Abstract
Phytoremediation is an in situ, low-cost strategy for cleanup of the sites contaminated with heavy metals. Experiments were conducted to assess the impact of synthetic chelators and plant growth-promoting rhizosphere bacteria (Herbaspirillum sp. GW103) on heavy metal lead (Pb) uptake in Z. mays cultivated in Pb-contaminated soil. The present study investigated the Pb phytoaccumulation rate and plant antioxidant enzyme activities in Z. mays exposed to 100 mg/kg of PbNO3. The combination of gluconic acid (GA) with Herbaspirillum sp. GW103 treatment showed higher Pb solubility (18.9 mg/kg) compared with other chelators. The chemical chelators showed the significant difference in phytoaccumulation as well as antioxidant enzyme activities. The antioxidant enzymes such as catalase, peroxidase and superoxide dismutase activities changed under Pb stress. The study indicated that increased activity of antioxidant enzymes may play as signal inducers to fight against Pb.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The practice of modern industrialization causes environmental imbalances due to the discharging of pollutants in the ecosystem (Govarthanan et al. 2015a, b). Heavy metals are the primary pollutants, which are normally found in the soil and water ecosystem. Toxicity and pollution is one of the major environmental problems because of its increasing level caused mainly by anthropogenic activities. The continuous discharging of heavy metals into the soil can cause serious problems to the ecosystem. Among the heavy metals, lead is relatively mobile in the soils and is one of the most toxic metals. Several studies and reviews reported the Pb toxicity and its high environmental persistence (Govarthanan et al. 2013).
Bioremediation of heavy metals which has been emerging is an innovative technology for cleaning up contaminated soil and maintaining the pollution free environment. Several studies have reported the physicochemical methods of remediation and its disadvantages (Yang et al. 2008; Ok et al. 2011). Bioremediation, particularly phytoremediation using plants, is an eco-friendly and efficient method for the removal of metals (Shin et al. 2012). Among phytoremediation, phytoextraction of heavy metals from contaminated soils has been receiving great attention. Metal hyper-accumulator plants have been demonstrated to be potentially useful in soil cleanup, as they can take up significant amounts of metals from contaminated soils, but their low annual biomass production tends to limit their phytoextraction ability (Zhuang et al. 2007). The hyper-accumulator plant shoots accumulate the heavy metals and produce considerable amount of biomass (Luo et al. 2006). The hyper-accumulator plants usually accumulate only a specific metal in small amount. Baker et al. (2000) reported on the Pb hyper-accumulator plants and their Pb uptake abilities. With advances in stress biology, some of the high biomass crops such as Z. mays, Pisum sativum, Avena sativa and Brassica juncea plants were used as the promising alternative to hyper-accumulating plants such as Viola baoshanensis, Sedum alfredii and Typha latifolia can accumulate heavy metals in their tissues (Zhuang et al. 2007; Chandra and Yadav 2011).
Synthetic chemical chelators are the most common amendments used in chemical-assisted phytoextraction of heavy metals from contaminated soils. The chemical chelators are capable of forming chemical complexes with metal ions, thereby modifying the bioavailability of heavy metals in soils (Wu et al. 2004; Quartacci et al. 2006; Liu et al. 2008). The application of chelators has been shown to enhance the phytoextraction of heavy metals from contaminated soil. Synthetic chelators such as EDTA, EDDS (ethylenediamine-N-disuccinic acid) and organic acids have been used extensively in Pb remediation and to enhance micronutrient supply to plants (Evangelou et al. 2007; Meers et al. 2009). Several studies have reported the synthetic chelators inducing metal accumulation in plants (Lombi et al. 2001; Kos and Lestan 2004; Wu et al. 2004; Liu et al. 2008). However, there are no reported studies on combination of synthetic chelators- with microorganisms-assisted phytoextraction of heavy metals in soils.
Recently, the plant growth-promoting bacteria associated with heavy metal hyper-accumulators have attracted attention due to their potential applications for assisting phytoaccumulation of metal-contaminated soil. The plant growth-promoting activity of the strain was reported. The most recent mechanism for enhancing phytoextraction is inoculating the soil with bacteria producing siderophores, which assist in chelating metals, suggesting that microorganisms may play a major role in successful phytoextraction.
Production of reactive oxygen species (ROS) is increased by stress conditions. The ROS are highly cytotoxic and can disrupt cell metabolism via oxidative damage to cellular components (Halliwell 1982). Plants have evolved protective mechanisms to reduce the risk of ROS, which are effective at different levels of stress-induced deterioration (Salin 1987; Foyer et al. 1994). Among the ROS protective mechanisms of plants, antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD) and catalases (CAT) have received much attention against ROS. Thus, these antioxidants play an important role in the cellular defense strategy of plants against oxidative stress, inducing resistance for metals by protecting labile macromolecules (Chandra and Yadav 2010). In the present study, we investigated on the (1) role of synthetic chelators with plant growth-promoting Herbaspirillum sp. GW103 on phytoextraction of Pb by Z. mays in artificially contaminated soil and (2) antioxidant enzymes activities in Z. mays exposed to Pb stress.
Materials and methods
Bacterial strain
The heavy metal-resistant, plant growth-promoting bacterium Herbaspirillum sp. GW103 (Lee et al. 2012) was kindly provided by Prof. Kui-Jae Lee, Chonbuk National University, Korea. Our previous study identified the Pb resistance of GW103 (Govarthanan et al. 2014). Thus, the strain was used for the assessment of Pb removal with synthetic chelators. All chemicals used in the study were of analytical grade.
Soil collection and characterization
Soil sample was collected from a trial agricultural field of Chonbuk National University, Iksan, Republic of Korea, in the spring season (April 2014). The samples were sieved through a 2-mm sieve and autoclaved for 20 min at 121 °C under 105 Pa of pressure and allowed to stabilize at room temperature. The soil was artificially contaminated with 100 mg/kg of Pb.
Effects of application of synthetic chelators
Seeds of Z. mays L. were surface sterilized with 70 % ethanol and washed with sterile water. After surface sterilization, the seeds were placed in Petri dishes filled with sterile distilled water and were allowed to germinate at room temperature for 3–4 days. The chelators ethylene diamine tetra acetic acid (EDTA), gluconic acid (GA), malic acid (MA), boric acid (BA) and oxalic acid (OA) were applied on the soil surface of the pots with five different concentrations (20, 40, 60, 80 and 100 mM). Each chelator was used same concentration. All experiments were carried out in growth chamber at 23 °C. The roots and shoots of the plants were washed with tap water and rinsed with distilled water, and separated into two equal volumes. One group was dried at 60–70 °C followed by HNO3 digestion. The digested plant samples were used to analyze Pb accumulation using inductively coupled plasma mass spectrometry (ICP) (150-00191-1, Rev. A, Leemans Labs, USA), after appropriate dilution. The ICP measurement conditions were as follows: nebulizer gas flow rate: 50 psi; auxiliary gas flow: 16 lpm; plasma gas flow: 16 lpm; ICP RF power: 1.4 kW. Another group was used to study the antioxidant enzymes. A control group without chelate treatment was used in the experiment. Each treatment was replicated three times. The higher Pb accumulation and antioxidant enzyme levels of each concentration of chelator were determined (data not shown), and the favorable concentration was used for further combination studies with plant growth-promoting Herbaspirillum sp. GW103.
Growth studies of the Herbaspirillum sp. GW103 with synthetic chelators
Log phase culture (5 ml) of the strain GW103 was aseptically inoculated in 100 ml of M9 minimal media (g/l) composed of Na2HPO4, 64 g; KH2PO4, 15 g; NaCl, 5 g; NH4Cl, 10 g; 2 ml of 1 M MgCl2; 2 ml of 20 % fructose; and 0.1 ml of 1 M CaCl2) supplemented with specific concentration of EDTA (60 mM), MA (60 mM), GA (40 mM), BA (60 mM) and OA (80 mM). The flasks were incubated in a shaking incubator (180 rpm) at 25 ± 2 °C, and the growth was measured at the prescribed time intervals (72 h) in terms of increase in optical density at 600 nm using a UV–Vis spectrophotometer (UV-1800, Shimadzu, Japan). Cultures grown in the absence of chelators were used as a control.
Effects of synthetic chelators combined with Herbaspirillum sp. GW103
In this experiment, the combined application of synthetic chelators and plant growth-promoting Herbaspirillum sp. GW103 was studied. Z. mays seedlings were grown for 14 days. The chelates and Herbaspirillum sp. GW103 was applied to the soil (250 g) surface of the pots (diameter 11 cm) with different concentrations (EDTA—60 mM; MA—60 mM; GA—40 mM; BA—60 mM; OA—80 mM with constant of 5 ml of bacterial culture). A control group without bacterial treatment was used in the experiment. Each treatment was replicated three times. All experiments were carried out in growth chamber at 23 °C. Plants were harvested after 2 weeks following the application of the chelates. The roots and shoots of the plants were washed with tap water and rinsed with distilled water, and separated into two equal volumes. One set was dried at 60–70 °C followed by HNO3 digestion. The digested plant samples were used to analyze Pb accumulation using ICP.
Antioxidant enzymes activity analysis
The sample preparation and analysis of antioxidant enzyme activity of the plant samples were done according to Singh et al. (2009) with minor modifications. Briefly, 2 g of plant material was ground with 10 ml of phosphate buffer (pH 7.0) containing 0.1 mM EDTA and 1 % polyvinylpyrrolidone. The homogenate was centrifuged at 15,000 rpm for 15 min at 4 °C, and the supernatant was collected for the determination of SOD, POD, CAT activities.
Superoxide dismutase (SOD) activity
SOD activity of the plant sample was estimated according to the method specified in Dhindsa et al. (1981). The complete reaction mixture contained 1.5 ml of 0.1 M potassium phosphate buffer (pH 7.0), 0.2 ml of 200 mM methionine, 0.1 ml of nitro-blue tetrazolium chloride (NBT), 0.1 ml of EDTA, 0.1 ml of 1.5 M sodium carbonate, 0.1 ml of enzyme solution and 0.8 ml of distilled water. The reaction is based on the formation of blue-colored formazone by nitro-blue tetrazolium chloride and O ¯2 radical. The absorbance was read at 560 nm using a UV–Vis spectrophotometer (UV-1800, Shimadzu, Japan). Tubes containing reaction mixture in the absence of enzyme extract were used as control. One unit of enzyme activity was defined as the quantity of SOD required to produce a 50 % inhibition of reduction of NBT.
Peroxidase (POX) activity
POX of the plant extract was estimated based on the method described by Castillo et al. (1984). The 0.1 ml of reaction solution was mixed with 1 ml of 100 mM phosphate buffer, 0.5 ml of 96 mM guaiacol, 0.5 ml of H2O2, 0.4 ml of double-distilled water. Addition of H2O2 converted guaiacol into the tetraguaiacol due to the oxidation reaction. The changes in the absorbance were determined at 470 nm. One unit of POX activity was determined as an absorbance change of 0.001 U/min.
Catalase (CAT) activity
Plant catalase was determined according to the method described by Piero et al. (1980) with minor modifications. Briefly, the enzyme mixture was mixed with 10 ml of 30 % H2O2. The reaction was initiated by adding H2O2 into the enzyme mixture. The resultant product absorbance was analyzed by UV spectrophotometer at 240 nm. The catalase activity was defined as amount of enzyme catalyzing the decomposition of 1 mmol of H2O2/min.
Results and discussion
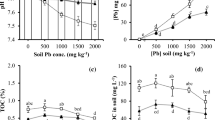
The physical and chemical parameters of the soil are given in Table 1. The pH value of the soil represents the neutral condition, and the soil contains 10.2 % of organic carbon. The minimal inhibitory concentration of Pb of the isolate GW103 was reported by Govarthanan et al. (2014). In order to study the synergistic effects of chelators and Herbaspirillum sp. GW103 on solubility of Pb was investigated. Accordingly, dose-dependent extraction studies were conducted. In the chelator concentration-dependent experiment with GW103, Pb accumulation was observed after treatment with different chelators. EDTA, MA, GA, BA, OA combined with GW103 treatments showed Pb accumulation in the plant as presented in Fig. 1. Compared with the control group, the application of EDTA, MA, GA, BA and OA to the soil significantly increased the concentrations of Pb accumulation in Z. mays L. The combination of GA with GW103 treatment showed higher Pb solubility (18.9 mg/kg) compared with other chelators. Saravanan et al. (2007) reported that the GA and its derivatives effectively solubilize the metals by endophyte G. diazotrophicus.
The application of EDTA, MA and BA with GW103 showed significant effects on Pb solubilization. In chemical-mediated phytoextraction process, the increased uptake of Pb induced by the application of EDTA can be explained by the effect of improved solubility of Pb, and the uptake of the Pb–EDTA complex by plants (Huang et al. 1997; Vassil et al. 1998; Epstein et al. 1999; Shen et al. 2002). It has been established that there is a specific threshold concentration of EDTA, which is required to induce the accumulation of metals in plant shoots (Vassil et al. 1998). Romkens et al. (2002) reported that the uptake and accumulation of metals by plants can be enhanced by addition of chemical chelators including EDTA. Ramachandran et al. (2006) reported that among the organic acids, gluconic acid, oxalic acid and citric acid have received much attention as potential chelators to improve the bioavailability of heavy metals in the soil (Sauer et al. 2008). Kos and Lestan (2004) reported that the addition of different concentration of organic acids increased soil respiration, presumably due to the microbial use of citric acid as an additional carbon and energy source. The efficiency of combination of microbe- and synthetic chelator-assisted phytoextraction is dependent on the survival of the inoculants in the presence of chelators. Thus, the present study showed the survival of the isolate GW103 with the addition of different concentration of chelating agents (Fig. 2). The growth studies clearly indicate the potential of GW103 to tolerate and survive in the presence of chelators.
Generally in plant system, the ROS are inevitable by-products of plant cell aerobic metabolism. Under the normal growth conditions, favorable growth environment causes amounts of ROS to be modest while cells experience only mild oxidative stress, which enhance ROS production (Inze and Van-Montagu 1995; Mittler et al. 2004). In particular, the heavy metal stress is of special interest to enhance the ROS production in the plant system. Heavy metals present in the environment generally cause damages to the plants and microbes either direct and/or indirect manner via reactive oxygen species formation (Mittler et al. 2004; Apel and Hirt 2004; Nehnevajova et al. 2012). The activities of enzymes scavenging ROS such as SOD, CAT, POX were measured in the plants. The results show differences in enzyme activities between the treatments, as well as between plants grown on a control and Pb-contaminated soil. The SOD activity was enhanced by EDTA amendment and inoculation with GW103. The highest level of SOD activity (55 ± 2.0 U/mg) was found in EDTA amended with GW103 plants. SOD is one of the ubiquitous enzymes, which play a key role in cellular defense mechanism against ROS. A significant increased in SOD activity was observed in all treatments compared to control (Table 2). The order of SOD activity was found to be EDTA + GW > BA + GW103 > MA + GW103 > OA + GW103 > GA + GW103 > control. The CAT activity was also found to be higher in all treatments compared to control. Maximum activity of CAT (44.3 U/mg) was observed in EDTA + GW treated plants. The order of CAT activity was found to be EDTA + GW > OA + GW103 > BA + GW103 > MA + GW103 > GA + GW103 > control whereas, the POD activity was found to be EDTA + GW > BA + GW103 > OA + GW103 > MA + GW103 > GA + GW103 > control. Gallego et al. (1999) reported that an increased antioxidant enzymes activity was observed under the heavy metal cadmium stress.
The antioxidant enzymes were significantly increased in the plants amended with EDTA + GW. SOD catalyzes disproportion of superoxide anion (O2 −) to H2O2. CAT and POD further scavenge H2O2 and convert to O2 + H2O and some other phenolic compounds. The variations of the antioxidant enzymes are due to the application of various amendments in the presence of GW103. The metal stress condition increase the formation of reactive oxygen species that damage plants by oxidizing photosynthetic pigments and nucleic acids (Mittler et al. 2004; Nehnevajova et al. 2012).
Conclusion
The heavy metal-resistant Herbaspirillum sp. GW103 with chelators shows a promising alternative to enhance the metal extraction ability in Z. mays. The extraction ability of Z. mays to remove Pb was considerable, due to its higher biomass. Addition of GA and EDTA enhanced the accumulation of Pb in Z. mays. The SOD activity was enhanced by EDTA amendment and inoculation with GW103. The highest level of SOD activity (55 ± 2.0 U/mg) was found in EDTA amended with GW103 plants. The activities of antioxidant enzymes (SOD, POD and CAT) were significantly increased in all treatments compared to control. The application of GA and EDTA with GW103 to contaminated soils greatly enhanced the uptake of Pb by Z. mays.
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Baker AJM, McGrath SP, Reeves RD, Smith JAC (2000) Metal hyperaccumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In: Terry N, Bañuelos G (eds) Phytoremediation of contaminated soil and water. CRC Press LLC, USA, pp 85–107
Castillo FI, Penel I, Reppin H (1984) Peroxidase release induced by ozone in sedum album leaves. Plant Physiol 74: 846–851
Chandra R, Yadav S (2010) Potential of Typha angustifolia for phytoremediation of heavy metals from aqueous solution of phenol and melanodin. Ecol Eng 36:1277–1284
Chandra R, Yadav S (2011) Phytoremediation of Cd, Cr, Cu, Mn, Fe, Ni, Pb and Zn from aqueous solution using Phragmites cummunis, Typha angustifolia and Cyperus esculentus. Int J Phytorem 13:580–591
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide disniutase and catalase. J Exp Bot 32:93–101
Epstein AL, Gussman CD, Blaylock MJ, Yermiyahu U, Huang JW, Kapulnik Y, Orser CS (1999) EDTA and Pb–EDTA accumulation in Brassica juncea grown in Pb-amended soil. Plant Soil 208:87–94
Evangelou MWH, Ebel M, Schaeffer A (2007) Chelate assisted phytoextraction of heavy metals from soil effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 68:989–1003
Foyer CH, Lelandais M, Kunert KJ (1994) Photooxidative stress in plants. Physiol Plant 92:696–717
Gallego SM, Benavides MP, Tomaro ML (1999) Effect of cadmium ions on antioxidant defence system in sunflower cotyledons. Biol Plant 42:4955
Govarthanan M, Lee KJ, Cho M, Kim JS, Kamala-Kannan S, Oh B-T (2013) Significance of autochthonous Bacillus sp. KK1 on biomineralization of lead in mine tailings. Chemosphere 90:2267–2272
Govarthanan M, Lee GW, Park JH, Kim JS, Lim SS, Seo SK, Cho M, Myung H, Kamala-Kannan S, Oh BT (2014) Bioleaching characteristics, influencing factors of Cu solubilization and survival of Herbaspirillum sp. GW103 in Cu contaminated mine soil. Chemosphere 109:42–48
Govarthanan M, Park SH, Park YJ, Myung H, Krishnamurthy RR, Lee SH, Lovanh N, Kamala-Kannan S, Oh BT (2015a) Lead biotransformation potential of allochthonous Bacillus sp SKK11 with sesame oil cake extract in mine soil. RSC Adv 5:54564
Govarthanan M, Shim J, Kim SA, Kamala-Kannan S, Oh BT (2015b) (b). Isolation and characterization of multi-metal resistant Halomonas sp. MG from Tamil Nadu magnesite ore soil in India. Curr Microbiol 71:618–623
Halliwell B (1982) The toxic effects of oxygen on plant tissues. In: Oberley LW (ed) Superoxide Dismutase, vol I. CRC Press, Boca Raton, pp 89–123
Huang JW, Chen J, Berti WR, Cunningham SD (1997) Phytoremediation of lead contaminated soils: role of synthetic chelates in lead phytoextraction. Environ Sci Technol 3:800–805
Inze D, Van-Montagu M (1995) Oxidative stress in plants. Curr Opin Biotechnol 6:153–158
Kos B, Lestan D (2004) Chelator induced phytoextraction and in situ soil washing of Cu. Environ Pollut 132:333–339
Lee GW, Lee KJ, Chae JC (2012) Genome sequence of Herbaspirillum sp. strain GW103, a plant growth promoting bacterium. J Bacteriol 194:4150
Liu D, Islam E, Li T, Yang X, Jin X, Mahmood Q (2008) Comparison of synthetic chelators and low molecular weight organic acids in enhancing phytoextraction of heavy metals by two ecotypes of Sedum alfredii Hance. J Hazard Mater 153:114–122
Lombi E, Zhao FJ, Dunham SJ, McGrath SP (2001) Phytoremediation of heavy metal-contaminated soils: natural hyperaccumulation versus chemically enhanced phytoextraction. J Environ Qual 30:1919–1926
Luo CL, Shen ZG, Li XD, Baker AJM (2006) Enhanced phytoextraction of Pb and other metals from artificially contaminated soils through the combined application of EDTA and EDDS. Chemosphere 63:1773–1784
Meers SE, Qadir M, de Caritat P, Tack FMG, Du Laing G, Zia MH (2009) EDTA-assisted Pb phytoextraction. Chemosphere 74(2009):1279–1291
Mittler R, Vanderauwera S, Gollery M, Van-Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Nehnevajova E, Lyubenova L, Herzig R, Schroder P, Schwitzguebel JP, Schmulling T (2012) Metal accumulation and response of antioxidant enzymes in seedlings and adult sunflower mutants with improved metal removal traits on a metal-contaminated soil. Environ Exp Bot 76:39–48
Ok YS, Kim SC, Kim DK, Skousen JG, Lee JS, Cheong YW, Kim SJ, Yang JE (2011) Ameliorants to immobilize Cd in rice paddy soils contaminated by abandoned metal mines in Korea. Environ Geochem Health 33:23–30
Piero F, Lorenzo P, Giovanni B (1980) Use of 3, 5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem 26:227–231
Quartacci MF, Argilla A, Baker AJM, Navari-Izzo F (2006) Phytoextraction of metals from a multiply contaminated soil by Indian mustard. Chemosphere 63:918–925
Ramachandran S, Fontanille P, Pandey A, Larroche C (2006) Gluconic acid: properties, applications and microbial production. Food Technol Biotechnol 44:185–195
Romkens P, Bouwman L, Japenga J, Draaisma C (2002) Potentials and drawbacks of chelate-enhanced phytoremediation of soils. Environ Pollut 116:109–121
Salin ML (1987) Toxic oxygen species and protective systems of the chloroplast. Physiol Plant 72:681–689
Saravanan VS, Kalaiarasan P, Madhaiyan M, Thangarju M (2007) Solubilization of insoluble zinc compounds by Gluconacetobacter diazotrophicus and the detrimental action of zinc ion (Zn2+) and zinc chelates on root knot nematode Meloidogyne incognita. Lett Appl Microbiol 44:235–241
Sauer M, Porro D, Mattanovich D, Branduardi P (2008) Microbial production of organic acids: expanding the markets. Trends Biotechnol 26:100–108
Shen ZG, Li XD, Wang CC, Chen HM, Chua H (2002) Lead phytoextraction from contaminated soil with high biomass plant species. J Environ Qual 31:1893–1900
Shin MN, Shim J, You Y, Myung H, Bang KS, Cho M, Kamala-Kannan S, Oh BT (2012) Characterization of lead resistant endophytic Bacillus sp. MN3-4 and its potential for promoting lead accumulation in metal hyperaccumulator Alnus firma. J Hazard Mater 199–200:314–320
Vassil AD, Kapulnik Y, Raskin I, Salt DE (1998) The role of EDTA in lead transport and accumulation in Indian mustard. Plant Physiol 117:447–544
Wu LH, Luo YM, Xing XR, Christie P (2004) EDTA-enhanced phytoremediation of heavy metal contaminated soil with Indian mustard and associated potential leaching risk. Agric Ecosyst Environ 102:307–318
Yang JE, Ok YS, Kim WI, Lee JS (2008) Heavy metal pollution, risk assessment and remediation in paddy soil environment: research and experiences in Korea. In: Sanchez ML (ed) Cause and effects of heavy metal pollution. Nova Science Publishers, New York
Zhuang P, Yang QW, Wang HB, Shu WS (2007) Phytoextraction of heavy metals by eight plant species in the field. Water Air Soil Pollut 184:235–242
Acknowledgments
This work was supported by the BK21 plus program through the National Research Foundation (NRF)funded by the Ministry of Education of Korea.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
About this article
Cite this article
Govarthanan, M., Kamala-Kannan, S., Kim, S.A. et al. Synergistic effect of chelators and Herbaspirillum sp. GW103 on lead phytoextraction and its induced oxidative stress in Zea mays . Arch Microbiol 198, 737–742 (2016). https://doi.org/10.1007/s00203-016-1231-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-016-1231-7