Abstract
Sucrose accumulation has been observed in some methylotrophic bacteria utilizing methane, methanol, or methylated amines as a carbon and energy source. In this work, we have investigated the biochemical pathways for sucrose metabolism in the model halotolerant methanotroph Methylomicrobium alcaliphilum 20Z. The genes encoding sucrose-phosphate synthase (Sps), sucrose-phosphate phosphatase (Spp), fructokinase (FruK), and amylosucrase (Ams) were co-transcribed and displayed similar expression levels. Functional Spp and Ams were purified after heterologous expression in Escherichia coli. Recombinant Spp exhibited high affinity for sucrose-6-phosphate and stayed active at very high levels of sucrose (K i = 1.0 ± 0.6 M). The recombinant amylosucrase obeyed the classical Michaelis–Menten kinetics in the reactions of sucrose hydrolysis and transglycosylation. As a result, the complete metabolic network for sucrose biosynthesis and re-utilization in the non-phototrophic organism was reconstructed for the first time. Comparative genomic studies revealed analogous gene clusters in various Proteobacteria, thus indicating that the ability to produce and metabolize sucrose is widespread among prokaryotes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sucrose is one of the most abundant products of photosynthesis. Until recently, sucrose was thought to be accumulated mainly in phototrophic organisms, including plants, unicellular algae, and cyanobacteria (Salerno and Curatti 2003; Klähn and Hagemann 2011). Sucrose synthesis upon the salt stress has been also demonstrated for the marine planctomycete Rhodopirellula baltica (d’Avó et al. 2013). The biochemical pathways for sucrose metabolism have been investigated mostly in higher plants and cyanobacteria where the disaccharide biosynthesis involves the sucrose-phosphate synthase (Sps, UDP glucose: d-fructose-6-phosphate 2-α-d-glucosyltransferase, EC 2.4.1.14) and the sucrose-phosphate phosphatase (Spp, sucrose-6F-phosphohydrolase, E.C. 3.1.3.24) (Bruneau et al. 1991; Page-Sharp et al. 1999; Lunn et al. 2000). The new pathway for sucrose breakdown was described in the cyanobacterium Synechococcus sp. PCC 7002 (Perez-Cenci and Salerno 2014). However, little is known about the enzymes catalyzing the biosynthesis and further metabolism of sucrose in non-phototrophic prokaryotes (Chua et al. 2008).
Our earlier studies have shown that a number of methylotrophic bacteria utilizing methane, methanol, or methylated amines can accumulate sucrose as a primary or secondary solute (Khmelenina et al. 1999; Doronina et al. 2003a, b; But et al. 2013b). Methylomicrobium alcaliphilum strain 20Z, a halotolerant gammaproteobacterial methanotroph, is one of the prominent methanotrophic species detected in saline and alkaline ecosystems (Khmelenina et al. 1997, 2010). The methanotroph synthesizes sucrose in response to the increased salinity of growth media (Khmelenina et al. 1999). A cluster of four genes encoding the putative Sps, Spp, fructokinase (FruK), and amylosucrase (Ams) has been identified in the genome of the strain (But et al. 2012, 2013b). The colocation of the four genes implied an unusual organization of sucrose metabolism in M. alcaliphilum 20Z. It has been shown that the mutation of sps abolished the ability of the methanotroph to accumulate sucrose, thus confirming the key role of Sps in sucrose biosynthesis (But et al. 2013b). The fruK gene product has been characterized as an ATP-dependent fructokinase (But et al. 2012). Here, we have further refined the sucrose metabolic pathway in M. alcaliphilum 20Z via biochemical and genetic studies. Additionally, we have surveyed the distribution of analogous functional modules among different bacterial phyla.
Materials and methods
Bacteria and growth conditions
Methylomicrobium alcaliphilum 20Z (VKM B-2133 = NCIMB 14124) was grown at 30 °C under methane–air atmosphere (1:1) in a mineral medium containing (g/L) KNO3 (2), MgSO4 (0.2), CaCl2 (0.02), Na2-EDTA (0.01), FeSO4·7H2O (0.004), ZnSO4·7H2O (0.0002), MnCl2·4H2O (0.00006), CuCl2·5H2O (0.0002), CoCl2·6H2O (0.0004), NiCl2·6H2O (0.00004), Na2MoO4 (0.00006), H3BO3 (0.0006) with the addition of 0.1 M NaHCO3 and 3 % (w/v) NaCl (Khmelenina et al. 1999). Escherichia coli cells were routinely grown at 37 °C in the Luria–Bertani (LB) medium (Sambrook and Russell 2001). The following antibiotics were added if required such as kanamycin, 50–100 μg/ml; chloramphenicol, 25 μg/ml; and tetracycline, 10 μg/ml.
DNA manipulations
Plasmid isolation and cleavage, agarose gel electrophoresis, ligation, and transformation of E. coli cells were performed according to the standard protocols (Sambrook and Russell 2001). Restriction enzymes, T4 DNA ligase, Taq DNA polymerase, and dNTP mixture were purchased from Thermo scientific (Lithuania).
Expression and purification of Spp and Ams
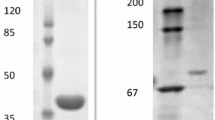
Chromosomal DNA from M. alcaliphilum cells was prepared as described previously (Kalyuzhnaya et al. 1999). The putative spp (MALCv4_0615) and ams (MALCv4_0617) genes were amplified by PCR using primer pairs Spp20zF-Spp20zR and Ams20zF-Ams20zR (Suppl. Table 1). PCR products were purified by the Wizard kit (Promega, USA). The fragments were digested with endonucleases, the NdeI and HindIII sites for the ams gene, and the NcoI and HindIII sites for the spp gene to generate sticky-ends and ligated into the expression vectors pET30(a)+ or pET28b (Novagen, USA), respectively. The resulting constructs were used to transform E. coli Rosetta (DE3) (Novagen, USA). E. coli strains harboring the ams or spp expression systems were grown overnight at 37 °C in 20 mL of the LB medium and then transferred into a fresh LB medium containing 50 μg/ml kanamycin and 25 μg/ml chloramphenicol and cultivated until OD600 0.6–0.7. The cells were transferred to 17 °C, and the protein expression was induced by isopropyl β-d-1-thiogalactopyranoside at a final concentration of 0.5 mM. After overnight incubation at 17 °C, the cells were harvested by centrifugation at 6,000g for 20 min (4 °C). The His6-tagged proteins were purified by affinity chromatography on a Ni2+-NTA agarose column as described earlier (But et al. 2012), and their purity was analyzed by 12 % SDS–PAGE (Laemmli 1970).
Sucrose-phosphate phosphatase activity assay
The activity of sucrose-phosphate phosphatase was measured by determining the concentration of orthophosphate released from sucrose-6P by the following two methods: (1) the reaction mixture containing 50 mM 2-(N-morpholino)ethanesulfonate (MES) buffer, pH 6.5, and 5 mM MgCl2, and 400 μM sucrose-6-phosphate (total volume 200 ml) was incubated at 30 °C and stopped by adding 600 μl of the Bencini reagent (Bencini et al. 1983). This procedure was used to follow the effects of Mg2+, pH, and temperature on the enzyme activity. The effect of pH was tested using the following buffers (50 mM): MES–KOH (pH 5.0–7.0), Tris–HCl (pH 7.5–9.0), and sodium carbonate (pH 9.0–10.0); (2) the kinetics of the enzyme was studied by measuring its activity at 35 °C in 1 ml of the reaction mixture containing 50 mM MES buffer (pH 6.5), 8–400 μM sucrose-6-phosphate, 1 mM fructose-1,6-bisphosphate, 5 mM MgCl2, 0.5 mM NADP+, 0.25 U of phosphoglucoisomerase (PGI), 2 U of glucose-6-phosphate dehydrogenase (GPDH), and 2 U pyrophosphate-dependent 6-phosphofructokinase obtained as a His6-tagged protein from M. alcaliphilum 20Z as described earlier (Rozova et al. 2010). The reduction of NADP+ at 340 nm was monitored with a Shimadzu UV-1700 spectrophotometer (Japan).
Amylosucrase activity assay
The total activity of amylosucrase was measured at 30 °C by determining the velocity of fructose formation with the auxiliary enzymes PGI, GPDH, and recombinant fructokinase (FruK-His6) obtained from M. alcaliphilum 20Z as indicated (But et al. 2012). 1 ml of the standard assay mixture contained 50 mM MES–KOH buffer (pH 7.0), 5 mM ATP, 5 mM MgCl2, 200 mM sucrose, 0.5 mM NADP+, 1 U of FruK-His6, 0.25 U PGI, 2 U GPDH, and 0.1 mg glycogen (Fermentas) if required. The hydrolytic activity of amylosucrase was determined by measuring the rate of glucose formation using the coupling enzymes hexokinase (HK) and GPDH in 1 ml of the reaction mixture containing 50 mM MES–KOH buffer (pH 7.0), 5 mM ATP, 5 mM MgCl2, 200 mM sucrose, 0.5 mM NADP+, 0.1 mg glycogen if required, 1 U HK and 2 U GPDH. The NADP+ reduction rate was registered at 340 nm. One unit of total or hydrolytic activity of amylosucrase corresponded to the amount of the enzyme that catalyzed the production of 1 μmol of fructose or glucose, respectively, per min under the assay conditions. Transglycosylation activity of amylosucrase was calculated as a difference between the total and hydrolytic activities. The effect of pH on the activity was tested by using the following buffers (50 mM): potassium phosphate (pH 5.5–6.5), MES–KOH (pH 6.0–7.0), N-Tris(hydroxymethyl)methyl-3-aminopropanesulfonate–KOH (pH 7.5–9.0), and sodium carbonate (pH 9.0–10.0). The kinetic parameters were determined by measuring the enzyme activities with different concentrations of sucrose (1–400 mM). The enzyme kinetics module of the SigmaPlot 11 software was used for the calculation of V max and K m.
The ability of amylosucrase to catalyze the formation of polyglucan from sucrose in vivo was verified using the iodine test. Briefly, the ams gene from M. alcaliphilum 20Z was cloned into the low copy number vector pHSG575 (Takeshita et al. 1987) under the lac promoter, and the cells of E. coli Top 10 (Invitrogen, USA) were transformed by the resulting plasmid pHSG575/ams. E. coli harboring the plasmid was grown on LB plates containing 150 mM sucrose and 1 mM IPTG, and the colonies were stained with iodine vapors as described (Buttcher et al. 1997).
Transcriptomic studies
In this study, the RNAseq data obtained from the previously described RNAseq experiments were used (NCBI’s GEO accession number GSE51145). Transcriptional organization of sucrose-metabolizing genes was additionally confirmed by RT-PCR. Total RNA was isolated from exponentially grown M. alcaliphilum cells (OD600 = 0.8) as described earlier (Reshetnikov et al. 2006). For cDNA synthesis, a mixture of 2 μg total RNA and 0.1 μM reverse primer was heated for 5 min at 70 °C and immediately placed on ice. The mixture was supplemented with 4 μl of the reverse transcriptase buffer (5×), 1 mM of each dNTP, 10 units RNase inhibitor (Fermentas), and 10 units HMinus M-MuLV reverse transcriptase (Fermentas), and the sample was incubated at 44 °C for 1 h and then at 70 °C for 15 min. For cDNA amplification, 5 μl of this reaction mixture was added to 50 μl of the amplification buffer containing 0.2 mM of each dNTP, 0.25 μM of the forward primer, and 1 unit of Taq DNA polymerase (Fermentas). In each case, PCR performed without the RT step was used for controlling DNA contamination in the RNA preparations. After 1-min incubation at 94 °C, the samples were subjected to 35 amplification cycles (30 s at 94 °C, 20 s at 65 °C, and 2 min at 72 °C) followed by the final incubation at 72 °C for 2 min. The reaction products were resolved in 1 % agarose gel and quantified using the Gene Ruler™ 100-bp DNA Ladder Plus (Fermentas). The primers used in this study are specified in Suppl. Table 1.
Phylogenetic analysis
The concatenated full-length amino acid sequences of Sps, FruK, and Ams, as well as the full-length sequences of Spp from the protein databases in the National Center for Biotechnology Information (NCBI), were used for phylogenetic analyses. The sequences were aligned using Clustal X software (version 1.8) (Thompson et al. 1997). The phylogenetic tree was generated with MEGA 4 using maximum parsimony, neighbor-joining, and UPGMA methods. The topologies of the trees constructed using different approaches were similar.
Results
Characterization of the recombinant sucrose-phosphate phosphatase
The putative Spp (ORF MALCv4_0615) was purified after heterologous expression in E. coli Rosettta (DE3). The molecular mass of the Spp-His6-tagged protein determined by SDS–PAGE was in a good agreement with the value calculated from the predicted amino acid sequence (32.7 kDa). The recombinant Spp catalyzed the hydrolysis of sucrose-6-phosphate to sucrose and inorganic phosphate with apparent K m = 36 ± 4 μM and V max = 18.9 ± 0.6 U/mg. The enzyme activity depended on Mg2+; the highest activity was obtained at 5 mM Mg2+. The enzyme did not hydrolyze fructose-1-phosphate, fructose-6-phosphate, fructose-1,6-bisphosphate, glucose-1-phosphate, glucose-6-phosphate, ribose-5-phosphate, ribulose-5-phosphate, or erythrose-4-phosphate as a substrate. The maximal Spp activity was found at 35 °C and pH 6.5 (Fig. 1a, b). Unlike Spp from other bacteria, the M. alcaliphilum Spp showed a very high tolerance toward sucrose, the end product of the reaction (K i = 1 ± 0.6 M) (Table 1).
Characterization of the recombinant amylosucrase
The genome of M. alcaliphilum 20Z has only one copy of the gene homologous to ams (MALCv4_0617). It consists of 1956 nucleotides encoding a 651 amino acid protein with a theoretical molecular mass of 76.6 kDa. The gene was expressed in E. coli Rosettta (DE3) as described in “Materials and methods.” The molecular mass of the purified His6-tagged protein calculated from electrophoresis under both native and denaturing conditions was ~75 kDa.
The recombinant Ams catalyzed the following two reactions: (i) sucrose cleavage into fructose and glucose, and (ii) transglycosylation with the transfer of the glycopyranosyl residue of sucrose to the glycan polymer accompanied by the formation of free fructose. Both Ams activities were maximal at pH 8.0 and 30 °C (Fig. 1c, d). In contrast, the described amylosucrases have the higher temperature optima (Table 2). The dependence of Ams activity on sucrose concentrations obeyed the classical Michaelis–Menten kinetics (Suppl. Fig. 1). The M. alcaliphilum amylosucrase showed the twice lower K sucrosem value (6 mM) for transglycosylation reaction compared to the value for hydrolysis (K sucrosem = 11 mM). In the absence of glycogen, the transglycosylation and hydrolytic activities were comparable (60 and 53 mU/mg, respectively). The addition of 0.1 mg/ml glycogen primer increased transglycosylation activity to 98 mU/mg, while the hydrolysis rate remained constant. Earlier, we showed that inactivation of the ams gene using insertion mutagenesis resulted in a 30 % increase in sucrose level in M. alcaliphilum cells, thus indicating the in vivo involvement of amylosucrase in sucrose cleavage (But et al. 2013b).
Additional test was performed in order to validate the transglycosylation activity of the enzyme in vivo. The E. coli Top10 cells were transformed by the pHSG575/ams plasmid and grown in the presence of sucrose. Only the cells harboring the pHSG575/ams plasmid were stained blue with iodine vapors, while the cells carrying the empty pHSG575 vector remained non-colored (Suppl. Fig. 2). This fact confirmed the participation of Ams from M. alcaliphilum 20Z in polyglycan formation from sucrose.
Analysis of transcriptional organization of the sucrose-metabolizing genes
In a single DNA locus of M. alcaliphilum 20Z, an 18-bp intergenic region separates the sps and spp genes. The spp and fruK genes overlap by 4 bp, and fruK is separated from ams by 82 bp. Two additional Orfs, predicted to encode a putative ABC transporter (orf1, 102 bp downstream of ams) and a conserved membrane protein (orf2, overlapped with orf1by 13 bp), were identified (Suppl. Fig. 3). The previously performed RNAseq experiments (GEO #GSM 1239656-1239658) showed good mapping coverage for the whole cluster sps–spp–fruK–ams–orf1–orf2 (Suppl. Fig. 3). The transcriptional start site predicted from the RNA transcript mapping data is located 25 bp upstream of the putative translation start site. The promoter elements −10 (TACAAT) and −35 (TTGAGA) were similar to the sigma-70 factor. RT-PCR analysis was used to verify an operon organization. The RT-PCR products of the expected size with correct sequences were obtained across the sps–spp, spp–fruK, fruK–ams, and fruK–orf1 gene pairs (Fig. 2). The controls for DNA contamination of the RNA preparations using direct PCR without the RT step were negative. These data confirmed that all six genes are co-transcribed.
Organization of genes for sucrose metabolism in Methylomicrobium alcaliphilum 20Z. a Localization of the genes in cluster and positions of the oligonucleotide primers used for cDNA synthesis and amplification in RT-PCR assays. b Gel electrophoresis of the RT-PCR products obtained with the primers sps-F/spp2R (lanes 1, 2, 3); sppF/fruk2R (lanes 4, 5, 6); AS-F/As-R (lanes 7, 8, 9); ABC-R2/ABC-R1 (lanes 10, 11, 12). Primers spp1R, fruk1R, AS-RT, and ABCrc were used for cDNA amplification. The negative controls for PCR containing the total RNA, primers, and Taq-polymerase, but not reverse transcriptase, are shown on lanes 3, 6, 9, 12. The positive controls for PCR containing the genomic DNA and the same primers are shown on lanes 1, 4, 7, 10. The numbers in brackets indicate DNA nucleotides downstream of the first sps nucleotide. The expected sizes of cDNA are indicated in squares. The sequences of oligonucleotide primers used for cDNA synthesis and amplification are listed in Suppl. Table 1
Distribution and organization of the sucrose-metabolizing genes in bacteria
The predicted amino acid sequences for the functionally validated sucrose-phosphate synthase from two cyanobacteria Synechocystis sp. PCC 6803 (Lunn et al. 2003) and Synechococcus sp. 7002 (Cumino et al. 2010), two methylotrophs Methylobacillus flagellatus KT and M. alcaliphilum 20Z (But et al. 2013a, b), and the Gram-positive heterotrophic bacterium Halothermothrix orenii (Huynh et al. 2005) were used to search for similar proteins in the sequenced microbial genomes using the NCBI database. The BLAST search revealed Sps-like ORFs in 101 bacterial genomes (Suppl. Table 2). Most of them belonged to Proteobacteria (64 genomes) and Cyanobacteria (22). The rest were Planctomycetes (6 genomes), Firmicutes (6), Deferribacteres (1), Nitrospinae (1), and Chrysiogenetes (1). However, only 37 of them had the same set of the four genes (sps, spp, fruK, and ams); in 27 bacteria, they clustered analogously to M. alcaliphium 20Z.
The gene order varied throughout the Proteobacteria (see Suppl. Fig. 6 for full details of the gene organization in 50 bacteria). The Sps homologs were identified in 11 out of 19 sequenced genomes of gammaproteobacterial methanotrophs. Eight of them possess the four-gene cluster sps–spp–fruK–ams (Suppl. Fig. 6). The concatenated full-length protein sequences of Sps, FruK, and Ams of methanotrophs comprise a separate branch on the phylogenetic tree (Suppl. Fig. 4) being closely related to the enzyme from Synechococcus sp. 7002 (55 % AA sequence identity). The sequences from the genus Methylophaga (methanol-utilizing gammaproteobacterial methylotrophs) form a separate branch together with the proteins from non-methylotrophic members of beta- and deltaproteobacteria (Suppl. Fig. 4).
Surprisingly, the spp gene was not found in genomes of 44 sps-possessing bacteria (Suppl. Table 2). We showed earlier that the bifunctional Sps of the methylotrophic bacterium M. flagellatus KT catalyzed both phosphorylation of sucrose and dephosphorylation of sucrose-6-phosphate to free sucrose (But et al. 2013a). Notably, the Sps–FruK–Ams polypeptides of the bacteria lacking Spp are separated on the phylogenetic tree from those of the species possessing the four enzymes (Suppl. Fig. 4). The methanotrophic Spp sequences are divided into two main groups. The first group combines the Spp proteins from halotolerant methanotrophs, such as M. alcaliphilum 20Z, Methylobacter marinus A45, and Methylomonas methanica MC09. The second group includes the sequences of salt-independent methanotrophs (Methylomicrobium album BG8, Methylobacter tundripaludum SV96, and Methylosarcina fibrata AML-C10). The only exception is Methylobacter luteus IMV-B-3098T isolated from soil; its Spp is related to the proteins of halotolerant methanotrophs (Suppl. Fig. 5). All methanotrophic Spp proteins are well conserved, and they share 63–93 % AA sequence identities but display very low similarity to the enzyme from Zea mays and Nicotiana tabacum (15.9 and 16.7 % identity, respectively) (Lunn et al. 2000; Chen et al. 2005).
The Ams-like sequences were revealed in 53 bacterial species: 21 Proteobacteria, 18 Cyanobacteria, and all Firmicutes lacked the ams genes. The function of Ams has been proved mainly in the sucrose-utilizing heterotrophic bacteria Neisseria polysaccharea, Deinococcus radiodurans, and Deinococcus geothermalis (Jung et al. 2009; van der Veen et al. 2006; Pizzut-Serin et al. 2005; Emond et al. 2008; Ha et al. 2009), and in the sucrose-synthesizing cyanobacterium Synechococcus sp. 7002 (Perez-Cenci and Salerno 2014). Many alpha-, beta-, gamma-, and deltaproteobacteria, including the halophilic methanotroph Methylohalobius crimeensis, possess a three-gene cluster, which includes fruK, sps, and sus, the latter coding for the reversible sucrose synthase (Sus) functionally replacing Ams. Ams from M. alcaliphilum 20Z shared about 37 % AA identity with the characterized enzymes of heterotrophs and 62 % identity with the enzyme from Synechococcus sp. 7002. The amino acid sequence alignment confirmed that the methanotrophic Ams possesses the consensus motifs characteristic of the glycoside hydrolase family 13 of the enzymes (Svensson 1994) and conserved residues (Asp394, Arg415, Phe436) in the active site (in the B’-domain), which are characteristic for amylosucrases from various microorganisms (Pizzut-Serin et al. 2005).
The absolute majority of bacteria possessing the ams or sus genes also contain fruK with the exceptions of Desulfocapsa sulfexigens and Planctomyces brasiliensis (Suppl. Table 2). The M. alcaliphilum FruK has the highest similarity to the putative FruK of other methanotrophs, i.e., M. methanica MC09, M. album BG8, and M. tundripaludum SV96 (54.4–61.4 % AA identities). Other close relatives of M. alcaliphilum FruK are the putative proteins from methylotrophic bacteria unable to grow under methane: Methylophaga thiooxydans DMS010, Methylophaga lonarensis MPL, Methylophaga aminisulfidivorans MP, Methylophaga sp. JAM1, Methylobacillus flagellatus KT, and Methylovorus glucosetrophus SIP3-4 (40.3–47.6 %), as well as from the autotrophic Thiomicrospira halophila (43.8 %) and Cyanobium gracile (46.6 % identity). FruK has been functionally characterized in Synechococcus sp. 7002 (Perez-Cenci and Salerno 2014).
Two additional Orfs (an ABC transporter and a conserved membrane protein of unknown function found in M. alcaliphilum 20Z) were revealed only in the halotolerant methanotroph M. buryatense 5G.
Discussion
The ability of halotolerant methylotrophs to accumulate sucrose is well documented (Khmelenina et al. 1999; Doronina et al. 2003a, b; But et al. 2013a, b). However, the pathways for sucrose production and further metabolism in non-phototrophic microbes remain elusive. Recently, we have demonstrated that the Sps and FruK enzymes are involved in sucrose conversion in the halotolerant methane-utilizing bacterium M. alcaliphilum 20Z (But et al. 2012, 2013b). In this work, we finalize the reconstruction of the sucrose metabolic pathway in this strain via additional biochemical, genomic, and transcriptomic studies. We have shown that the kinetic characteristics of the two enzymes, Spp and Ams, predicted to be involved in sucrose conversions in M. alcaliphilum 20Z, differ from those investigated previously. The activity of Spp is inhibited by very high sucrose concentrations, while the described cyanobacterial enzymes are more sensitive to sucrose. For example, much lower K i values for sucrose were reported for Spp from Anabaena sp. PCC 7120 and Synechocystis sp. 6803 (80 and 161 mM, respectively) (Cumino et al. 2001; Lunn 2002). The enhanced resistance of the enzyme to sucrose probably allows the halophilic methanotroph to accumulate high intracellular concentrations of the disaccharide under osmotic stress. Contrary to the sucrose-utilizing D. radiodurans and N. polysacharea, with amylosucrases displaying the higher K m for transglycosylation activity compared to sucrose hydrolysis (Pizzut-Serin et al. 2005; van der Veen et al. 2006), the kinetic properties of the M. alcaliphilum Ams indicate that the enzyme mostly contributes to the polyglycan biosynthesis in vivo.
Herewith, we have shown that sucrose production and cleavage in M. alcaliphilum 20Z are determined by a single operon. The metabolic network includes two stages. Similarly to higher plants and cyanobacteria, the methanotroph converts fructose-6-phosphate and UDP glucose into sucrose with the involvement of Sps and Spp. At the next stage, a glycogen-like polymer is synthesized, and free fructose is formed with the participation of Ams. The fructose can be converted back into fructose-6-phosphate via the ATP-dependent FruK (Fig. 3). Thus, the metabolic network probably represents a ‘sucrose cycle.’
Overview of sucrose metabolism in M. alcaliphilum 20Z. MMO methane monooxygenase, MDH methanol dehydrogenase, FOP formaldehyde oxidation pathways, FDH formate dehydrogenase, HPS hexulose-phosphate synthase, HPI hexulose-phosphate isomerase, PGI phosphoglucoisomerase, PGM phosphoglucomutase, UGP UDP glucose pyrophosphorylase, AGP ADP glucose pyrophosphorylase, GS glycogen synthase
Glycogen synthesis via sucrose as an intermediate requires two NTP molecules for attaching the glucosyl residue to the glycogen primer. This pathway is less energy efficient compared to the glucose pyrophosphorylase (GlgC)/glycogen synthase (GlgA) route, which requires only one NTP molecule for glycogen elongation. The genome of M. alcaliphilum 20Z possesses and expresses the necessary genes being inventory for the latter route (Suppl. Table 3). Intracellular accumulation of sucrose in M. alcaliphilum 20Z is one of the responses to the decrease in water activity (Khmelenina et al. 1999). It would be reasonable to suggest that the main function of the pathway is de novo sucrose biosynthesis. This suggestion is supported by the atypical kinetic behavior of Spp with respect to the inhibition of the enzyme activity by an extremely high sucrose level. Nevertheless, the genes involved in sucrose biosynthesis (Sps/Spp) and sucrose breakdown/reutilization (Ams/FruK) display similar relative expression levels (Suppl. Fig. 3). It is possible that the sucrose cycle could be envisioned as a dynamic mechanism that balances the internal concentration of sucrose via posttranscriptional activation or repression of the Sps/Spp or Ams/FruK branches of the cycle.
All of the sequenced genomes of halotolerant methanotrophs and three of the four genomes of methanotrophs isolated from soils possess the sucrose biosynthesis genes. On the other hand, in the nine genomes of methanotrophs isolated from freshwater sediments or sewage systems, the sucrose biosynthetic genes were identified only in Methyloglobulus morosus DSM 22980. However, organization of the genes for sucrose metabolism in this species is different, since the corresponding gene cluster lacks fruK (Suppl. Fig. 6). It might be speculated that the distribution of sucrose metabolism in methanotrophic bacteria is driven by the conditions of natural habitat such as salinity and/or seasonal exposure to a low-water activity under dry conditions or high temperature.
No sps-homologous genes were found in the alpha- or betaproteobacterial methylotrophs assimilating the reduced C1 compounds via the serine pathway and the methanotrophs belonging to Verrucomicrobia and candidatus “Methylomirabilis oxyfera” utilizing methane carbon at the level of CO2 via the CBB cycle (Khadem et al. 2011; Rasigraf et al. 2014). Thus far, the ability to produce sucrose is restricted to methylotrophic gamma- and betaproteobacteria employing the energy-efficient RuMP pathway for C1 assimilation.
Interestingly, the majority of the sequenced genomes of Methylophilaceae, mostly of freshwater species, possess the sucrose-metabolizing genes (Suppl. Fig. 6). The salt- and temperature-dependent sucrose biosynthesis has been observed in M. flagellatus KT (But et al. 2013a). It should be noted that sucrose accumulation provides only moderate salt and temperature tolerance of this methylotroph. On the other hand, all species of Methylophilaceae capable of sucrose biosynthesis display highly active growth on methanol, while the sucrose biosynthesis genes are not present in those species (Methylotenera mobilis JLW8, Methylotenera versatilis 301, Methylotenera sp 1P/1, and marine bacterium Methylophilaceae spp. HTCC 2181) showing no growth or very poor growth on methanol. It is tempting to speculate that sucrose metabolism provides bacteria with some advantages for efficient and active assimilation of reduced C1 compounds, since the sucrose synthesis in all members of the Methylophilaceae is linked to the initial steps of formaldehyde assimilation via the RuMP pathway (Fig. 3). The conversion of fructose-6-phosphate into sucrose can stimulate the incorporation of formaldehyde into glycogen, the main carbon storage compound. It is also possible that glycogen synthesis via the sucrose cycle may function like a “futile cycle” for dissipation of energy excess.
Among non-methylotrophic bacteria, the homologs of the sps genes were identified in the genomes of photo- and chemolithotrophs capable of fixing CO2 via the CBB cycle (such as Nitrosomonas europaea), the Wood–Ljungdahl pathway (Desulfobacterium autotrophicum), and the reverse Krebs cycle (Magnetococcus marinus). The genes for sucrose-synthesizing enzymes can also be identified in the genomes of Bacteroidetes, Chloroflexi, Planctomycetes, and Firmicutes. The exact role of sucrose biosynthesis/utilization genes in these poorly characterized microbial clades still has to be elucidated. Bearing in mind that these bacterial phyla were isolated from a variety of marine, freshwater, and terrestrial ecosystems, a more intricate picture of sucrose as an intermediate of microbial metabolic networks might be evolved in the future.
References
Bencini DA, Wild JR, O’Donovan GA (1983) Linear one-step assay for the determination of orthophosphate. Anal Biochem 132:254–258
Bruneau JM, Worrell AC, Cambou B, Lando D, Voelker TA (1991) Sucrose phosphate synthase, a key enzyme for sucrose biosynthesis in plants. Plant Physiol 96:473–478
But SY, Rozova ON, Khmelenina VN, Reshetnikov AS, Trotsenko YA (2012) Properties of recombinant ATP dependent fructokinase from the halotolerant methanotroph Methylomicrobium alcaliphilum 20Z. Biochemistry (Moscow) 77:372–377
But SY, Khmelenina VN, Reshetnikov AS, Trotsenko YA (2013a) Bifunctional sucrose phosphate synthase/phosphatase is involved in the sucrose biosynthesis by Methylobacillus flagellatus KT. FEMS Microbiol Lett 347:43–51
But SY, Khmelenina VN, Mustakhimov II, Trotsenko YA (2013b) Construction and characterization of Methylomicrobium alcaliphilum 20Z knockout mutants defective in sucrose and ectoine biosynthesis genes. Microbiology (Moscow) 82(2):253–255
Buttcher V, Welsh T, Willmitzer L, Kossmann J (1997) Cloning and characterization of the gene for amylosucrase from Neisseria polysaccharea: production of a linear alpha-1,4-glucan. J Bacteriol 179:3324–3330
Chen S, Hajirezaei M, Börnke F (2005) Differential expression of sucrose-phosphate synthase isoenzymes in tobacco reflects their functional specialization during dark-governed starch mobilization in source leaves. Plant Physiol 139(3):1163–1174
Chua TK, Bujnicki JM, Tan TC, Huynh F, Patel BK, Sivaraman J (2008) The structure of sucrose phosphate synthase from Halothermothrix orenii reveals its mechanism of action and binding mode. Plant Cell 20:1059–1072
Cumino A, Ekeroth C, Salerno GL (2001) Sucrose-phosphate phosphatase from Anabaena sp. strain PCC 7120: isolation of the protein and gene revealed significant structural differences from the higher-plant enzyme. Planta 214:250–256
Cumino AC, Perez-Cenci M, Giarrocco LE, Salerno GL (2010) The proteins involved in sucrose synthesis in the marine cyanobacterium Synechococcus sp. PCC 7002 are encoded by two genes transcribed from a gene cluster. FEBS Lett 584:4655–4660
d’AvÓ AF, Cunha S, Mingote A, Lamosa P, da Costa MS, Costa J (2013) A unique pool of compatible solutes in Rhodopirellula baltica, member of the deep-branching phylum Planctomycetes. PLoS One 8(6):e68289. doi:10.1371/journal.pone.0068289
Doronina NV, Darmaeva TD, Trotsenko YA (2003a) Methylophaga alcalica sp. nov., a novel alkaliphilic and moderately halophilic, obligately methylotrophic bacterium from an East Mongolian saline soda lake. Int J Syst Evol Microbiol 53:223–229
Doronina NV, Darmaeva TD, Trotsenko YA (2003b) Methylophaga natronica sp. nov., a new alkaliphilic and moderately halophilic, restricted-facultative methylotrophic bacterium from soda lake of the Southern Transbaikal region. Syst Appl Microbiol 26:382–389
Emond S, Mondeil S, Jaziri K, André I, Monsan P, Remaud-Siméon M, Potocki-Véronèse G (2008) Cloning, purification and characterization of a thermostable amylosucrase from Deinococcus geothermalis. FEMS Microbiol Lett 285:25–32
Ha SJ, Seo DH, Jung JH, Cha J, Kim TJ, Kim YW, Park CS (2009) Molecular cloning and functional expression of a new amylosucrase from Alteromonas macleodii. Biosci Biotechnol Biochem 73:1505–1512
Huynh F, Tan TC, Swaminathan K, Patel BKC (2005) Expression, purification and preliminary crystallographic analysis of sucrose phosphate synthase (SPS) from Halothermothrix orenii. Acta Cryst F 61:116–117
Jung JH, Seo DH, Ha SJ, Song MC, Cha J, Yoo SH, Kim TJ, Baek NI, Baik MY, Park CS (2009) Enzymatic synthesis of salicin glycosides through transglycosylation catalyzed by amylosucrases from Deinococcus geothermalis and Neisseria polysaccharea. Carbohydr Res 344:1612–1619
Kalyuzhnaya M, Khmelenina VN, Kotelnikova S, Holmquist L, Pedersen K, Trotsenko YA (1999) Methylomonas scandinavica sp. nov., a new methanotrophic psychrotrophic bacterium isolated from deep igneous rock ground water of Sweden. Syst Appl Microbiol 22:565–572
Khadem AF, Pol A, Wieczorek A, Mohammadi SS, Francoijs KJ, Stunnenberg HG, Jetten MS, Op den Camp HJ (2011) Autotrophic methanotrophy in Verrucomicrobia: Methylacidiphilum fumariolicum SolV uses the Calvin-Benson-Bassham cycle for carbon dioxide fixation. J Bacteriol 193:4438–4446
Khmelenina VN, Kalyuzhnaya MG, Starostina NG, Suzina NE, Trotsenko YA (1997) Isolation and characterization of halotolerant alkaliphilic methanotrophic bacteria from Tuva soda lakes. Curr Microbiol 35:257–261
Khmelenina VN, Kalyuzhnaya MG, Sakharovsky VG, Suzina NE, Trotsenko YA, Gottschalk G (1999) Osmoadaptation in halophilic and alkaliphilic methanotrophs. Arch Microbiol 172:321–329
Khmelenina VN, Shchukin VN, Reshetnikov AS, Mustakhimov II, Suzina NE, Eshinimaev BT, Trotsenko YA (2010) Structural and functional features of methanotrophs from hypersaline and alkaline lakes. Microbiology (Moscow) 79:472–482
Klähn S, Hagemann M (2011) Compatible solute biosynthesis in cyanobacteria. Environ Microbiol 13:551–562
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lunn JE (2002) Evolution of sucrose synthesis. Plant Physiol 128:1490–1500
Lunn JE, Ashton AR, Hatch MD, Heldt HW (2000) Purification, molecular cloning, and sequence analysis of sucrose-6-phosphate phosphohydrolase from plants. Proc Natl Acad Sci USA 97:12914–12919
Lunn JE, Gillespie VJ, Furbank RT (2003) Expression of a cyanobacterial sucrose-phosphate synthase from Synechocystis sp. PCC 6803 in transgenic plants. J Exp Bot 54:223–237
Page-Sharp M, Behm CA, Smith GD (1999) Involvement of the compatible solutes trehalose and sucrose in the response to salt stress of a cyanobacterial Scytonema species isolated from desert soils. Biochim Biophys Acta 1472:519–528
Perez-Cenci M, Salerno GM (2014) Functional characterization of Synechococcus amylosucrase and fructokinase encoding genes discovers two novel actors on the stage of cyanobacterial sucrose metabolism. Plant Sci 224:95–102
Pizzut-Serin S, Potocki-Véronèse G, van der Veen BA, Albenne C, Monsan P, Remaud-Simeon M (2005) Characterisation of a novel amylosucrase from Deinococcus radiodurans. FEBS Lett 579:1405–1410
Rasigraf O, Kool DM, Jetten MS, Sinninghe Damsté JS, Ettwig KF (2014) Autotrophic carbon dioxide fixation via the Calvin–Benson–Bassham cycle by the denitrifying methanotroph Methylomirabilis oxyfera. Appl Environ Microbiol 80:2451–2460
Reshetnikov AS, Khmelenina VN, Trotsenko YA (2006) Characterization of the ectoine biosynthesis genes in obligate haloalkalotolerant methanotroph Methylomicrobium alcaliphilum 20Z. Arch Microbiol 184:286–296
Rozova ON, Khmelenina VN, Vuilleumier S, Trotsenko YA (2010) Characterization of recombinant pyrophosphate-dependent 6-phosphofructokinase from halotolerant methanotroph Methylomicrobium alcaliphilum 20Z. Res Microbiol 161:861–868
Salerno GL, Curatti L (2003) Origin of sucrose metabolism in higher plants: when, how and why? Trends Plant Sci 8:63–69
Sambrook J, Russell DW (2001) Molecular Cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, New-York
Svensson B (1994) Protein engineering in the α-amylase family: catalytic mechanism, substrate specificity, and stability. Plant Mol Biol 25:141–157
Takeshita S, Sato M, Toba M, Masahashi W, Hashimoto-Gotoh T (1987) High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63–74
Thomson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res 24:4876–4882
Van der Veen BA, Skov LK, Potocki-Véronèse G, Gajhede M, Monsan P, Remaud-Simeon M (2006) Increased amylosucrase activity and specificity, and identification of regions important for activity, specificity and stability through molecular evolution. FEBS J 273:673–681
Whitaker DP (1984) Purification and properties of sucrose-6-phosphatase from Pisum sativum shoots. Phytochemistry 23:2429–2430
Acknowledgments
The work was supported by the grants of the Russian Foundation for Basic Research (13-04-01119-a), the Ministry of Education and Science of the Russian Federation (6.749.2014/k), the Russian Science Foundation (14-04-01045), and the US National Science Foundation (MCB-0842686). The authors are grateful to D. Beck for his assistance with the IGV toolbox.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Michael Rother.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
But, S.Y., Khmelenina, V.N., Reshetnikov, A.S. et al. Sucrose metabolism in halotolerant methanotroph Methylomicrobium alcaliphilum 20Z. Arch Microbiol 197, 471–480 (2015). https://doi.org/10.1007/s00203-015-1080-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-015-1080-9