Abstract
In spite of potentially being an important source of rhizobial diversity and a key determinant of common bean productivity, there is a paucity of data on Rhizobium genetic variation and species composition in the important bean producing area of Chile and only one species has been documented (Rhizobium leguminosarum). In this study, 240 Rhizobium isolates from Torcaza bean (Phaseolus vulgaris L.) nodules established in the highest bean producing area in Chile (33°34′S–70°38′W and 37°36′S–71°47′W) were characterized by PCR-RFLP markers for nodC gene, revealing eight banding patterns with the polymorphic enzyme Hinf I. The locality of San Agustín de Aurora in Central Chile (35°32′S–71°29′W) had the highest level of diversity. Isolates were classified by species using PCR-RFLP markers for 16S rDNA gene and were confirmed by sequencing an internal fragment of the 16S rDNA gene. The results confirmed the presence of R. leguminosarum and three other species of rhizobia nodulating beans in South Central Chile (R. etli, R. tropici and R. leucaenae). R. tropici and R. leucaenae showed the least genetic variation and were most commonly identified in acid soils, while R. etli was the most common species in slightly acidic to moderately alkaline soils, with higher levels of organic matter content. R. leguminosarum was identified in almost all soils, was the most genetically diverse, and was the most common, being documented in soils with pH that ranged between 5.3 and 8.2, and with organic matter content between 2.1 and 4 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The common bean (Phaseolus vulgaris L.) is the most frequently consumed legume in Central and South America. Mesoamerica and the Andean region of South America were the centers of origin and/or domestication of P. vulgaris (Gepts and Debouck 1991; Kaplan 1965), and the southern Andes of Argentina was an important secondary origin of P. vulgaris var. aborigineus Burk (Baudet) (Gepts and Debouck 1991). From the Andean center, three strains have been identified. Of these, “the Chilean strain” has been distinguished as an important source of genetic diversity (Bascur and Tay 2005). An important feature of the common bean is that it establishes a symbiotic partnership with a group of bacteria species, collectively known as “rhizobia.” These bacteria establish symbiotic associations with many legumes and, most importantly, form root nodules that catalyze the fixation of atmospheric nitrogen (N2). Interest in utilizing rhizobia as biofertilizers in agriculture has prompted studies of their diversity and the description of a large number of rhizobial species. In addition to contributing to our knowledge of soil biodiversity and increasing the utility of rhizobial collections, assessments of rhizobial genetic diversity are important in helping develop long-term strategies to increase the role of biological N2 fixation in improving agricultural productivity.
To date, no studies have been done in Chile on native rhizobia species diversity and their association with the nodulating common bean and only one species R. leguminosarum sv. phaseoli has been described (Urzúa 2005; Urzúa and Tesser 1998). However, the common bean is very promiscuous in its relationship with rhizobia (Aserse et al. 2012; Herrera-Cervera et al. 1999; Martínez-Romero 2003) and establishes symbiotic associations with a wide range of rhizobial bacteria, including the most commonly studied species: R. leguminosarum sv. phaseoli (Jordan 1984), R. etli sv. phaseoli (Segovia et al. 1993), R. gallicum (sv. phaseoli and sv. gallicum), R. giardinii (sv. phaseoli and sv. giardinii) (Amarger et al. 1997), R. tropici (Martínez-Romero et al. 1991), R. lusitanum (Valverde et al. 2006), R. multihospitium (Han et al. 2008), R. phaseoli (Ramirez-Bahena et al. 2008), R. rizogenes (Velázquez et al. 2010), R. vallis (Wang et al. 2011), R. leucaenae (Ribeiro et al. 2012), R. grahamii and R. mesoamericanum (López-López et al. 2012). R. etli is the dominant microsymbiont in the Mesoamerican and the Andean centers of origin (Aguilar et al. 1998, 2004; Bernal and Graham 2001; Martínez-Romero 2003; Rodriguez-Navarro et al. 2000; Segovia et al. 1993; Souza et al. 1997) and is commonly associated with beans in Mexico, Colombia and the southern Andes and Argentina (Aguilar et al. 1998, 2004; Bernal and Graham 2001), as well as Europe, Africa and Oceania (Herrera-Cervera et al. 1999; Mhamdi et al. 2002; Sessitsch et al. 1997). R. leguminosarum sv. phaseoli was first identified in Europe, and it was suggested that the symbiotic plasmid may have been transferred to native R. leguminosarum from introduced seeds containing R. etli sv. phaseoli from America (Pérez-Ramírez et al. 1998; Segovia et al. 1993). R. tropici, a promiscuous rhizobia, with hosts that include several species of the three legume subfamilies (Hungria and Vargas 2000), is very effective at fixing N2 in association with Andean and Mesoamerican common bean genotypes. They are also competitive against indigenous rhizobia, genetically stable, and are hypothesized to be native to tropical regions of South America, since they are adapted to stressful conditions such as high temperature and low soil pH (Graham et al. 1994; Hungria and Vargas 2000, 2003; Martínez-Romero et al. 1991; Pinto et al. 2007). They have been reported in French acidic soils (Amarger et al. 1994) and in Kenya (Anyango et al. 1995), but are poorly represented in soils with neutral pH from Africa, Argentina, México and Spain (Aguilar et al. 1998; Anyango et al. 1995; Rodriguez-Navarro et al. 2000; Vásquez-Arroyo et al. 1998). In contrast, R. gallicum and R. giardinii are predominant in French soils (Amarger et al. 1997), as well as soils in Spain (Herrera-Cervera et al. 1999) and Australia (Sessitsch et al. 1997).
The main objective of this study was to assess the diversity and distribution of native populations of rhizobia from the main bean producing area in Chile, including moderately alkaline to acidic soils under Mediterranean climate conditions, and to determine the existence of other strains that nodulate beans in addition to R. leguminosarum described only in Chile. This study was performed using RFLP markers from nodC and 16S rDNA by sequencing a partial fragment of the 16S rDNA gene.
Materials and methods
Bacteria were isolated from nodules extracted from bean plants (Torcaza INIA cv.) from five locations in the Central and South Central zones of Chile (Table 1). The region has a Mediterranean climate that ranges from 300 mm of precipitation per year and an 8-month dry period in the north to 1,200 mm of rainfall and a 4-month dry period in the south. From each site measures of soil pH, electric conductivity, organic matter, total nitrogen, and carbon to nitrogen ration were estimated (Table 1) following Sadzawka et al. (2004). From each plant, 3–6 nodules were randomly excised and surface sterilized with ethanol and hydrogen peroxide. Rhizobia were isolated axenically on YEM–Congo red agar medium as described by Vincent (1970). R. tropici CIAT899 and R. etli CFN 42, provided by the Universidad Politécnica de Madrid, Spain and R. leguminosarum TAL1121 (IPAGRO Brazil), provided by CIFA-Las Torres, Spain) were used as reference species. Molecular and fixing efficiency analyses were conducted at the Faculty of Agricultural Sciences of the University of Chile.
PCR-RFLP of nodC gene
DNA extraction was conducted following the protocol described by Aguilar et al. (2001). The isolated DNA was used for PCR amplification of an internal fragment of the nodC gene using primers NodCF (forward): 5′-AYGTHGTYGAYGACGGTTC-3′ and NodCI (reverse): 5′-CGYGACAGCCANTCKCTATTG-3′ and an amplification pattern of 95 °C for 3 min, 35 cycles of 94 °C for 1 min, 55 °C for 1 min and 72 °C for 2 min, 72 °C for 10 min (Laguerre et al. 2001). PCR product was digested with the restriction enzyme HinfI (Aguilar et al. 2001).The gels were evaluated by visual inspection recording “1” or “2” to indicate the presence or absence of each RFLP fragment size. A binary data matrix was built using the “PC—NTSYS version 2.02” program to construct similarity and hierarchical clustering matrices among isolates using the index “DICE”. To determine the genetic similarity among the PCR-RFLP patterns, a dendrogram based on the cluster analysis was built using the UPGMA algorithm (Rohlf 1998).
PCR-RFLP amplified 16S rDNA gene
From the previous step, eight different RFLP patterns were identified and from each a representative isolate was characterized by PCR-RFLP of the 16S rDNA gene, following the Young et al. (1991) protocol using primers Y1 (5′-TGGCTCAGAACGAACGCTGGCGGC-3′) and Y2 (5′-CCCACTGCTGCCTCCCGTAGGAGT-3′) and the following amplification conditions: 93 °C for 45 s, 35 cycles of 93 °C for 45 s, 62 °C for 45 s and 72 °C for 2 min, 72 °C for 5 min. PCR products were digested with the restriction enzyme RsaI (Laguerre et al. 1994).
Sequencing the 16S rDNA internal fragment and phylogenetic analyses
For more detailed analyses at the nucleotide level, the eight 16S rDNA internal fragments from the RFLP analyses were sequenced after PCR amplification. The 307-bp fragment was purified using the “QIAquick PCR Purification Kit (250)” from Qiagen. If the amplification showed additional bands, the target band was isolated from the agarose gel and purified using the commercial kit “Gene Clean Turbo” from MP Biomedicals. The purified PCR products were sequenced using Applied Biosystem sequencer ABI3700 and ABI3730XL at Macro gen Inc., (Seoul, Korea), using the previously described primers. The 16S rDNA sequences from the rhizobia strains corresponded with positions 20 through 338 of the Echerichia coli numbering system. Taxonomic identity of each fragment was determined using the BLAST tool of the nucleotide DNA database of NCBI (National Center for Biotechnology Information). Additional DNA sequences corresponding with previously isolated homologous fragments in related taxa were also downloaded from GenBank for reference. Our eight sequences from Chile and 24 additional GenBank DNA sequences were aligned in a single matrix using CLUSTALX (Thompson et al. 1997), and the alignments were inspected visually using the Mesquite 2.75 software package (Maddison and Maddison 2014). Neighbor joining and maximum-likelihood phylogenetic trees were built using the software MEGA5.2 (Tamura et al. 2011). Models of sequences evolution were inferred with Modeltest (Posada and Crandall 1998), and statistical support for clades was estimated with 1,000 bootstrap replicates. Trees were visualized using the Tree View software (Page 1996).
Results
PCR-RFLP of nodC gene
Samples were collected from 240 isolates from five locations (Table 1). The internal fragments of nodC ranged from 950–980 bp. Digestion of this fragment with HinfI produced eight RFLP patterns that were designated by the letters A, B, C, G, H, K, L and N (Tables 2, 3; Figs. 1, 2). San Agustín de Aurora (SAA) displayed the highest genetic diversity, with four patterns (K, C, G, B, Fig. 2; Table 3). In contrast, Ñiquen (NI) samples had only two restriction patterns (L and B). Pattern B also appeared in San Agustín de Aurora and Santa Barbara, (SBAR) while pattern L was only found in NI and had an identical patterns as the R. tropici reference strain (CIAT899) (Fig. 2; Table 3). The H pattern only occurred in Antumapu and was identical to R. etli and R. leguminosarum reference strains (CFN42 and TAL1121, respectively) (Figs. 1, 2; Table 3). The G, A and B patterns were the most common, representing 34, 21 and 20 % of total analyzed isolates, respectively (Table 3). Pattern G was the most common in Antumapu (ANT), San Fernando (SFDO) and San Agustin de Aurora, while A was the most common isolate in Santa Barbara and B the most common in San Agustín de Aurora and Ñiquén. Patterns L, N and K were less common and together only represented 10 % of the analyzed isolates (Table 3).
RFLP patterns from the internal fragment of the nodC gene, resulting from the enzyme digestion of the rhizobia isolates nodulating beans in Chile (A, B, C, G, H, K, L, N) and three reference strains [T1 R. tropici (CIAT899), T2 R. etli (CFN42) and T3 R. leguminosarum (TAL1121)] with HinfI. MM size marker (100 bp). Sizes are indicated on the left and right of the picture in bp
PCR-RFLP of the nodC gene sequence (≈960 bp) of rhizobia nodulating beans from South Central Chile and reference strains. 1 Antumapu (ANT), 2 San Fernando (SDO), 3 San Agustín de Aurora (SAA), 4 Ñiquén (NI), 5 Santa Bárbara (SBAR), 6 Reference strains, T1 R. tropici (CIAT899), T2 R. etli (CFN42) and T3 R. leguminosarum (TAL1121). In lane 1 of all the pictures, MM indicates size marker (100 bp) and numbers to the left of each picture indicated fragment sizes in bp
The similarity and hierarchical clustering dendrograms among the different isolates and the three reference strains are represented in Fig. 3. CIAT899 (R. tropici) and isolates B, N, K and L grouped together and represents 30 % of the isolates. According to the UPGMA algorithm, there was an 80 % similarity coefficient between B and N and a similarity coefficient of 60 % between the (B, N) clade and the clade composed of type K, R. tropici (CIAT899) and type L (Fig. 3). A 100 % similarity coefficient was observed between the isolate CIAT899 and type L (Fig. 3). The clade composed of CFN42 reference strains (R. etli) and TAL1121 (R. leguminosarum) includes the pattern type H with 100 % similarity coefficient. Patterns A and G form a sister clade with the clade previously described, with 47 % similarity coefficient with respect to the (R. etli, R. leguminosarum, H) clade. This two sister groups contained 65 % of the isolates, with the G band pattern being the most common. In addition, the H band pattern had an identical PCR-RFLP pattern to those of CFN42 and TAL1121. The third and most divergent group (15 % similarity coefficient), consisted of only 5 % of the samples and corresponded to isolates with the type C band pattern.
PCR-RFLP from the 16S ribosomal gene
The internal fragment of the 16S ribosomal gene obtained for the eight selected isolates (Table 2) consisted of around 320 bp. PCR products were digested with RsaI, digesting only ANT 12.3 (pattern type H) and BAR 21.3 (pattern type A) isolates and the reference strain CFN42 Rhizobium etli (Fig. 4; lanes 4, 6 and 9, respectively). In all three cases, the obtained fragments were similar and ≈225 and 100 bp.
Fragments of 16S ribosomal gene isolated from rhizobia digested with RsaI. MM: 100-bp size molecular marker (Fermentas®), 1 SAA 60.3, 2 SAA 27.4, 3 SAA 15.3, 4 ANT 12.3, 5 SFDO 41.3, 6 SBAR 21.3, 7 NI 17.3, 8 NI 14.3, 9 CFN42 (R. etli), 10 TAL 1121 (R. leguminosarum), 11 CIAT 899 (R. tropici). Numbers to the left and right of the picture indicate fragment sizes in bp
Phylogenetic analysis of the 16S rDNA gene
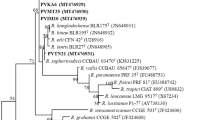
The phylogenetic tree from the sequence of a 307-bp internal fragment of the 16S rDNA of the selected isolates is presented in Fig. 5. Several related sequences available in GenBank were also used for more accurate comparison of our results. The phylogeny shows consistent results with the previous RFLP analyses and helps elucidate some patterns that were not clear in either of the RFLPs performed. The phylogeny clearly separates R. leguminosarum, R. tropici and R. etli and further confirms the identities of the strains found in Chile, clustering within well-supported monophyletic groups (Fig. 5). The strains found in NI appear to be of different origin (i.e., represent different species), one of them more closely related to R. leucaenae (NI 14.3) and the other one, NI 17.3, was identified here as R. tropici. Antumapu (ANT12.3) and Santa Bárbara (BAR21.3) isolates were identified as R. etli and San Agustín de Aurora (SAA 15.3), San Fernando (SFDO 41.3), SAA 27.4 and SAA 60.3 as R. leguminosarum.
Neighbor joining phylogram derived from internal sequence of 16S rDNA gene from eight isolates classified according to the patterns obtained through the nodC gene PCR-RFLP marker, showing the relationships between the strains evaluated in this study and other type or reference strains from closely related species. GenBank accession numbers from reference strains are indicated in parentheses. Numbers above nodes represent support for clades obtained with 1,000 bootstrap replicates. (R Rhizobium, B Bradyrhizobium, A Azorhizobium, E Escherichia)
Discussion
Several authors have demonstrated that there is a large amount of genetic variability among bean symbiotic bacteria (Aguilar et al. 2004; Aserse et al. 2012; Grange et al. 2007; Martínez-Romero 2003; Shamseldin and Werner 2005). In spite of there previously being only species described in Chile, this variability was also observed in Central and South Central Chile (33°34′–37°36′S), where there are a wide range of conditions and soil types in the different studied areas, including soils of coarse to fine textural class Mollisols of alluvial origin (Antumapu, San Fernando), fine textural class Alfisols of alluvial–colluvial origin (San Agustín de Aurora) and silt loam textural class Andisols (Santa Bárbara and Ñiquen) of volcanic origin with eolian and alluvial transport (Luzio 2010). This heterogeneity in the soil origin, as demonstrated by ranges from 2.3 to 6.3 % organic matter levels and pH between 5.2 and 8.2 (Table 1), may influence cell development, survival and nodule formation (Ballen et al. 1998), although there is no consensus on whether soil characteristics help determine the presence and absence of different rhizobia species (Martínez-Romero 2003). From a total of 240 isolates, eight nodC gene PCR-RFLP patterns were differed (Fig. 1; Table 3). Similar studies conducted by Aguilar et al. (2001) determined the presence of three different PCR-RFLP patterns among 80 isolates from northeastern Argentina.
San Agustín de Aurora was the area with the highest level of dgene PCR-RFLP patterns, showing patterns B, C, G and K (Fig. 2; Table 3), even though the 16S genotypes of these were not closely related based on phylogenetically analyses (Fig. 3). R. leguminosarum and R. etli are closely related and share more than 97 % sequence identity (Ribeiro et al. 2013), and thus could not be discriminated by the nodC gene PCR-RFLP marker, as has been observed by other studies (Aguilar et al. 2001; Gutierrez and Barraquio 2010; Laguerre et al. 1994; Silva et al. 2007). This could be because there have been nodC type alleles that were laterally transferred from one species to another (Aguilar et al. 2004). An alternative perspective, argued by Beyene et al. (2004), is that there is no clear rationale to separate R. leguminosarum from R. etli into different species, because the differences between them represent the variation extremes of a single species. However, here we confirm that the 16S rDNA PCR-RFLP marker can discriminate both species (Fig. 4), as observed by Aguilar et al. (2004). These same authors, evaluating strains from a wide range of geographical origins, identified four nodC gene PCR-RFLP marker patterns designated as α, γ, δ and β, where the first three were R. etli. Patterns α and γ match patterns H and A herein, identified also as R. etli in the present study. Moreover, the β pattern was identified only in wild exemplars of common beans and corresponded to a R. leguminosarum type pattern C in our study (isolate SAA15.3, Fig. 5). The nodC gene PCR-RFLP marker classified pattern L as R. tropici (Fig. 3), which was confirmed by the sequence analyses (Fig. 5). It is noteworthy that originally R. tropici comprised two phenotypically distinct groups, named type A and B, but recently a new species, R. leucaenae, has been proposed to describe type A strains, with strain CFN 299 selected as the representative of the species (Ribeiro et al. 2012). In this study, the isolated NI 14.3 (pattern B) was the only one that corresponded to this species (Fig. 5).
Rhizobium leguminosarum was present in virtually all soil types and was also the most genetically diverse species (patterns C, G, K, N, Fig. 6), and the most common of the documented isolates (43 %; Table 3). Most of these isolates were located between coordinates 33°34′S–70°38′W and 35° 32′S–71°29′W, within 247 km of each other (Table 1; Fig. 6), and with pattern G isolates being the most abundant (34 % of total). Note that the variety of beans, from which the nodules were extracted, corresponded to a variety created in Chile with indigenous ecotypes, which may partially explain this diversity (Laguerre et al. 1994). Even though the study area has Mediterranean climate, there is a north–south gradient rainfall increase (from 300 mm year−1 in Antumapu to 1,200 mm year−1 in Santa Bárbara) and decreasing average temperatures (from 14.2 to 12.2) with a marked dry season in summer (Uribe et al. 2012). From Ñiquén to the south, andisols appear in Central Valley with a marked increase in the organic matter content (Padarian et al. 2012). The soil from this area is very heterogeneous, varying in organic matter (2.1–4 %) and pH (from 5.3 in San Fernando to 8.2 in Antumapu) (Table 1), and clay content from 8.2 % in Antumapu to 38.7 in San Agustín de Aurora, according to CIREN (1996, 1997). Some studies have reported relatively low diversities of R. leguminosarum in acid soils relative to lime soils (Andrade et al. 2002; Lapinskas 2007), because soil pH hypothesizing that they are less tolerant to low pH soils (Andrade et al. 2002; Bala et al. 2001; Bala and Giller 2007). However, in this study, the greatest diversity of R. leguminosarum was observed in acid (San Fernando) and slightly acid (San Agustín de Aurora) soils (Table 1; Fig. 6). The presence of R. leguminosarum in acid soils is not surprising, however. Although most studies associated such soils with R. tropici (Amarger et al. 1994; Anyango et al. 1995; Stocco et al. 2008), other studies have also demonstrated the presence of R. leguminosarum in low pH soils (Andrade et al. 2002; Bernal et al. 2004; Gutierrez and Barraquio 2010).
The most dominant and diverse species in Chile was R. leguminosarum, which differs from what was observed in other bean domestication centers (e.g. Mexico, Ecuador, Peru and Argentina) where R. etli are the most dominant (Aguilar et al. 2001, 2004; Grange et al. 2007; Martínez-Romero 2003; Souza et al. 1997) and diverse species (Bernal and Graham 2001; Caballero-Mellado and Martínez-Romero 1999; Segovia et al. 1991). This has been explained by Aguilar et al. (2004), whom investigated the distribution of nodC alleles in a wide collection of R. etli populations from worldwide, that the presence and dominance of R. etli in these regions may be due to the introduction of these during the importation of seeds. Similar conclusions were obtained by Gutierrez and Barraquio (2010) in the Philippines and by Rodriguez-Navarro et al. (2000) in Spain. Therefore, since Chile is a sub-center of bean genetic diversity (Bascur and Tay 2005), and since most of the varieties used are native, it is reasonable that R. etli is not the dominant species. Another possibility is that R. etli share symbiotic genes with the R. leguminosarum native bacteria, as was observed with R. etli in Europe (García-Fraile et al. 2010; Segovia et al. 1993). In our data, R. etli showed only two PCR-RFLP patterns for nodC gene (A and H) and only 31 % of the isolates corresponded to this species (Table 3). However, the observed patterns were from distant locations (≈490 km from each other). Populations representing pattern H were located in the north central region (33°34′S–70°38′W) in soils of the great group Haploxeroll, with alkaline pH and low organic matter content. In contrast, type A was mostly found in the south central region (37°36′S–71°47′W, Fig. 6) in soils from the great group Haploxerand, with greater levels of organic matter (6.3 %) and slightly acidic pH (6.1). This contrasting pattern has been observed in other studies in which R. etli is found in alkaline pH (pH 9) soils (Shamseldin and Werner 2005), but also in acidic soils (Gutierrez and Barraquio 2010; Mostasso et al. 2001), where genetic diversity of the rhizobia was lower (Gutierrez and Barraquio 2010), and is consistent with the observation that there is a large amount of genetic variability among bean symbiotic bacteria (Rodriguez-Navarro et al. 2000).
Rhizobium leucaenae accounted for 20 % of the isolates, and it was distributed in San Agustín de Aurora (35°25′S–71°40′W), Ñiquén (36°19′S–72°20′W) and Santa Barbara (37°36′S–71°47′W) but centered mainly in Ñiquén (Fig. 6). This species has been characterized as persisting in soils with pH between 5 and 7 (Ribeiro et al. 2012), in several regions and ecosystems of Brazil (Grange and Hungria 2004; Martínez-Romero et al. 1991; Pinto et al. 2007), and establishing symbiosis with both Leucaene leucocephala and with Phaseolus vulgaris. It has also been reported nodulating beans in Mexico (Acosta-Durán and Martínez Romero 2002; Ribeiro et al. 2012). Meanwhile, R. tropici was found only in Ñiquén, representing only 6 % of the isolates. However, this soil type, Andisol type (Typic Haploxerands) and acidity pH (5.2), being the lowest on all analyzed locations (Table 1). The finding of this species in Chile follows the observations of several authors who correlate predominance of R. tropici strains nodulating beans in acid soils (Pinto et al. 2007; Andrade et al. 2002; Graham et al. 1994). However, it is noteworthy that although San Fernando soils are equally acidic (5.3), R. tropici was not detected. In our study areas, all soils had a xeric moisture regime (USDA 2003), implying that there would be water stress if irrigation is not available in the summer. Therefore, perhaps the most important factors regulating the distribution of Rhizobium species might be the water storage capacity and aeration conditions of the soil (Tajini et al. 2012), which tend to be better in Andisols (Luzio 2010).
Conclusions
Although only one species, R. leguminosarum, had been previously documented in Chile, our results indicate that there are at least four Rhizobium species associated with nodulating beans, R. leguminosarum, R. etli, R. tropici and R. leucaenae. Of these four species, R. leguminosarum was the most common and had the highest genetic observed diversity in areas with both acidic and alkaline soils of the central and south central regions of the country. Only two genetic groups of R etli we observed, but each was the most common haplotype observed in the two very contrasting soil and climatic zones that they were found. R. leucaenae and R. tropici demonstrated the least diversity R. leucaenae was documented in three locations with soils with pH fluctuating between 5.2 and 6, whereas R. tropici was most common only in acidic soils (pH 5.2) of volcanic origin located in the South Central Chile.
References
Acosta-Durán C, Martínez Romero E (2002) Diversity of rhizobia from nodules of the leguminous tree Gliricidia sepium, a natural host of Rhizobium tropici. Arch Microbiol 178:161–164
Aguilar OM, López MV, Riccillo PM, Gonzalez RA, Pagano M, Grasso DH, Pühler A, Favelukes G (1998) Prevalence of the Rhizobium etli-like allele in genes coding for 16S rDNA among the indigenous rhizobial populations found associated with wild beans from the Southern Andes in Argentina. Appl Environ Microbiol 64:3250–3524
Aguilar OM, López MV, Riccillo PM (2001) The diversity of rhizobia nodulating beans in Northwest Argentina as a source of more efficient inoculant strains. J Biotechnol 91:181–188
Aguilar OM, Riva O, Peltzer E (2004) Analysis of Rhizobium etli and of its symbiosis with wild Phaseolus vulgaris supports coevolution in centers of host diversification. Proc Natl Acad Sci 101:13548–13553
Amarger N, Bours M, Revoy F, Allard MR, Laguerre G (1994) Rhizobium tropici nodulates field-grown Phaseolus vulgaris in France. Plant Soil 161:147–156
Amarger N, Macheret V, Laguerre G (1997) Rhizobium gallicum sp. nov. y Rhizobium giardinii sp. nov., from Phaseolus vulgaris nodules. Int J Syst Bacteriol 47:996–1006
Andrade DS, Murphy PJ, Giller KE (2002) The diversity of Phaseolus-nodulating rhizobial populations is altered by liming of acid soils planted with Phaseolus vulgaris L. in Brazil. Appl Environ Microbiol 68:4025–4034
Anyango B, Wilson KJ, Beynon JL, Giller KE (1995) Diversity of rhizobia nodulating Phaseolus vulgaris L. in two Kenyan soils with contrasting pHs. Appl Environ Microbiol 61:4016–4021
Aserse AA, Rasanen LA, Assefa F, Hailemariam A, Lindström K (2012) Phylogeny and genetic diversity of native rhizobia nodulating common bean (Phaseolus vulgaris L.) in Ethiopia. Syst Appl Microbiol 35:120–131
Bala A and Giller KE (2007) Relationships between rhizobial diversity and host legume nodulation and nitrogen fixation in tropical ecosystems. Conference information: biannual meeting on Advances in Integrated Soil Fertility Management in Sub-Saharan Africa—challenges and opportunities, May 2004, Yaounde, Cameroon. Advances in Integrated Soil Fertility Management in Sub-Saharan Africa: challenges and opportunities, pp 691–702
Bala A, Murphy P, Giller KE (2001) Genetic diversity of rhizobia from natural populations varies with the soil dilution sampled. Soil Biol Biochem 33:841–843
Ballen KG, Graham PH, Jones RK, Bowers JH (1998) Acidity and calcium interaction affecting cell envelope stability in Rhizobium. Can J Microbiol 44:582–587
Bascur G, Tay J (2005) Colecta, caracterización y utilización de la variabilidad genética en germoplasma chileno de poroto (Phaseolus vulgaris L.). Agric Técnica 65:135–146
Bernal G, Graham P (2001) Diversity in the rhizobia associated with Phaseolus vulgaris L. in Ecuador, and comparisons with Mexican bean rhizobia. Can J Microbiol 47:526–534
Bernal G, Tlusty B, Estevez De Jensen C, Van Berkum P, Graham PH (2004) Characteristics of rhizobia nodulating beans in the central region of Minnesota. Can J Microbiol 50:1023–1031
Beyene D, Kassa S, Ampy F, Asseffa A, Gebremedhin T, van Berkum P (2004) Ethiopian soils harbor natural populations of rhizobia that form symbioses with common bean (Phaseolus vulgaris L.). Arch Microbiol 181:129–136
Caballero-Mellado J, Martínez-Romero E (1999) Soil fertilization limits the genetic diversity of Rhizobium in bean nodules. Symbiosis 26:111–121
CIREN (1996) Estudio agrológico Región Metropolitana. Descripciones de suelos materiales y símbolos, vol 115. Publicación, Santiago, p 425
CIREN (1997) Estudio agrológico VII Región. Descripciones de suelos materiales y símbolos, vol 117. Publicación. Santiago, p 659
García-Fraile P, Mulas-García D, Peix A, Rivas R, González-Andrés F, Velázquez E (2010) Phaseolus vulgaris is nodulated in northern Spain by Rhizobium leguminosarum strains harboring two nodC alleles present in American Rhizobium etli strains: biogeographical and evolutionary implications. Can J Microbiol 56:657–666
Gepts P, Debouck D (1991) Origin, domestication and evolution of the common bean (Phaseolus vulgaris L.). In: van Schoonhoven A, Voysest O (eds) Common beans, research for crop improvement. CAB, Wallingford, pp 7–53
Graham PH, Draeger KJ, Ferrey ML, Conroy MJ, Hammer BE, Martínez E, Aarons SR, Quinto C (1994) Acid pH tolerance in strains of Rhizobium and Bradyrhizobium, and initial studies on the basis for acid tolerance of Rhizobium tropici UMR1899. Can J Microbiol 40:198–207
Grange L, Hungria M (2004) Genetic diversity of indigenous common bean (Phaseolus vulgaris) rhizobia in two Brazilian ecosystems. Soil Biol Biochem 36:1389–1398
Grange L, Hungria M, Graham PH, Martínez-Romero E (2007) New insights into the origins and evolution of rhizobia that nodulate common bean (Phaseolus vulgaris) in Brazil. Soil Biol Biochem 39:867–876
Gutierrez R, Barraquio W (2010) Acid-Tolerant Rhizobia of Phaseolus vulgaris L. from the intensively cropped soils of La Trinidad, Benguet, Philippines. Philipp J Sci 139:79–90
Han TX, Wang ET, Wu LJ, Chen WF, Gu JG, Gu CT, Tian CF, Chen WX (2008) Rhizobium multihospitium sp. nov., isolated from multiple legume species native of Xinjiang, China. Int J Syst Evol Microbiol 58:1693–1699
Herrera-Cervera JA, Caballero-Mellado J, Laguerre G, Tichy HV, Requena N, Amarger N (1999) At least five rhizobial species nodulate Phaseolus vulgaris in a Spanish soil. FEMS Microbiol Ecol 30:87–97
Hungria M, Vargas MAT (2000) Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crops Res 65:151–164
Hungria M, Campo RJ, Carvalho I (2003) Benefits of inoculation of the common bean (Phaseolus vulgaris) crop with efficient and competitive Rhizobium tropici strains. Biol Fertil Soils 39:88–93
Jordan DC (1984) Family III. Rhizobiaceae Conn 1938. In: Krieg NR, Holt JG (eds) Bergey’s manual of systematic bacteriology. Williams and Wilkins, Baltimore, pp 234–235
Kaplan L (1965) Archeology and domestication in American Phaseolus (beans). Econ Bot 19:358–368
Laguerre G, Allard MR, Revoy F, Amarger N (1994) Rapid identification of rhizobia by restriction fragment length polymorphism analysis of PCR-amplified 16S rDNA genes. Appl Environ Microbiol 60:56–63
Laguerre G, Nour SM, Macheret V, Sanjuan J, Drouin P, Amarger N (2001) Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology 147:981–993
Lapinskas EB (2007) The effect of acidity on the distribution and symbiotic efficiency of rhizobia in Lithuanian soils. Eurasian Soil Sci 40:419–425
López-López A, Rogel-Hernández MA, Barois I, Ortiz Ceballos AI, Martínez J, Ormeño-Orrillo E, Martínez-Romero E (2012) Rhizobium grahamii sp. nov., from nodules of Dalea leporina, Leucaena leucocephala and Clitoria ternatea, and Rhizobium mesoamericanum sp. nov., from nodules of Phaseolus vulgaris, siratro, cowpea and Mimosa pudica. Int J Syst Evol Microbiol 62:2264–2271
Luzio W (2010) Suelos de Chile. Maval Impresores, Santiago, p 364
Maddison WP, Maddison DR (2014) Mesquite: a modular system for evolutionary analysis. Version 2.75, http://mesquiteproject.org
Martínez-Romero E (2003) Diversity of Rhizobium-Phaseolus vulgaris symbiosis: overview and perspectives. Plant Soil 252:11–23
Martínez-Romero E, Segovia L, Mercante FM, Franco AA, Graham P, Pardo MA (1991) Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int J Syst Bacteriol 41:417–426
Mhamdi R, Laguerre G, Elarbia M, Mars M, Amarger N (2002) Different species and symbiotic genotypes of feld rhizobia can nodulate Phaseolus vulgaris in Tunisian soils. FEMS Microbiol Ecol 41:77–84
Mostasso L, Mostasso FL, Dias BG, Vargas MAT, Hungria M (2001) Selection of bean (Phaseolus vulgaris L.) rhizobial strains for the Brazilian Cerrados. Field Crops Res 73:121–132
Padarian J, Pérez-Quezada J, Seguel O (2012) Modelling the distribution of organic carbón in the soils of Chile. In: Minasny B, Malone BP, McBratney AB (eds) Proceeding of the fifth global workshop on digital soil mapping. Digital Soil assessments and beyond, Sydney pp 329–333
Page RDM (1996) TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Pérez-Ramírez NO, Rogel MA, Wang E, Castellanos JZ, Martínez-Romero E (1998) Seeds of Phaseolus vulgaris bean carry Rhizobium etli. FEMS Microbiol Ecol 26:289–296
Pinto F, Hungria M, Mercante F (2007) Polyphasic characterization of Brazilian Rhizobium tropici strains effective in fixing N2 with common bean (Phaseolus vulgaris L.). Soil Biol Biochem 39:1851–1864
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Ramirez-Bahena MH, Garcia-Fraile P, Peix A, Valverde A, Rivas R, Igual JM, Mateos PF, Martinez-Molina E, Velazquez E (2008) Revision of the taxonomic status of the species Rhizobium leguminosarum (Frank 1879) Frank 1889AL, Rhizobium phaseoli Dangeard 1926AL and Rhizobium trifolii Dangeard 1926AL. R. trifolii is a later synonym of R. leguminosarum. Reclassification of the strain R. leguminosarum DSM 30132 (¼NCIMB 11478) as Rhizobium pisi sp. nov. Int J Syst Evol Microbiol 58:2484–2490
Ribeiro RA, Rogel MA, López-López A, Ormeño-Orrillo E, Gomes Barcellos F, Martinez J, Lopes Thompson F, Martínez-Romero E, Hungria M (2012) Reclassification of Rhizobium tropici type A strains as Rhizobium leucaenae sp. nov. Int J Syst Evol Microbiol 62:1179–1184
Ribeiro RA, Ormeño-Orrillo E, Fuzinatto Dall’Agnol R, Graham PH, Martinez-Romero E, Hungria M (2013) Novel Rhizobium lineages isolated from root nodules of the common bean (Phaseolus vulgaris L.) in Andean and Mesoamerican areas. Res Microbiol. doi:10.1016/j.resmic.2013.05.002
Rodriguez-Navarro DN, Buendia AM, Camacho M, Lucas MM, Santamaría C (2000) Characterization of Rhizobium spp. Bean isolates from south-west Spain. Soil Biol Biochem 32:1601–1613
Rohlf F (1998) NTSYS—PC numerical taxonomy and multivariate analysis system, version 2.02. Exeter, Setauket. p 142
Sadzawka A, Carrasco M, Grez R, Mora M (2004) Métodos de análisis recomendados para los suelos chilenos. Comisión de Normalización y Acreditación. Sociedad Chilena de la Ciencia del Suelo, Santiago, p 113
Segovia L, Piñero D, Palacios R, Martínez-Romero E (1991) Genetic structure of a soil population of nonsymbiotic Rhizobium leguminosarum. Appl Environ Microbiol 57:426–433
Segovia L, Young J, Martinez-Romero E (1993) Reclassification of American Rhizobium leguminosarum sv. phaseoli type I strains as Rhizobium etli sp. nov. Int J Syst Evol Bacteriol 43:374–377
Sessitsch G, Hardarson A, Arkermens ADL, De Vos WM (1997) Characterization of Rhizobium etli and other Rhizobium spp. that nodulate Phaseolus vulgaris L. in an Austrian soil. Mol Ecol 6:601–608
Shamseldin A, Werner D (2005) High salt and high pH tolerance of new isolated Rhizobium etli strains from Egyptian soils. Curr Microbiol 50:11–16
Silva F, Hungría M, Martins F (2007) Polyphasic characterization of Brazilian Rhizobium tropici strains effective in fixing N2 with common bean (Phaseolus vulgaris L.). Soil Biol Biochem 39:1851–1864
Souza V, Bain J, Silva C, Bouchet V, Valera A, Marquez E, Eguiarte LE (1997) Ethnomicrobiology: do agricultural practises modify the population structure of the nitrogen fixing bacteria Rhizobium etli biovar. phaseoli. J Ethnobiol 17:249–266
Stocco P, Santos JCP, Vargas VP, Hungria M (2008) Avaliação da biodiversidade de rizobios simbiontes do feijoeiro (Phaseolus vulgaris L.) em Santa Catarina. Rev Bras Ci Solo 31:1–9
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAl_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tajini F, Trabelsi M, Drevon JJ (2012) Comparison between the reference Rhizobium tropici CIAT899 and the native Rhizobium etli 12a3 for some nitrogen fixation parameters in common bean (Phaseolus vulgaris L.) under water stress. African J Microbiol Res 6:4058–4067
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Uribe JM, Cabrera R, de la Fuente A, Paneque M (2012) Atlas bioclimático de Chile. Universidad de Chile, Santiago, p 232
Urzúa H (2005) Beneficios de la fijación simbiótica de nitrógeno en Chile. Cien. Inv Agr 32(2):133–150
Urzúa H, Tesser B (1998) Efecto de características químicas de suelos aluviales de la zona central de Chile en la concentración de ADN plasmidial de cepas de Rhizobium leguminosarum sv. phaseoli. Cien Inv Agr 25:185–187
USDA (2003) Keys to soil taxonomy, 9th edn. Natural Resources Conservation Service, Soil Survey Division Staff, Washington, DC, USA, p 332
Valverde A, Igual JM, Peix A, Cervantes E, Vela´zquez E (2006) Rhizobium lusitanum sp. nov. a bacterium that nodulates Phaseolus vulgaris. Int J Syst Evol Microbiol 56:2631–2637
Vásquez-Arroyo J, Sessitsch A, Martínez E, Peña-Cabriales JJ (1998) Nitrogen fixation and nodule occupancy by native strains of Rhizobium on different cultivars of common bean (Phaseolus vulgaris L.). Plant Soil 204:147–154
Velázquez E, Palomo JL, Rivas R, Guerra H, Peix A, Trujillo ME, García-Benavides P, Mateos PF, Wabiko H, Martínez-Molina E (2010) Analysis of core genes supports the reclassification of strains Agrobacterium radiobacter K84 and Agrobacterium tumefaciens AKE10 into the species Rhizobium rhizogenes. Syst Appl Microbiol 33:247–251
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. Blackwell Scientific, Oxford, p 15
Wang FQ, Wang ET, Wu LJ, Sui XH, YL Jr, Chen WX (2011) Rhizobium vallis sp. nov., isolated from nodules of three leguminous species. Int J Syst Evol Microbiol 61:2582–2588
Young J, Downer L, Eardly D (1991) Phylogeny of the phototrophic Rhizobium strain BTAil by polymerase chain reaction-based sequencing of a 16S rDNA gene segment. J Bacteriol 173:2271–2277
Acknowledgments
We thank the staff from the “Departamento de Biotecnología E.T.S. Ingenieros Agrónomos, Universidad Politécnica de Madrid” and the “Centro de Investigación y Formación Agraria—Las Torres” for providing the reference strains. Also the authors thank the “Dirección de Investigación - Universidad de Chile”, for financing this study under Project DI REIN 04/05.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
About this article
Cite this article
Baginsky, C., Brito, B., Scherson, R. et al. Genetic diversity of Rhizobium from nodulating beans grown in a variety of Mediterranean climate soils of Chile. Arch Microbiol 197, 419–429 (2015). https://doi.org/10.1007/s00203-014-1067-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-014-1067-y