Abstract
We aimed to gain a better understanding of cold adaption in Mortierella isabellina M6-22 by using proteomics approaches. The temperature range and optimal temperature for M6-22 growth were investigated, and composition changes in fatty acids were analyzed. Accompanied with the 2-D gel electrophoresis, MALDI-TOF/TOF–MS analysis was conducted to characterize alterations in protein profiling in M6-22 cultured at 30 °C for 24 h and 15 °C for another 24 h when compared with those cultured at 30 °C for 48 h. Gene Ontology (GO) cluster analysis was finally conducted for successfully identified proteins. M6-22 cells could grow well at temperatures ranging from 15 to 30 °C. As temperature decreased from 30 to 15 °C, LA and GLA significantly increased from 11.63 to 17.85 % and from 9.12 to 13.19 %, respectively, while oleic acid significantly decreased from 47.25 to 36.53 %. Proteomics analyses revealed 111 differentially expressed protein spots, among which 5 unique proteins (A38, A40, A47, A49 and A58), 29 up-regulated proteins and 10 down-regulated proteins were identified by MALDI-TOF/TOF–MS. GO enrichment analysis demonstrated that these proteins mainly involved in glycolytic pathway (A34 and A50), electron transport (A28), ATP production (A35 and B39) and protein modification (A38). A total of 44 differentially expressed proteins have been successfully identified in M. isabellina M6-22 cultured at 15 °C. These proteins may play important roles in cold adaption via regulation of ATP synthesis, activation of cold-adaptive proteins, degradation of needless protein, accumulation of PUFAs, etc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial lipophilic compounds, namely single cell oils (SCO), present potential industrial and financial interests due to their superiority. Mortierella isabellina (M. isabellina) is a kind of microorganism producing SCO, in which lipid accumulation occupies approximately 80 % of cell biomass (Chatzifragkou et al. 2010). M. isabellina is considered as a feedstock for biodiesel production, since its fatty acid composition is similar to those of common plants (Vicente et al. 2009; Economou et al. 2011). What is more, the lipids produced by M. isabellina contain a large amount of high-valued polyunsaturated fatty acids (PUFAs) such as γ-linolenic acid (GLA) and arachidonic acid (AA) (Aki et al. 2001; Mamatha et al. 2008), both of which are of nutritional importance and can be used for treating human diseases (Watkins et al. 2002; Calder 2008). These declared that M. isabellina was a promising candidate for lipid production in industry.
Temperature is one of the most important environmental factors affecting the growth and survival of organisms, and low temperature is always considered as a stress since cell viability or organism’s fitness may decrease in such an environment (Tesei et al. 2012). Recent studies have revealed that certain kinds of organisms can adapt to low temperature through modulation of membrane fluidity, accumulation of compatible solutes, synthesis of cold adaption protein, as well as alterations in DNA, RNA and protein conformations (Shivaji and Prakash 2010; Tesei et al. 2012). Among these mechanisms, modulation of membrane fluidity is of high importance (Zhang and Rock 2008). Under low temperature, microorganisms can produce more PUFAs to composite membrane lipids and maintain membrane fluidity which is essential for cold adaption of the microorganisms (Weinstein et al. 2000; Kang et al. 2002; Lu et al. 2005).
Our preliminary study has demonstrated that M. isabellina can survive at low temperatures (≥5 °C), indicating its cold adaption. However, the cold-adapted mechanisms of M. isabellina are still not clear. Proteomics analysis is helpful for better understanding the dynamics of cellular function and the responses in the microbial system and therefore a powerful tool to investigate potential proteins involving in the cold-adapted mechanisms (Bakermans et al. 2007; Garnier et al. 2010). Thus, we conducted this present study to examine the response strategy developed by M. isabellina strain M6-22 to overcome cold stress by using proteomics approaches of 2-D gel electrophoresis (2-DE) and matrix-assisted laser desorption/ionization (MALDI) time-of-flight/time-of-flight (TOF/TOF) mass spectrometry (MS) analysis, as well as the correlation between cold adaptation of M6-22 and content of PUFAs.
Materials and methods
Strain and culture
Mortierella isabellina M6-22 was kindly presented by Nankai University and cultured in liquid medium containing 2 % glucose, 1 % Bacto yeast extract, 0.2 % KH2PO4 and 0.1 % MgSO4 at pH 6.0. To investigate the temperature range and optimum temperature for M6-22 growth, M6-22 was statically cultured at 5, 10, 15, 20, 25, 30 and 35 °C for 48 h, respectively. To explore the protein profiling under cold stress, M6-22 strains cultured at 30 °C for 24 h and at 15 °C for another 24 h were severed as treatment group, while those cultured at 30 °C for 48 h were served as control. Mycelia were collected by filtration, washed with poly butylenes succinate (PBS) for three times, quickly frozen in liquid nitrogen and then stored at −80 °C.
Fatty acids analysis
Fatty acids of M6-22 were analyzed according to the method described by Yang et al. (2014). Briefly, approximately 100 mg of mycelia powder was firstly saponified in 5 mL 5 % KOH in methanol at 70 °C for 5 h, followed by methyl etherification with 4 mL 14 % boron trifluoride in methanol at pH 2.0 for another 1.5 h. After adding saturated sodium chloride solution, fatty acid methyl esters (FAME) were finally extracted by hexane, analyzed using gas chromatography (GC, model GC-9A, Shimadzu, Kyoto, Japan) and identified via comparing with their peaks of standards (Sigma, St. Louis, USA).
Protein extraction
Mycelium proteins were extracted using phenol extraction and ammonium acetate precipitation (Isaacson et al. 2006). In brief, the mycelia were transferred into a pre-cooled mortar, ground into powder and lysed in 5 mL Tris-saturated phenol. After mixed with equal volume of extraction buffer (0.7 M sucrose; 0.1 M KCl; 0.5 M Tris–HCl; 50 mM ethylenediaminetetraacetic acid, EDTA; 1 mM phenylmethanesulfonyl fluoride, PMSF; pH 7.5), the cell lysate was cooled in a water–ice bath for 15 min and then centrifuged at 6000 rpm for 20 min at 4 °C. Subsequently, the upper phenol phase was pipetted to a new microtube and incubated for precipitation at −20 °C overnight after added with five volumes of cold methanol contained 0.1 M ammonium acetate. The mixture was centrifuged at 6000 rpm for 20 min at 4 °C, after which the supernatant was carefully removed. The protein pellets were washed twice with 5 mL methanol and 5 mL acetone, respectively. The residual organic solvent was further removed by freeze-drying method. Protein pellets were then collected and stored at −80 °C.
Protein preparation and 2-DE
For 2-DE, protein samples were firstly obtained from the collected protein pellets via re-suspending in lysis buffer containing 0.7 M urea, 2 M thiourea and 4 % 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonic acid, CHAPS). To remove insoluble materials, the suspension was centrifuged at 10,000g for 20 min. The total protein concentration was then measured using bicinchoninic acid (BCA) assay. To obtain statistically reliable results, the 2-DE was repeated in triplicate as described previously (Strocchi et al. 2006). Briefly, 120 μg of protein samples for analytical gels and 1200 μg of protein samples for preparative gels were loaded onto immobilized pH gradient (IPG) strips (pH 4–7, Bio-Rad, CA, USA) for the first-dimension isoelectric focusing (IEF), after which the strips were transferred to 12.5 % vertical sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for the second-dimension electrophoresis. Finally, the gels with coomassie blue staining were visualized a UMAX Power look 1100 scanner (Maximum Tech, Taiwan, China) at 150 dpi (dots/in), and quantitative intensity analysis was conducted by ImageMaster 2D platinum 5.0 (GE) software. The 2-D pattern matching was conducted by the ImageMaster 2D platinum 5.0 (GE Healthcare, Uppsala, Sweden). Protein spots of interest were selected if they were differentially expressed with greater than twofold change between strains cultured at 15 and 30 °C or just expressed at 15 °C.
Gel preparation, MALDI-TOF/TOF–MS analysis and database searching
For detection, protein spots of interest were cut from the coomassie blue staining 2-DE gels, destained with methyl alcohol/50 mM NH4HCO3 (1:1 v/v) for 30 min and washed twice with 120 μL Milli-Q water. Each spots were incubated in 50 μL 100 mM NH4HCO3 for 30 min, dehydrated twice with 100 μL 50 % acetonitrile (ACN) and then digested overnight 4–8 μL 20 ng/μL trypsin in 25 mM NH4HCO3. The peptides were finally extracted with 60 μL 90 % ACN/2.5 % trifluoroacetic acid (TFA) in double-distilled water and completely dried by a vacuum centrifuge.
MS data for protein identification were obtained by using an Ultraflex III TOF/TOF instrument (Bruker Daltonics, Bremen, Germany). Instrument parameters were set as follows: wave length 355 nm, faster laser repetition rate 200 Hz, acceleration voltage 20,000 V, mass range from 700 to 3200 Da and a focus mass of 2000 Da. The MS spectra were recorded in the default mode and analyzed using flexAnalysis 2.0 software (Bruker Daltonics, Bremen, Germany). Autolysis peaks of trypsin were used as internal calibrates for MS calibration. Using BioTools 2.2 (Bruker Daltonics, Bremen, Germany), the obtained peptide fingerprints were aligned against the Swiss-Prot/TREMBL and NCBInr databases with parameters as described previously (Strocchi et al. 2006).
The Gene Ontology (GO) cluster analysis of differentially expressed proteins
GO analysis is widely applied to demonstrate biological process, cellular component and molecular function of gene coding proteins (Tweedie et al. 2009). The interested proteins were analyzed by GO cluster through the GoMiner Web site (http://discover.nci.nih.gov/gominer/index.jsp).
Statistical analysis
SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) was performed for all statistical analyses. Student’s t test was used to select differentially expressed proteins between the treatment group and control group. Proteins with p value <0.05 or quantitative intensity ratio ≥1.5 were considered as differentially expressed ones.
Results
Optimum temperature for M6-22 growth
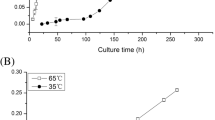
In order to identify the temperature range and optimum temperature, M. isabellina M6-22 were separately cultured for 48 h at different temperatures (5, 10, 15, 20, 25, 30 and 35 °C). According to the temperature effect on M6-22 biomass (Table 1), M6-22 could grow well at temperatures ranging from 15 to 30 °C with an optimum temperature 30 °C. When the temperature reached to 35 °C, M6-22 exhibited retarded growth.
Influence of low temperature on compositions of fatty acids in M6-22
Cellular fatty acids were extracted from M6-22 cultured at 15 or 30 °C, and then analyzed by GC–MS. Results showed that LA and GLA significantly increased from 11.63 to 17.85 % and from 9.12 to 13.19 %, respectively, when the growth temperature decreased from 30 to 15 °C. On the contrary, the content of oleic acid (OA) significantly decreased from 47.25 to 36.53 % (Fig. 1; Table 2).
2-DE analysis
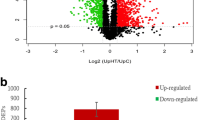
Figure 2 shows the 2-DE maps of M6-22 in the treatment group. Approximately 1800 gel spots were detected and 111 displayed altered abundance. Totally 63 gel spots were found to be unique or different in protein level by at least twofold, and these spots were regarded as interested proteins and further identified by MALDI-TOF/TOF–MS.
Identification of differentially expressed proteins
Totally 63 differentially expressed protein spots were selected for identification by mass spectrometry. Finally, 44 spots were successfully identified based on alignment against Swiss-Prot/TREMBL and NCBInr databases, including 5 unique proteins (A38, A40, A47, A49 and A58), 29 up-regulated proteins and 10 down-regulated proteins. These identified 44 protein spots contained three pairs of same proteins, including transitional endoplasmic reticulum ATPase–transitional endoplasmic reticulum ATPase, A7–A10; tryptophanyl-tRNAsynthetase–tryptophanyl-tRNAsynthetase, A18–A20; and heat-shock 70-kDa protein 2–Hsp70 protein 2, A46-B3, and 11 hypothetical proteins (Table 3). These proteins were involved in various functions, including transmembrane signal transduction, protein folding (heat-shock 70-kDa protein 2, A46; hsp71-like protein, A48; heat-shock 70-kDa protein 3, A49; and Hsp70 protein 2, B3), ATP synthesis, amino acid synthesis (carbamoyl phosphate synthetase, A5; 3-isopropylmalate dehydratase large subunit, A14; phosphoserine aminotransferase, A16), transcription regulation (E3 ubiquitin-protein ligase BRE1, A12; and histone acetyltransferase GCN5, A32) and protein synthesis (tryptophanyl-tRNAsynthetase, A18; tryptophanyl-tRNAsynthetase, A20; and glycyl-tRNAsynthetase, A21).
The GO cluster analysis of differentially expressed proteins
To investigate the potential functions of the 44 successfully identified proteins, GO enrichment analysis was performed for annotation and classification based on cellular component, molecular function and biological process, respectively (Fig. 3). The results showed that these differentially expressed proteins were involved in metabolism, transcription, translation, cell structure and response. In detail, functions in biological process mainly included nitrogen compound metabolic process, biosynthetic process and localization. Functions in molecular function mainly included nucleotide binding, ion binding, oxidoreductase activity and transferase activity. The differentially expressed proteins were mainly located in cytoplasm, membrane, organelle, cytoskeletal and nucleus.
Discussion
In this study, the 2-DE results of mycelium protein extracts from M6-22 revealed clear differences in protein compositions between strains cultured at 30 and 15 °C. Further MALDI-TOF/TOF–MS analysis demonstrated that 44 proteins were significantly altered in low-temperature-treated strains. GO enrichment analysis revealed that most of the differentially expressed proteins were involved in metabolism, transcription, translation, cell structure and response. Besides, fatty acids analysis indicated that temperature decreasing contributed to the conversion of OA to LA and GLA.
Under low temperature, the need of mycelium for ATP will significantly increase. To compensate for an overall lower energetic capacity, the decreased enzyme-catalyzed reaction rates due to cold temperatures can be offset by inducing some glycolytic enzymes. For instance, it has been reported that fructose-bisphosphate aldolase is a cold-induced protein in Lactococcus piscium (Garnier et al. 2010). In antarctic fungi, UDP-N-acetyl-hexosamine pyrophosphorylase can enhance glycolysis and ATP production (Kostadinova et al. 2011). As located in the inner mitochondrial membrane, cytochrome c oxidase acts as the terminal complex of the electron transport chain and is a key enzyme in cell respiration (Chen and Pervaiz 2010). The alpha and epsilon chains of ATP synthase and ATPase α-subunit, β-subunit, γ-subunit, δ-subunit are overexpressed under cold stress (Cui et al. 2005). In our study, the differentially expressed proteins of M. isabellina M6-22 cultured under lower temperature at 15 °C were predicted to involve in glycolytic pathway (e.g., A34; fructose-bisphosphate aldolase, A50), electron transport (e.g., cytochrome c oxidase polypeptide 5B, A28) and ATP production (e.g., ATP synthase subunit beta, A35; ATP synthase d subunit, B39). It is therefore speculated that these proteins, especially ATP synthase subunits, may play important roles to maintain the proton concentration in cold adaption via glycolytic pathway, electron transport and ATP production.
As a cold-adaptive proteins, E3 ubiquitin-protein ligase BRE1 can ubiquitylate the histone H2B to H2BK123ub1 which is a flag of epigenetic transcriptional activation, and degrade the misfolded proteins dependent on the cytosolic ubiquitin–proteasome pathway (Imai et al. 2000; Wood et al. 2003). In Cryptococcus neoformans, histone acetyltransferase GCN5 acts as a transcriptional adaptor for many transcription factors by directly contacting DNA-bound activators, and histone acetyltransferase complexes are recruited to modify local chromatin structure for regulating transcription of specific genes (O’Meara et al. 2010). In this study, A12 (E3 ubiquitin-protein ligase BRE1) and A32 (histone acetyltransferase GCN5) were found to be differentially expressed in M. isabellina M6-22 under cold stress, which declared that the A12 and A32 may affect cold adaptation through regulating transcription of genes.
Many researches have demonstrated that protein degradation plays an important role in cold adaptation. The transitional endoplasmic reticulum ATPase (TER ATPase) is an essential component of 26S proteasome which is built from approximately 31 different subunits and acts as a molecular machine for catalyzing protein degradation (Voges et al. 1999). In the present study, TER ATPases (A7 and A10) of M. isabellina M6-22 were found to be dysregulated at 15 °C, indicating that they may promote degradation of needless proteins under low temperature for survival. Among molecular chaperones, HSPs are ubiquitous and could cope with stress-induced denaturation with other proteins (Lee and Vierling 2000; Kregel 2002). It is known that HSPs are induced not only by heat but also by low temperatures (Beere and Green 2001; Kosová et al. 2013). In our study, most HSPs (A46, A48 and A49) were up-regulated under 15 °C, suggesting that HSPs could help key proteins to overcome cold condition.
For many psychrophiles, increasing the percentage of unsaturated fatty acids in membrane lipid can maintain an optimal membrane fluidity and is a well-known strategy of cold adaptation (Gentile et al. 2003). In one of our previous studies, the percentage of PUFAs, including LA and ALA, were found to be obviously increased in membrane of Rhodotorula glutinis YM25079 with the culture temperature decreasing from 25 to 15 °C (He et al. 2015). Previous study demonstrates that PUFAs in membrane phospholipids of deep-sea and cold-adapted bacteria, such as Shewanella olleyana sp. nov., are increased (Skerratt et al. 2002). Moreover, changes in membrane lipid unsaturation have correlation with cold adaptation in Methanococcoides burtonii (Nichols et al. 2004). In the present study, LA and GLA, respectively, increased from 11.63 to 17.85 % and from 9.12 to 13.19 % as temperature decreased from 30 to 15 °C, indicating the accumulation of PUFAs during cold address.
Conclusions
Using proteomics approaches, totally 111 protein spots were detected to be differentially expressed in M. isabellina M6-22 cultured at 15 °C, among which 44 were successfully identified. This set of proteins may stand a good chance of being key factors for cold adaption in M. isabellina M6-22 through regulation of ATP synthesis, activation of cold-adaptive proteins, degradation of needless protein, accumulation of PUFAs, etc. Future studies are needed to verify the roles of these proteins in combating low temperature and identify the complete cold-adapted proteome of M. isabellina.
References
Aki T et al (2001) Production of arachidonic acid by filamentous fungus, Mortierella alliacea strain YN-15. J Am Oil Chem Soc 78:599–604
Bakermans C, Tollaksen SL, Giometti CS, Wilkerson C, Tiedje JM, Thomashow MF (2007) Proteomic analysis of Psychrobacter cryohalolentis K5 during growth at subzero temperatures. Extremophiles 11:343–354
Beere HM, Green DR (2001) Stress management–heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol 11:6–10
Calder PC (2008) Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res 52:885–897
Chatzifragkou A, Fakas S, Galiotou-Panayotou M, Komaitis M, Aggelis G, Papanikolaou S (2010) Commercial sugars as substrates for lipid accumulation in Cunninghamella echinulata and Mortierella isabellina fungi. Eur J Lipid Sci Technol 112:1048–1057
Chen Z, Pervaiz S (2010) Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell Death Differ 17:408–420
Cui S, Huang F, Wang J, Ma X, Cheng Y, Liu J (2005) A proteomic analysis of cold stress responses in rice seedlings. Proteomics 5:3162–3172
Economou CN, Aggelis G, Pavlou S, Vayenas D (2011) Single cell oil production from rice hulls hydrolysate. Bioresour Technol 102:9737–9742
Garnier M, Matamoros S, Chevret D, Pilet M-F, Leroi F, Tresse O (2010) Adaptation to cold and proteomic responses of the psychrotrophic biopreservative Lactococcus piscium strain CNCM I-4031. Appl Environ Microbiol 76:8011–8018
Gentile G, Bonasera V, Amico C, Giuliano L, Yakimov M (2003) Shewanella sp. GA-22, a psychrophilic hydrocarbonoclastic antarctic bacterium producing polyunsaturated fatty acids. J Appl Microbiol 95:1124–1133
He J et al (2015) Correlation of polyunsaturated fatty acids with the cold adaptation of Rhodotorula glutinis. Yeast 32:683–690
Imai Y, Soda M, Takahashi R (2000) Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem 275:35661–35664
Isaacson T et al (2006) Sample extraction techniques for enhanced proteomic analysis of plant tissues. Nat Protoc 1:769–774
Kang Y, Xian M, Wang J, Cheng T, Li W, Bi W (2002) Effects of mycelial biomembrane fluidity on the activity of desaturases. J Mol Catal 16:1–4
Kosová K, Vítámvás P, Sb Planchon, Renaut J, Vanková R, Prášil IT (2013) Proteome analysis of cold response in spring and winter wheat (Triticum aestivum) crowns reveals similarities in stress adaptation and differences in regulatory processes between the growth habits. J Proteome Res 12:4830–4845
Kostadinova N, Vassilev S, Spasova B, Angelova M (2011) Cold stress in antarctic fungi targets enzymes of the glycolytic pathway and tricarboxylic acid cycle. Biotechnol Biotechnol Equip 25:50–57
Kregel KC (2002) Invited review: heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92:2177–2186
Lee GJ, Vierling E (2000) A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol 122:189–198
Lu H, Zhang X, Li J, T-g LEI (2005) Effects of adversity on fatty acid composition in fungal membrane. J Microbiol 25:1–3
Mamatha S, Ravi R, Venkateswaran G (2008) Medium optimization of gamma linolenic acid production in Mucor rouxii CFR-G15 using RSM. Food Bioprocess Technol 1:405–409
Nichols DS, Miller MR, Davies NW, Goodchild A, Raftery M, Cavicchioli R (2004) Cold adaptation in the Antarctic archaeon Methanococcoides burtonii involves membrane lipid unsaturation. J Bacteriol 186:8508–8515
O’Meara TR, Hay C, Price MS, Giles S, Alspaugh JA (2010) Cryptococcus neoformans histone acetyltransferase Gcn5 regulates fungal adaptation to the host. Eukaryot Cell 9:1193–1202
Shivaji S, Prakash JS (2010) How do bacteria sense and respond to low temperature? Arch Microbiol 192:85–95
Skerratt JH, Bowman JP, Nichols PD (2002) Shewanella olleyana sp. nov., a marine species isolated from a temperate estuary which produces high levels of polyunsaturated fatty acids. Int J Syst Evolut Microbiol 52:2101–2106
Strocchi M, Ferrer M, Timmis KN, Golyshin PN (2006) Low temperature-induced systems failure in Escherichia coli: insights from rescue by cold-adapted chaperones. Proteomics 6:193–206
Tesei D, Marzban G, Zakharova K, Isola D, Selbmann L, Sterflinger K (2012) Alteration of protein patterns in black rock inhabiting fungi as a response to different temperatures. Fungal Biol 116:932–940
Tweedie S et al (2009) FlyBase: enhancing Drosophila gene ontology annotations. Nucleic Acids Res 37:D555–D559
Vicente G et al (2009) Biodiesel production from biomass of an oleaginous fungus. Biochem Eng J 48:22–27
Voges D, Zwickl P, Baumeister W (1999) The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem 68:1015–1068
Watkins S, German J, Akoh C, Min D (2002) Unsaturated fatty acids. In: Akoh CC, Min DB (eds) Food Lipids Chem Nutr Biotechnol, Marcel Dekker, New York, pp 559–588
Weinstein RN, Montiel PO, Johnstone K (2000) Influence of growth temperature on lipid and soluble carbohydrate synthesis by fungi isolated from fellfield soil in the maritime Antarctic. Mycologia 92:222–229
Wood A et al (2003) Bre1, an E3 ubiquitin ligase required for recruitment and substrate selection of Rad6 at a promoter. Mol Cell 11:267–274
Yang Z, Li L, Hu B, Lin L, Wei Y, Ji X (2014) Correlation of polyunsaturated fatty acids and cold adaptation of Rhodotorula glutinis. Chin J Appl Environ Biol 20:233–237
Zhang Y-M, Rock CO (2008) Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 6:222–233
Acknowledgments
This study was supported by a grant from National Natural Science Foundation of China (Nos. 31160016 and 31260034). (1) National Natural Science Foundation of China (31160016): “Effects of biosynthesis inhibition of polyunsaturated fatty acids on the cold adaptation of M. isabellina M6-22 to low temperature.” (2) National Natural Science Foundation of China (31260034): “Studies on the temporal regulation of viral transcription during the development of Sulfolobus virus STSV2.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interests to state.
Additional information
Communicated by Erko Stackebrandt.
Binbin Hu and Minzhou Luo should be regarded as co-first authors.
Rights and permissions
About this article
Cite this article
Hu, B., Luo, M., Ji, X. et al. Proteomic analysis of Mortierella isabellina M6-22 during cold stress. Arch Microbiol 198, 869–876 (2016). https://doi.org/10.1007/s00203-016-1238-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-016-1238-0