variety of bacteria, except gram-negative bacteria (Cassir et al. 2016). Consistent with previous studies, our study found that the bacteriocin of C. butyricum did not exhibit a strong antimicrobial effect against A. baumannii, gram-negative bacteria. Therefore, our findings suggest that planktonic cell growth of A. baumannii was inhibited by organic acids formed by C. butyricum rather than bacteriocin-like inhibitory substance.
Butyric acid, which constitutes the majority of the short-chain fatty acids (SCFAs) secreted by C. butyricum, exhibits amphipathic properties (Gill et al. 2018). The amphipathic interaction between the biofilm and butyric acid is facilitated by the significant water content (97%) of the biofilms (Cordeiro et al. 2019). CFS derived from C. butyricum is more effectively absorbed in biofilms of A. baumannii. Based on these studies, our findings suggest that the antimicrobial and antibiofilm effects of C. butyricum CFS are related to action of butyric acid.
The experiment used A. baumannii strains isolated from patients with respiratory system and bloodstream infections, which account for the largest proportion of A. baumannii infection. According to Saranya et al., the respiratory isolates formed robust and thicker biofilm compared with the blood isolates (Vijayakumar et al. 2016). Similarly, our data also showed that P2713 strain (a respiratory isolate) produced more biofilm than P3000 strain (a blood isolate).
The biofilm formation of A. baumannii is associated with various virulence factors. The biosynthesis of pili in A. baumannii is mediated via expression of csuA/BABCDE chaperone–usher assembly system, which is essential for twitching motility (Luo et al. 2015). The simultaneous expression of pili and twitching motility facilitates the adherence of A. baumannii to abiotic surfaces and occurs in the early stage of biofilm development (Tomaras et al. 2003; Luo et al. 2015). Based on these studies, our study suggests that the antibiofilm effect of C. butyricum CFS is closely associated with the inhibition of motility in A. baumannii. However, the mechanism of biofilm development and motility of A. baumannii has yet to be clearly identified and requires further study.
The RND-type efflux systems, another virulence factor, play key roles in gram-negative bacteria: (1) resistance to antibiotic and antibacterial substances; (2) modulation of virulence factors involved in the expression of quorum sensing systems; (3) neutralization of intracellular metabolites; and (4) regulation of cellular homeostasis and intercellular communication (Beceiro et al. 2013). The RND-type efflux systems in A. baumannii are classified into AdeABC, AdeFGH and AdeIJK types. Among the three RND-type efflux systems, the AdeABC is the major efflux pump system related to antibiotic resistance of A. baumannii. The AdeABC efflux pump system consists of three components: AdeA, which synthesizes the inner membrane fusion protein; AdeB, which produces the trans-membrane segment; and AdeC, which generates the outer membrane protein channel (Marchand et al. 2004). The structural genes promote the efflux of the drug out of the cell across the inner and outer membranes, resulting in resistance to various antibiotics (Modarresi et al. 2015). A previous study reported that biofilm formation in A. baumannii closely involves the genes encoding RND-type efflux pump system (He et al. 2015). More specifically, the overexpression of AdeABC efflux pump genes contributed to increased biofilm development (He et al. 2015; Yoon et al. 2015), whereas the downregulation of AdeABC efflux pump genes was associated with decreased biofilm, along with the increase in antimicrobial susceptibility and reduction in virulence factors (Richmond et al. 2016). Consistent with previous studies, our findings suggest that C. butyricum CFS suppresses the A. baumannii biofilm by inhibiting the expression of RND-type efflux pump-related genes, indicating that the expression of AdeABC efflux pump genes is closely related to A. baumannii biofilm formation.
In conclusion, our findings demonstrate that CFS derived from C. butyricum exerts antimicrobial and antibiofilm effects on A. baumannii. Also, these effects are closely related to the inhibition of motility and RND-type efflux pump-related adeABC genes in A. baumannii. This study reinforced the value of C. butyricum as a probiotic and suggested the potential of C. butyricum as a new therapeutic alternative against A. baumannii. However, further studies are needed to determine the mechanisms of C. butyricum CFS regulating A. baumannii biofilms. Such studies expand our insight into the development of new antimicrobial and antibiofilm agents to treat biofilm-associated infection by multidrug-resistant A. baumannii.
References
Antunes LC, Visca P, Towner KJ (2014) Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis 71:292–301. https://doi.org/10.1111/2049-632X.12125
Beceiro A, Tomas M, Bou G (2013) Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world? Clin Microbiol Rev 26:185–230. https://doi.org/10.1128/CMR.00059-12
Boucher HW et al (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. https://doi.org/10.1086/595011
Cady NC et al (2012) Inhibition of biofilm formation, quorum sensing and infection in Pseudomonas aeruginosa by natural products-inspired organosulfur compounds. PLoS ONE 7:e38492. https://doi.org/10.1371/journal.pone.0038492
Cassir N, Benamar S, La Scola B (2016) Clostridium butyricum: from beneficial to a new emerging pathogen. Clin Microbiol Infect 22:37–45. https://doi.org/10.1016/j.cmi.2015.10.014
Clinical and Laboratory Standards Institute (CLSI) (2018) Performance standards for antimicrobial susceptibility testing: 29th edn. CLSI document M31–A3
Cordeiro RA et al (2019) Sodium butyrate inhibits planktonic cells and biofilms of Trichosporon spp. Microb Pathog 130:219–225. https://doi.org/10.1016/j.micpath.2019.03.013
Courvalin P (2006) Antibiotic resistance: the pros and cons of probiotics. Dig Liver Dis 38(Suppl 2):S261–S265. https://doi.org/10.1016/S1590-8658(07)60006-1
Coyne S, Guigon G, Courvalin P, Perichon B (2010) Screening and quantification of the expression of antibiotic resistance genes in Acinetobacter baumannii with a microarray. Antimicrob Agents Chemother 54:333–340. https://doi.org/10.1128/AAC.01037-09
Dijkshoorn L, Nemec A, Seifert H (2007) An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5:939–951. https://doi.org/10.1038/nrmicro1789
Eze EC, Chenia HY, El Zowalaty ME (2018) Acinetobacter baumannii biofilms: effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect Drug Resist 11:2277–2299. https://doi.org/10.2147/IDR.S169894
FAO, WHO (2001) Joint FAO/WHO Working Group on drafting guidelines for the evaluation of probiotics in food: health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Publishing Management Service, Information Division, Rome
Fischer J, Prosenc MH, Wolff M, Hort N, Willumeit R, Feyerabend F (2010) Interference of magnesium corrosion with tetrazolium-based cytotoxicity assays. Acta Biomater 6:1813–1823. https://doi.org/10.1016/j.actbio.2009.10.020
Gantois I et al (2006) Butyrate specifically down-regulates salmonella pathogenicity island 1 gene expression. Appl Environ Microbiol 72:946–949. https://doi.org/10.1128/AEM.72.1.946-949.2006
Gill PA, van Zelm MC, Muir JG, Gibson PR (2018) Review article: short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther 48:15–34. https://doi.org/10.1111/apt.14689
Gunn JS, Bakaletz LO, Wozniak DJ (2016) What’s on the outside matters: the role of the extracellular polymeric substance of gram-negative biofilms in evading host immunity and as a target for therapeutic intervention. J Biol Chem 291:12538–12546. https://doi.org/10.1074/jbc.R115.707547
Hager CL et al (2019) Effects of a novel probiotic combination on pathogenic bacterial-fungal polymicrobial biofilms. MBio. https://doi.org/10.1128/mBio.00338-19
Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108. https://doi.org/10.1038/nrmicro821
He X et al (2015) Biofilm formation caused by clinical Acinetobacter baumannii isolates is associated with overexpression of the AdeFGH efflux pump. Antimicrob Agents Chemother 59:4817–4825. https://doi.org/10.1128/AAC.00877-15
Huyck L, Ampe C, Van Troys M (2012) The XTT cell proliferation assay applied to cell layers embedded in three-dimensional matrix. Assay Drug Dev Technol 10:382–392. https://doi.org/10.1089/adt.2011.391
Kashiwagi I et al (2015) Smad2 and Smad3 inversely regulate TGF-beta autoinduction in Clostridium butyricum-activated dendritic cells. Immunity 43:65–79. https://doi.org/10.1016/j.immuni.2015.06.010
Kaur S, Sharma P, Kalia N, Singh J, Kaur S (2018) Anti-biofilm properties of the fecal probiotic lactobacilli against Vibrio spp. Front Cell Infect Microbiol 8:120. https://doi.org/10.3389/fcimb.2018.00120
Kim H, Kang SS (2019) Antifungal activities against Candida albicans, of cell-free supernatants obtained from probiotic Pediococcus acidilactici HW01. Arch Oral Biol 99:113–119. https://doi.org/10.1016/j.archoralbio.2019.01.006
Kim MK et al (2018) Antibacterial and antibiofilm activity and mode of action of magainin 2 against drug-resistant Acinetobacter baumannii. Int J Mol Sci. https://doi.org/10.3390/ijms19103041
Komolafe OO (2003) Antibiotic resistance in bacteria—an emerging public health problem. Malawi Med J 15:63–67. https://doi.org/10.4314/mmj.v15i2.10780
Kuroiwa T, Kobari K, Iwanaga M (1990) Inhibition of enteropathogens by Clostridium butyricum MIYAIRI 588. Kansenshogaku Zasshi 64:257–263. https://doi.org/10.11150/kansenshogakuzasshi1970.64.257
Lewis K (2001) Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999–1007. https://doi.org/10.1128/AAC.45.4.999-1007.2001
Luo LM et al (2015) Enhancing pili assembly and biofilm formation in Acinetobacter baumannii ATCC19606 using non-native acyl-homoserine lactones. BMC Microbiol 15:62. https://doi.org/10.1186/s12866-015-0397-5
Marchand I, Damier-Piolle L, Courvalin P, Lambert T (2004) Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob Agents Chemother 48:3298–3304. https://doi.org/10.1128/AAC.48.9.3298-3304.2004
Mendonca FH, Santos SS, Faria Ida S, Goncalves e Silva CR, Jorge AO, Leao MV (2012) Effects of probiotic bacteria on Candida presence and IgA anti-Candida in the oral cavity of elderly. Braz Dent J 23:534–538. https://doi.org/10.1590/s0103-64402012000500011
Modarresi F, Azizi O, Shakibaie MR, Motamedifar M, Valibeigi B, Mansouri S (2015) Effect of iron on expression of efflux pump (adeABC) and quorum sensing (luxI, luxR) genes in clinical isolates of Acinetobacter baumannii. APMIS 123:959–968. https://doi.org/10.1111/apm.12455
Nait Chabane Y et al (2014) Virstatin inhibits biofilm formation and motility of Acinetobacter baumannii. BMC Microbiol 14:62. https://doi.org/10.1186/1471-2180-14-62
Nosyk O, ter Haseborg E, Metzger U, Frimmel FH (2008) A standardized pre-treatment method of biofilm flocs for fluorescence microscopic characterization. J Microbiol Methods 75:449–456. https://doi.org/10.1016/j.mimet.2008.07.024
Peleg AY, Adams J, Paterson DL (2007) Tigecycline efflux as a mechanism for nonsusceptibility in Acinetobacter baumannii. Antimicrob Agents Chemother 51:2065–2069. https://doi.org/10.1128/AAC.01198-06
Pierce CG et al (2008) A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 3:1494–1500. https://doi.org/10.1038/nport.2008.141
Raorane CJ, Lee JH, Kim YG, Rajasekharan SK, Garcia-Contreras R, Lee J (2019) Antibiofilm and antivirulence efficacies of flavonoids and curcumin against Acinetobacter baumannii. Front Microbiol 10:990. https://doi.org/10.3389/fmicb.2019.00990
Reid G, Jass J, Sebulsky MT, McCormick JK (2003) Potential uses of probiotics in clinical practice. Clin Microbiol Rev 16:658–672. https://doi.org/10.1128/cmr.16.4.658-672.2003
Richmond GE et al (2016) The Acinetobacter baumannii two-component system AdeRS regulates genes required for multidrug efflux, biofilm formation, and virulence in a strain-specific manner. MBio 7:e00430–16. https://doi.org/10.1128/mBio.00430-16
Roy A, Chaudhuri J, Sarkar D, Ghosh P, Chakraborty S (2014) Role of enteric supplementation of probiotics on late-onset sepsis by Candida species in preterm low birth weight neonates: a randomized, double blind, placebo-controlled trial. N Am J Med Sci 6:50–57. https://doi.org/10.4103/1947-2714.125870
Silva CCG, Silva SPM, Ribeiro SC (2018) Application of bacteriocins and protective cultures in dairy food preservation. Front Microbiol 9:594. https://doi.org/10.3389/fmicb.2018.00594
Takahashi M, Taguchi H, Yamaguchi H, Osaki T, Kamiya S (2000) Studies of the effect of Clostridium butyricum on Helicobacter pylori in several test models including gnotobiotic mice. J Med Microbiol 49:635–642. https://doi.org/10.1099/0022-1317-49-7-635
Takahashi M, Taguchi H, Yamaguchi H, Osaki T, Komatsu A, Kamiya S (2004) The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice. FEMS Immunol Med Microbiol 41:219–226. https://doi.org/10.1016/j.femsim.2004.03.010
Tomaras AP, Dorsey CW, Edelmann RE, Actis LA (2003) Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology 149:3473–3484. https://doi.org/10.1099/mic.0.26541-0
Vijayakumar S, Rajenderan S, Laishram S, Anandan S, Balaji V, Biswas I (2016) Biofilm formation and motility depend on the nature of the Acinetobacter baumannii clinical isolates. Front Public Health 4:105. https://doi.org/10.3389/fpubh.2016.00105
Wasfi R, Abd El-Rahman OA, Zafer MM, Ashour HM (2018) Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J Cell Mol Med 22:1972–1983. https://doi.org/10.1111/jcmm.13496
Yoon EJ et al (2015) Contribution of resistance-nodulation-cell division efflux systems to antibiotic resistance and biofilm formation in Acinetobacter baumannii. MBio. https://doi.org/10.1128/mBio.00309-15
Acknowledgements
This study was supported by the Soonchunhyang University Research Fund and the Basic Science Research Program via the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A1B03032960).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest relevant to this article.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shin, DS., Eom, YB. Antimicrobial and antibiofilm activities of Clostridium butyricum supernatant against Acinetobacter baumannii. Arch Microbiol 202, 1059–1068 (2020). https://doi.org/10.1007/s00203-020-01823-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-01823-0