Abstract
Many researchers proved that plant endophytes manage successful issues to synthesize active chemicals within plant cells. These bioactive compounds might support a range of plant defense mechanism against many pathogenic microorganisms. In this study, a total of 22 isolates representing 21 fungal species belonging to 15 fungal genera in addition to one variety were isolated and identified for the first time from Euphorbia geniculate plants. The genus Aspergillus was the most common fungus isolated from the studied plant. The fungus Isaria feline was recorded in both leaves and stem, while Aspergillus flavus, A. ochraceus, A. terreus var. terreus, Emercilla nidulans var. acristata, Macrophomina phaseolina colonized both stem and root. The isolated fungi showed antagonistic activities against six strains of plant pathogenic fungi viz., Eupenicillium brefeldianum, Penicillium echinulatum, Alternaria phragmospora, Fusarium oxysporum, Fusarium verticilloid, and Alternaria alternata in dual culture assay. The highest antagonistic activity fungal species (Aspergillus flavus, A. fumigatus, and Fusarium lateritium) and the lowest (Cladosprium herbarum, F. culomrum, and Sporotrichum thermophile) showed twining in their secondary metabolites especially terpens and alkaloids with that of their host E. geniculata. Three concentrations of (0.5, 1.0, and 2.0 mg/ml) of these secondary metabolites extracted by ethyl acetate and n-butanol from the above six endophytic fungal species were tested against three pathogenic fungi isolated from infected tomato plant (E. brefeldianum-EBT-1, P. echinulatum-PET-2, and A. phragmospora-APT-3), whereas these pathogens showed promising sensitivity to these fungal secondary metabolites. In conclusion, this is the first report on the isolation of endophytic fungi from E. geniculata and evaluation of their antifungal activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Euphorbia geniculata (syn. E. heterophylla) commonly known as spurge weed belongs to the family Euphorbiaceae which represented by trees, shrubs, and herbs. This plant was characterized by the presence of white milky latex which is more or less toxic (Kumar et al. 2010). It was locally abundant, erected about 3 ft. high and annual weed (Fred-Jaiyesimi and Abo 2010). In East Africa, E. geniculata is used for the treatment of gonorrhea, as a purgative, a lactogenic agent and as a cure for migraine and warts (Dokosi 1998; Yesilada et al. 1999; Falodun and Agbakwuru 2004; Falodun et al. 2003; James and Friday 2010). The latex of the plant is used as fish poison, insecticide, and poisons (Rodriguez et al. 1976). Moreover, the antibacterial and anti-inflammatory activities of E. geniculata have been reported (Falodun et al. 2003, 2006; Okoli et al. 2009).
Endophytic fungi are commonly found in almost all plants. Most endophytic fungi belong to the Ascomycetes and fungi Imperfecti. Under normal circumstances, they can survive in plants for all or part of their life without causing any damage or diseases (Petrini et al. 1993; Ali et al. 2018). Some endophytic fungi were isolated from Euphorbia species such as Chaetomium strumarium from E. hirta (Anitha and Mythili 2017), Fusarium sp. from E. pekinensis, and E. milii (Dai et al. 2008; Rao et al. 2018), while the endophytic fungal population in E. geniculata was not studied yet.
Many studies revealed that endophytes play an important role in host protection against pathogens (Azevedo et al. 2000; Firáková et al. 2007; Giménez et al. 2007). In this case, the endophytic fungi establish mutualistic symbiosis. Many are capable of synthesizing antimicrobial secondary metabolites, which support plants defense against pathogenic microorganisms, and enhance plant growth (Carrol 1988; Schulz et al. 2002; Strobel 2003; Corrado and Rodrigues 2004; Owen and Hundley 2004; Strobel et al. 2004; Giménez et al. 2007). It is believed that screening for antimicrobial compounds from endophytes is a convenient way to overcome the increasing threat of drug-resistant microbes of human and plant pathogen (Tan and Zou 2001; Yu et al. 2010).
In this study, we focus on the isolation of endophytic fungi from the medicinal herb E. geniculate. Then, to investigate the antagonism between these endophytic fungi and some selected plant pathogens, the antifungal activity of secondary metabolites produced by selected endophytes against some tomato pathogens. Finally, comparing the spiking secondary metabolites of the host plant (E. geniculata) and inhabiting endophyte.
Materials and methods
Plant materials
Euphorbia geniculata plant was collected from their growing habitat in the campus of Aswan University, Aswan governorate, Egypt. Fourteen healthy medicinal plants were selected from three different sites in Aswan university area. The climatic environment in this region ranged between moderately cold dry in winter to very hot summer (Abdelrahman et al. 2016; Ali et al. 2018).
Isolation and identification of endophytic fungi from Euphorbia geniculata
Euphorbia geniculata plants were collected and surface sterilized in 70% (v/v) ethanol for 1 min and then in 5% (v/v) sodium hypochlorite solution for 5 min. Sterilized plants were subsequently washed twice with sterilized distilled water (Rossman et al. 1998). The separated plant parts (roots, stems, and leaves) were longitudinally cut into 0.5–1.0 cm segments, and the resultant specimens were directly placed on a sterilized Petri dish containing Potato Dextrose Agar (PDA) medium. Three replicated plates were incubated at 28 °C and other three replicas at 45 °C for 2–3 weeks. The growing fungi were identified on the basis of their morphological characteristics according to Raper and Thom (1949), Raper and Fennell (1965), Ellis (1971, 1976), Booth (1977), Christensen and Raber (1978); Pit (1979, 1985), and Moubasher (1993).
Pathogenic fungi
Six plant pathogenic fungi, Eupenicillium brefeldianum-EBT-1, Penicillium echinulatum-PET-2 and Alternaria phragmospora-APT-3 isolated from the infected tomato leaves and fruits, in addition to, Alternaria alternata-AAP-1 from infected pepper fruits were obtained from mycology lab of faculty of science, Aswan University. Fusarium oxysporum-FOP-1 from the rotting roots of palm, Fusarium verticilloid-FVZ-1 from Zea maize seeds obtained from Agriculture Research Center in Aswan Governorate.
Dual-culture experiment
In vitro screening for antagonistic activity of all isolated endophytic fungi against six pathogenic fungal strains was evaluated by dual culture assay (Jinantana and Sariah 1998; Albert et al. 2011). Control plates were established by co-inoculating pathogens with PDA agar plug. All tests were conducted in three replicates at 28 ± 2 °C. The diameter of pathogen colony was measured regularly. The inhibition percentage (IP %) of pathogen was recorded after incubation for 5 days following the formula:
where Dc is the average increase in mycelial growth in control, and Ds is the Average increase in mycelial growth in treatment (Singh and Tripathi 1999).
Secondary metabolites extractions from selected endophytes
The most active isolates (A. flavus, A. fumigatus, and F. lateritium) and the lowest (F. culomrum, C. herbarum, and S. thermophile) were incubated with potato dextrose broth (PDB) in 500 ml flask under shaking condition (200 rpm) for 7 days. Ethyl acetate (EtOAc) was added to the fungal culture and incubated overnight, and then, the extract was separated and dried, n-butanol (n-BuOH) was added to the remaining media and kept overnight, and then, extraction was separated and dried (Abdel-Motaal et al. 2010a).
Control of some tomato pathogenic fungi experiment
Fungal ethyl acetate and n-butanol extracts which mentioned above were incorporated into the potato dextrose agar at different concentrations (0.5 mg/ml, 1.0 mg/ml, and 2.0 mg/ml), mixed well and kept at 4 °C overnight. The mycelial disc (0.7 cm in diameter), was deposited in the center of the plate (6.0 cm in diameter) according to Poisoned food method which used to evaluate the antifungal effect against pathogens (Balouiri et al. 2016). After further incubation for 7 days at 28 °C for the fungal strains tested, the diameters of fungal growth for control and treated plates were measured, and the inhibition percentage was calculated (Singh and Tripathi 1999).
The minimum inhibitory concentration (MIC) values of each extract for fungal growth compared to the control sample were determined according to Li and Rinaldi (1999) and Abdel-Motaal et al. (2010b).
Euphorbia geniculata plant extraction
The plant materials were collected, washed, and dried. The dried plant materials were powdered by grinding and extracted with EtOAc and n-BuOH similar to fungal extraction process (Abbasi et al. 2013).
Screening of secondary metabolites produced by E. geniculata and some associated endophytes
Detection of active secondary metabolites constituents was carried out for E. geniculata and the mentioned above fungal extracts, such as alkaloids, flavonoids, terpenoids, steroids, phenols, tannins, and saponins according to Visweswari et al. (2013).
Statistical analysis
Data obtained were subjected to a one-way analysis of variance (ANOVA). Significant differences between the control and treatments (P ≤ 0.05) obtained by student’s t test using SPSS software (SPSS20.0, SPSS Inc., USA). Values shown in the figures are the means ± standard errors (SEs) of four independent replicates. Correlation analysis was carried out using Corrplot in R program (R- 3.4.3, https://www.r-project.org/).
Results and discussion
Endophytic fungi associated with E. geniculata
From 14 individual plants corresponding to E. geniculata (roots, stems, and leaves), a total of 21 fungal species belonging to 15 fungal genera in addition to one variety were isolated. According to our knowledge, this is the first report describing the isolation of endophytic fungi associated with E. geniculata plants. Pela´ez et al. (1998); Schulthess and Faeth (1998) reported that many fungi were known to grow endophytically in many plants such as Festuca arizonica, Phragmites australis, and Scirpus maritimus. Aspergillus was the most predominant fungal genus in all plant parts of E. geniculata (36%), followed by Cladosporium and Emercicella (9% for each). Anith and Mythili (2017) isolated Chaetomium strumarium as endophytic fungi from E. hirta, also from Euphorbia pekinensis, Dai et al. (2008) isolated Fusarium sp. as endophytic fungi. Many endophytes restricted to a small tissue area or in specific plants parts such as roots, leaves, or twigs (Stone et al. 2004). In this study, the relative abundance of Aspergillus in the different parts of the studied plant was (28%, 60%, and 28% of the leaves, stem, and roots, respectively) represented by five species and two varieties of A. terreus. Distribution of fungal species was variable in different parts of the studied plant, whereas Cladosporium herbarum was the most common fungal species in leaves (4 colonies), A. terreus var. terreus in the stem (3 colonies), and A. terreus var. terreus, E. nidulans var. acristata, and Gibberella intricans were common in the root (3 colonies for each) (Fig. 1a, b). It was interesting to recognize that only one fungal species (Isaria feline) present in both stem and leaves, while A. flavus, A. ochraceus, A. terreus var. terreus, E. nidulans var. acristata, and Macrophomina phaseolina were in mutual occurrence in both stem and root (Fig. 1c).
Diversity profile of endophytic fungi associated with Euphorbia geniculata. a Total count of fungal species, asterisks indicate isolation temperatures (*temp = 28 °C; **temp = 45 °C); b relative abundance of fungal genera; c venn diagram of the total number and mutual occurrence of fungal species of the root, stem, and leaf
Some isolated endophytic fungal species were thermo-tolerant (A. fumigatus and A. terreus var. terreus) which are able to grow at 28 °C and 45 °C. Others were thermophilic fungi (A. terreus var. aureus, Ascotricha guamensis, E. nidulans var. acristata, Myricoccum sparsum, Melanocarpus sparsum, and Sporotrichum thermophile) growing at 45 °C only but the remaining fungi were mesophilic (grow at 28 °C only) (Fig. 1a).
Similar endophytic fungal species were isolated from variable plants, whereas Aspergillus fumigatus, A. flavus, A. niger, A. ochraeous, A. terreus, and C. herbarum isolated from Hyoscyamus muticus (Abdel-Motaal et al. 2010a), Emericella nidulans from Ipomea batatas plant (Hipol 2012), Fusarium lateritium from Yew plant (Strobel et al. 1996), Penicillium funiculosum from soybean plants (Khan and Lee 2013), and Phoma eupyrena isolated from leaves of Avicennia schaueriana (Costa et al. 2012).
Antagonistic activity of the isolated endophytic fungi against selected pathogenic fungi
The dual culture test revealed that all endophytic fungi isolated from E. geniculata could inhibit most of the studied pathogenic fungi. The serious pathogen APT-3 which could damage the tomato crop by causing leaf spot was controlled by the thermophilic endophytic fungal species, A. terreus var. aureus and the mesophilic fungal species Chaetomium variostiolatum and A. flavus with inhibition percentage 41.67%, 42.46%, and 41.32%, respectively. The other tomato pathogen, EBT-1 which spoil tomato fruit was inhibited by the mesophilic fungal species P. funiculosum, A. flavus, A. ochraceus, and thermo-tolerant fungal species A. fumigatus with inhibition percentage 56.11%, 42.50%, 40.09, and %50.20%, respectively. PET-2 (fruit tomato pathogen) growth was controlled by the thermo-tolerant fungus, A. fumigatus and the mesophilic fungus Fusarium lateritium with inhibition percentage of 42.63% and 40.88%, respectively. The palm rotting root pathogen (FOP-1) was inhibited by the mesophilic fungal species Gibberella intricans, Thanatephorus cucumeris, and the thermophilic fungal species Melanocarpus sparsum with percentage (41.93%, 41.67%, and 59.37%, respectively). The zea maize pathogen FVZ-1 was inhibited by A. niger with inhibition percentage 50.0%. Alternaria phragmospora was reported as pathogen that causes damage to the seedlings of radish (Emden 1970) and A. alternata caused rotting of tomato fruits (Abdel-Mallek et al. 1995). Berbee (2001) proved that F. oxysporum, Eupencillium sp., and A. alternata were plant and mammal pathogens. Penicillium echinulatum was recorded as pathogen which causes blue mold symptoms to grape fruits (Kim et al. 2007). Liu et al. (2001) reported that 21 endophytes isolated from Artimisia annua had strong antifungal activity against six phytopathogenic fungi causing wheat take-all, sharp eye spot, and common rot. Rhizospheric fungi such as A. flavus, A. niger, and A. terreus inhibit the growth of F. oxysporum (Alwathnani and Perveen 2012). Faba bean (Vicia faba L.) leaf spot disease caused by A. alternata has been limited by Trichoderma harzianum (Kayim et al. 2018). In this study, AAP-1 (pepper pathogen) was controlled by the mesophilic fungal species A. flavus and F. lateritium (40.17% and 47.29%, respectively). In vitro F. verticilloid was inhibited by Chaetomium zeae (Gao et al. 2010).
Figure 2b shows that the tested pathogenic fungi reaction to the endophytic fungi was variable. Whereas the pathogenic EBT-1 and FOP-1 in a clade, while AAP-1 and PET-2 in another clade and the last clade included FVZ-1 and APT-3. Each clade showed similar sensitivity and resistance to the endophytic fungi. Moreover, the endophytic fungi, A. flavus, A. fumigatus, and F. lateritium virulence, was clear as the most active effect against almost all pathogenic fungi.
Antagonistic activity of Endophytic fungi isolated from Euphorbia geniculata against different plant pathogens, a pathogen growth inhibition percentage, asterisks indicate significance of inhibition (*low; **moderate; ***high); ns (not significance), b heatmap illustrate the sensitivity of selected plant pathogenic fungi to the endophytic fungi
Antifungal activity of selected endophytic fungal extracts against three tomato’s pathogenic fungi
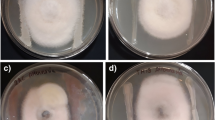
The percentage of growth inhibition of the selected phytopathogenic fungi (EBT-1, PET-2, and APT-3) by different concentrations (0.5, 1.0, and 2.0 mg/ml) broth of selected endophyte culture (A. flavus, A. fumigatus, C. herbarum, F. lateritium, F. culomrum, and S. thermophile) was determined by measuring the diameter of inhibition zones and minimum inhibitory concentration (MIC mg/ml) (Balouiri et al. 2016), and the data were shown in (Fig. 3) not paralleled to the antagonistic action mentioned above, the antifungal activities of selected endophyte cultures were significantly different. The three concentrations of the selected endophytes cultures were active against the three tested plant pathogens with variable inhibitory effects. Fusarium lateritium, an endophyte producing the most active substance, the EtOAc fractions of which exhibited abroad antifungal spectrum at a concentration of 0.5 mg/ml (MIC90) followed by n-BuOH fraction (MIC78.93) against all tomato pathogens. In contrast, n-BuOH fraction of A. fumigatus culture was more effective than EtOAc fraction especially against EBT-1 (MIC72-90, MIC74-80), respectively. However, the pathogen APT-3 had marked sensitivity towards both n-BuOH and EtOAc of A. flavus with the three concentrations which inhibited at 0.5 mg/ml with MIC50 n-BuOH, MIC90 at 1.0 mg/ml EtOAc and MIC95 at 2.0 mg/ml EtOAc. Furthermore, the MICs of EtOAc fraction of C. herbarum and F. culomrum against all tested pathogen was determined to be 50–75, while n-BuOH fraction possessed moderate activity 40–66 at 0.5 mg/ml for each of them. Sporotrichum thermophile had the lowest inhibition activity in comparison with endophyte cultures tested on phytopathogenic fungi, the n-BuOH fraction of which only detected with MIC50 at concentration 0.5 mg/ml against APT-3. Both EtOAc and n-BuOH fractions of A. flavus, A. fumigatus, and F. lateritium showed broader antifungal spectrum and stronger toxicity mycelium growth of APT-3.
In the microscopic examination, swollen in hyphae and formation of chlamydospores were recorded in APT-3 which treated with both extracts (EtOAc and n-BuOH) of A. flavus, A. fumigatus, and F. lateritium compared to the control. Sporulation also affected compared to control, whereas asexual spores were disappeared in treated colonies. In addition, melanin pigmentation of hypha under microscope was reduced in 2.0 mg/ml of both extracts in A. flavus, A. fumigatus, and F. lateritium. Pathogen melanins may act as a supportive weapon in host penetration and suppression of host’s responses (Mérillon and Ramawat 2017). Thus, reducing melanin formation makes the pathogen hypha very weak and easy to control. The pigmentation of the spores was reduced in PET-2 colonies which treated with 1.0 mg/ml n-BuOH fraction of A. flavus. The antifungal activity of the chemicals biosynthesized by the plant endophytes has been reported by several groups (Rojas et al. 1992). The present results indicate that the metabolites (EtOAc and n-BuOH fractions) of endophytic fungi from E. geniculata have extensive inhibition to the growth of phytopathogenic fungi but with variable inhibitory effect. This could be related to variation in quality and quantity of active compounds in their extracts and could be attributed to structural nature of the pathogen and endophytic fungi constituents (Yao and Moellering 1995).
Twinning between the secondary metabolites of E. geniculata plant and some selected endophytic fungi inhabiting it as a model
Natural products are naturally derived compounds present as metabolites or byproducts from microorganisms, plants, or animals. Microorganisms are one of the most important sources of these compounds. Recently, there is a special focus on endophytic fungi residing medicinal plants, whereas they are able to synthesize similar secondary metabolites of great potential for the discovery of new bioactive compounds (Kusari et al. 2008). Endophytes are synergistic to their host in producing secondary metabolites to avoid other microorganism and insects attack (Strobel and Daisy 2003). Some endophytic fungi would produce different bioactive compounds, such as alkaloids, terpenes, and flavonoids to increase the resistance to biotic and abiotic stresses for their host plants (Firáková et al. 2007; Rodriguez et al. 2009). Steroids, terpenoids, flavonoids, etc. were detected in many endophytes (Zhang et al. 2006). Flavonoids are able to inhibit spore germination of plant pathogens; therefore, they have been proposed for the use against fungal pathogens (Cushnie and Lamb 2005).
In the current study, we have tried to confirm this relationship between the host plant (E. geniculata) and selected endophytic fungi (A. flavus, A. fumigatus, C. herbarum, F. lateritium, F. culomrum, and S. thermophile) and their co-operation to produce similar secondary metabolites. The highest antagonistic activity fungi were (A. flavus, A. fumigatus, and F. lateritium) and that the lowest activity (C. herbarum, F. culomrum, and S. thermophile) against most studied pathogenic fungi in this work (Fig. 2).
Interestingly, the secondary metabolites, alkaloids, and terpens were detected in both EtOAc and n-BuOH extracts of E. geniculata herb and all the selected endophytes, while saponin was absent in both host plant and its associated tested fungi (Fig. 4). The most twinning fungus was A. flavus, whereas besides alkaloids and terpens, other chemical classes were detected such as flavenoids and tannins in both fractions and steroids in EtOAc fraction. In addition, the EtOAc fraction of A. fumigatus contains two more active chemical classes (flavenoids and steroids). While the n-BuOH fraction contains tannins and flavenoids in C. herbarum and S. thermophile, respectively (Fig. 4). These results may open a complete new dimension for the production of natural antimicrobial compounds in an extremely effective manner, stating that the relationship between endophytic fungi and their host medicinal plants is completely understood. A result that warrants more investigation in the future in our lab is that phenolic compounds were detected only in plant extract and how all selected fungi respond to these compounds.
Conclusion
This study reported for the first time the endophytic fungal community associated with the medicinal herb Euphorbia geniculata and screening their antifungal activity. Endophytic fungi associated with E. geniculata are a promising safe tool to control urgent plant disease. Twinning of secondary metabolites compounds between plant and its associated endophytes confirm the positive role of endophytes in supporting their host plant.
Further detailed studies of these endophytes after colonizing the tomato plant and their roles in protection against pathogens and improving plant morphology and physiology are currently being undertaken in our laboratory.
References
Abbasi AM, Saleem H, Aziz-ur-Rehman Riaz T, Ajaib M (2013) Determination of antioxidant activity and phytoconstituent screening of euphorbia heterophylla linn. Br J Pharm Res. 3(2):202–216
Abdel-Mallek YA, Hemida KS, Bagy KMM (1995) Studies on fungi associated with tomato fruits and effectiveness of some commercial fungicides against three pathogens. Mycopathologia 130:109–116. https://doi.org/10.1007/bf01103459
Abdel-Motaal FF, El-zayat AS, Kosaka Y, El-Sayed AM, Kashima R, Maeda Y, Nassar MSM, Ito SI (2010a) b) Antifungal activities of hyoscyamine and scopolamine against two major rice pathogens: Magnaporthe oryzae and Rhizoctonia solani. J Gen Plant Pathol 76:102–111
Abdel-Motaal FF, Nassar MSM, EL-Zayat AS, EL-Sayed AM, Ito SI (2010b) Antifungal activity of endophytic fungi isolated from Egyptian henbane (Hyoscyamus muticus L). Pak J Bot 42(4):2883–2894
Abdelrahman M, Abdel-Motaal F, El-Saved M, Jogaiah S, Shigyo M, Ito S, Tran LS (2016) Dissection of Trichoderma longibrachiatum-induced defense in onion (Allium cepa L.) against Fusarium oxysporum f. Sp. Cepa by target metabolite profiling. Plant Sci 245:128–138
Albert S, Chauhan D, Pandya B, Padhiar A (2011) Screening of Trichoderma spp. as potential fungal partner in co-culturing with white rot fungi for efficient bio-pulping. GJBBR 6:95–101
Ali A, Abdelrahman M, Radwan U, El-Zayat S, El- Sayed A (2018) Effect of Thermomyces fungal endophyte isolated from extreme hot desert adapted plant on heat stress tolerance of cucumber. App. Soil Eco 124:155–162
Alwathnani AH, Perveen K (2012) Biological control of fusarium wilt of tomato by antagonist fungi and cyanobacteria. Afr J Biotechnol 11(5):1100–1105. https://doi.org/10.5897/ajb11.3361
Anitha UKPG, Mythili S (2017) Antioxidant and hepatoprotective potentials of novel endophytic fungus Achaetomium sp., from Euphorbia hirta. Asian Pac J Trop Med 10(6):588–593. https://doi.org/10.1016/j.apjtm.2017.06.008
Azevedo LJ, Pereira OJ, Araújo LW (2000) Endophytic microorganisms: a review on insect control and recent advances on tropical plants. EJB 3:40–65. https://doi.org/10.1007/s13369-018-3431-8
Balouiri M, Sadiki M, Ibnsouda KS (2016) Methods for in vitro evaluating antimicrobial activity: a review. JPA 6:71–79
Berbee LM (2001) The phylogeny of plant and animal pathogens in the Ascomycota. Physiol Mol Plant Pathol 59(4):165–187. https://doi.org/10.1006/pmpp.2001.0355
Booth C (1977) Fusarium. Laboratory guide to the identification of major species. Common wealth Mycol Inst, Kew. https://doi.org/10.2307/3758956
Carrol G (1988) Fungal endophytes in stem and leave: from latent pathogen to mutualistic symbiont. Ecology 69(1):2–9. https://doi.org/10.2307/1943154
Christensen M, Raber BK (1978) Synoptic key to Aspergillus nidulans group species and related Emericella species. Trans Br Mycol Soc 71:177–191. https://doi.org/10.1016/s0007-1536(78)80097-7
Corrado M, Rodrigues KF (2004) Antimicrobial evaluation of fungal extracts produced by endophytic strains of Phomopsis sp. J Basic Microbiol 44(2):157–160. https://doi.org/10.1002/jobm.200310341
Costa WMPI, Maia CL, Cavalcanti AM (2012) Diversity of leaf endophytic fungi in mangrove plants of Northeast Brazil. Braz J of Microbiol. https://doi.org/10.1590/s1517-83822012000300044
Cushnie TPT, Lamb JA (2005) Antimicrobial activity of flavonoids. Int J Antimicrob Agents 26:343–356. https://doi.org/10.1016/j.ijantimicag.2005.12.002
Dai C, Yu B, Li X (2008) Screening of endophytic fungi that promote the growth of Euphorbia pekinensis. Afr J Biotechnol 7(19):3505–3510
Dokosi OB (1998) Herbs of Ghana book. Ghana Universities Press, Accra
Ellis MB (1971) Dematiaceous hyphomycetes. Commonwealth Mycol Inst, Kew
Ellis MB (1976) More dematiaceous hyphomycetes. Commonwealth Mycol Inst, Kew
Emden VHJ (1970) Alternaria phragmospora nov. Spec Acta Bot Neerl 19(3):393–400. https://doi.org/10.1111/j.1438-8677.1970.tb00660.x
Falodun A, Agbakwuru POE (2004) Phytochemical analysis and laxative activity of Euphorbia heterophylla Linn (Euphorbiaceae). Pak J Sci Ind Res 47(5):345–348
Falodun A, Agbakwuru EOP, Ukoh GC (2003) Antibacterial activity of Euphorbia heterophylla Linn (family Euphorbiaceae). Pac J Sci Res 46(6):471–472
Falodun A, Okunrobo OL, Uzoamaka N (2006) Phytochemical and Antiinflammatory evaluation of methanolic and aqueous extracts of Euphorbia heterophylla Linn (Euphorbiaceae). Afri J Biotech 5(5):529–531
Firáková S, Šturdíková M, Múčková M (2007) Bioactive secondary metabolites produced by microorganisms associated with plants. Biologia (Bratisl) 62(3):251–257
Fred-Jaiyesimi AA, Abo KA (2010) Phytochemical and Antimicrobial analysis of the crude extract, petroleum ether and chloroform fractions of Euphorbia heterophylla Linn Whole Plant. Pharmacognosy J 2(16):1–4. https://doi.org/10.1016/s0975-3575(10)80042-2
Gao KF, Dai CC, Liu ZX (2010) Mechanisms of fungal endophytes in plant protection against pathogens. Afr J Microbiol Res 4(13):1346–1351
Giménez C, Cabrera R, Reina M, González-Coloma A (2007) Fungal endophytes and their role in plant protection. Curr Org Chem 1:707–720. https://doi.org/10.2174/138527207780598765
Hipol MR (2012) Molecular identification and phylogenetic affinity of two growth promoting fungal endophytes of sweet potato (Ipomea batatas (L.) Lam.) from Baguio City, Philippines. Electron J Biol 8(3):57–61. https://doi.org/10.1016/j.jpha.2015.11.005
James O, Friday E (2010) Phytochemical composition, bioactivity and wound healing potential of Euphorbia Heterophylla (Euphorbiaceae) leaf extract. IJPBR 1(1):54–63
Jinantana J, Sariah M (1998) Potential for biological control of Sclerotium foot rot of Chilli by Trichoderma spp. Pertanika J Trop Agric Sci 21(1):1–10
Kayim M, Yones MA, Endes A (2018) Biocontrol of Alternaria alternata causing leaf spot disease on faba bean (Vicia faba L.) using some Trichoderma harzianum ısolates under in vitro condition. Harran Tarım ve Gıda Bilimleri Derg 22(2):169–178
Khan A, Lee IJ (2013) Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol. https://doi.org/10.1186/1471-2229-13-86
Kim KW, Sang KH, Woo KS, Park SM, Paul CN, Yu HS (2007) Six species of penicillium associated with blue mold of grape. Mycobiology 35(4):180–185
Kumar S, Malhotra R, Kumar D (2010) Euphorbia hirta: its chemistry, traditional and medicinal uses, and pharmacological activities. Pharmacogn Rev 4:58–61. https://doi.org/10.4103/0973-7847.65327
Kusari S, Lamshoft M, Zuhlke S, Spiteller M (2008) An endophytic fungus from Hypericum perforatum that produces hypericin. J Nat Prod 71(2):159–162. https://doi.org/10.1021/np070669k
Li KR, Rinaldi GM (1999) In vitro antifungal activity of nikkomycin Z in combination with fluconazole or itraconazole. Antimicrob Agents Chemother 43:1401–1405. https://doi.org/10.1128/aac.43.6.1401
Liu HC, Zou XW, Lu H, Tan XR (2001) Antifungal activity of Artemisia annua endophyte cultures against phytopathogenic fungi. J Biotechnol 88:277–282
Mérillon MJ, Ramawat GK (2017) Fungal metabolites. In: Belozerskaya AT, Gessler NN, Aver’yanov AA (eds) Melanin pigments of fungi. Springer International Publishing, Berlin, pp 263–291. https://doi.org/10.1007/978-3-319-25001-4_29
Moubasher AH (1993) Soil fungi in Qatar and other Arab countries. The scientific and applied research center. University of Qatar, Doha
Okoli IR, Turay AA, Mensah KJ, Aigbe OA (2009) Phytochemical and antimicrobial properties of four herbs from Edo state, Nigeria. Rep Opinion 1(5):67–73
Owen LN, Hundley N (2004) Endophytes-the chemical synthesizers inside plants. Sci Prog 87:79–99
Pelaez F, Collado J, Arenal F, Basilio A, Cabello A, Díez TM, Garcia BJ, González del Val A, González V, Gorrochategui J, Hernández P, Martín I, Platas G, Vicente F (1998) Endophytic fungi from plants living on gypsum soils as a source of secondary metabolites with antimicrobial activity. Mycol Res 102:755–761. https://doi.org/10.1017/s0953756297005662
Petrini O, Sieber T, Toti L, Viret O (1993) Ecology, metabolite production and substrate utilization in endophytic fungi. Nat Toxins 1:185–196. https://doi.org/10.1002/nt.2620010306
Pitt IJ (1979) The genus Penicillium and its telemorphic stato Eupenicillium and Talaromyces. Academic Press, London
Pitt IJ (1985) A laboratory guide to common Pencillium species. Commonwealth Scientific and Industrial Research Organization. Division of food research, North Ryde
Rao A, Ramakrishna N, Arunachalam S, Sathiavelu M (2018) Isolation, screening and optimization of laccase-producing endophytic fungi from Euphorbia milii. AJSE 44(1):51–64
Raper KB, Fennell DI (1965) The genus Aspergillus. Williams & Wilkins Co, Baltimore
Raper KB, Thom C (1949) A manual of the Penicillia. Baillière, Tindall and Cox, London
Rodriguez E, Twers NHG, Mitchell CJ (1976) Biological activities of sesquiterpene Lactones. Phytochemistry 15:1573–1580. https://doi.org/10.1016/s0031-9422(00)97430-2
Rodriguez JR, White JF Jr, Arnold EA, Redman SR (2009) Fungal endophytes: diversity and functional roles. New Phytol 182:314–330. https://doi.org/10.1111/j.1469-8137.2009.02773.x
Rojas A, Hernandezb L, Pereda-Miranda R, Mata R (1992) Screening for anti-icrubial activity of crude drug extracts and pure natural products from Mexican medicinal plants. J Ethnophurmacol 35:275–283
Rossman YA, Tulloss ER, O’Dell ET, Thorn GR (1998) Protocols for an all taxa biodiversity inventory of fungi in Costa Rican conservation area. Parkway publishers Inc, Boone. https://doi.org/10.2307/3761287
Schulthess MF, Faeth HS (1998) Distribution, abundances, and associations of the endophytic fungal community of Arizona fescue (Festuca arizonica). Mycologia 90:569–578. https://doi.org/10.2307/3761215
Schulz B, Boyle C, Draeger S, Römmert KA, Krohn K (2002) Endophytic fungi: a source of novel biologically active secondary metabolites. Mycol Res 106(9):996–1004. https://doi.org/10.1017/s0953756202006342
Singh J, Tripathi NN (1999) Inhibition of storage fungi of blackgram Vigna mungo by some essential oils. Flavour Fragr J 14(1):1–4. https://doi.org/10.1002/(sici)1099-1026(199901/02)14:1%3c1:aid-ffj735%3e3.0.co;2-r
Stone JK, Polishook JD, White JF (2004) Endophytic fungi. In: Mueller MG, Bills FG, Foster SM (eds) Biodiversity of fungi, inventory and monitoring methods. Elsevier Academic Press, San Diego, pp 241–270
Strobel GA (2003) Endophytes as sources of bioactive products. Microbes Infect 5(6):535–544. https://doi.org/10.1016/S1286-4579(03)00073-X
Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbial Mol Biol Rev 67(4):491–502. https://doi.org/10.1128/mmbr.67.4.491-502.2003
Strobel GA, Hess WM, Ford E, Sidhu RS, Yang X (1996) Taxol from fungal endophytes and the issue of biodiversity. J Ind Microbiol 17(5–6):417–423. https://doi.org/10.1007/bf01574772
Strobel G, Daisy B, Castillo U, Harper J (2004) Natural products from endophytic microorganisms. J Nat Prod 67:257–268
Tan XR, Zou WX (2001) Endophytes: a rich source of functional metabolites. Nat Prod Rep 18:448–459. https://doi.org/10.1039/b100918o
Visweswari G, Christopher R, Rajendra W (2013) Phytochemical screening of active secondary metabolites present in Withania somnifera root: role in traditional medicine. IJPSR 4(7):2770–2776
Yao J, Moellering R (1995) Antibacterial agents. In: In Versalovic J, Carroll K, Funke G, Jorgensen J, Landry M, Warnock D (eds) Manual of clinical microbiology. ASM Press, Washington, Manual of Clinical Microbiology, pp 1281–1290
Yeşilada E, Sezik E, Honda G, Takaishi Y, Takeda Y, Tanaka T (1999) Traditional medicine in Turkey IX, folk medicine in North West Anatolia. J Ethnopharmacol 64(3):195–201
Yu H, Zhang L, Li L, Zheng C, Guo L, Li W, Sun P, Qin L (2010) Recent developments and future prospects of antimicrobial metabolites produced by endophytes. Microbiol Res 165:437–449. https://doi.org/10.1016/j.micres.2009.11.009
Zhang WH, Song YC, Tan RX (2006) Biology and chemistry of endophytes. Nat Prod Rep 23:753–771. https://doi.org/10.1039/b609472b
Acknowledgements
We are grateful to the director of the Unit of Environmental Studies and Development (UESD) at Aswan University for providing facilities in the unit laboratories to accomplish this work. We are very thankful to the researcher Mr. Rasheed Zidan for his help in the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamel, N.M., Abdel-Motaal, F.F. & El-Zayat, S.A. Endophytic fungi from the medicinal herb Euphorbia geniculata as a potential source for bioactive metabolites. Arch Microbiol 202, 247–255 (2020). https://doi.org/10.1007/s00203-019-01740-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-019-01740-x