Abstract
Uridine triphosphate (UTP)-glucose-1-phosphate uridylyltransferase (GalU; EC 2.7.7.9) is an enzyme that catalyzes the formation of uridine diphosphate (UDP)-glucose from UTP and glucose-1-phosphate. GalU is involved in virulence in a number of animal-pathogenic bacteria since its product, UDP-glucose, is indispensable for the biosynthesis of virulence factors such as lipopolysaccharide and exopolysaccharide. However, its function in Xanthomonas campestris pv. campestris, the phytopathogen that causes black rot in cruciferous plants, is unclear. Here, we characterized a galU mutant of X. campestris pv. campestris and showed that the X. campestris pv. campestris galU mutant resulted in a reduction in virulence on the host cabbage. We also demonstrated that galU is involved in bacterial attachment, cell motility, and polysaccharide synthesis. Furthermore, the galU mutant showed increased sensitivity to various stress conditions including copper sulfate, hydrogen peroxide, and sodium dodecyl sulfate. In addition, mutation of galU impairs the expression of the flagellin gene fliC as well as the attachment-related genes xadA, fhaC, and yapH. In conclusion, our results indicate involvement of galU in the virulence factor production and pathogenicity in X. campestris pv. campestris, and a role for galU in stress tolerance of this crucifer pathogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uridine triphosphate (UTP)-glucose-1-phosphate uridylyltransferase (GalU; EC 2.7.7.9), which is also called uridine diphosphate (UDP)-glucose pyrophosphorylase, is an enzyme responsible for the production of UDP-glucose from UTP and glucose-1-phosphate. UDP-glucose is required to synthesize different surface structures, exopolysaccharide (EPS), and lipopolysaccharide (LPS) (Jiang et al. 2010). Since GalU is involved in producing glycosyl donors for EPS and LPS biosynthesis, the enzyme is essential for virulence in many bacterial pathogens, such as Actinobacillus pleuropneumoniae (Rioux et al. 1999), Aeromonas hydrophila (Vilches et al. 2007), Klebsiella pneumonia (Chang et al. 1996), Proteus mirabilis (Jiang et al. 2010), Pseudomonas aeruginosa (Priebe et al. 2004), Pseudomonas syringae (Deng et al. 2010), and Vibrio cholerae (Nesper et al. 2001).
Xanthomonas is a large genus of Gram-negative bacteria that cause disease in hundreds of plant hosts, including many economically important crops (Ryan et al. 2011). Xanthomonas species produce a characteristic EPS, xanthan, which leads to the mucoid appearance of the bacterial colonies (Buttner and Bonas 2010). A putative GalU encoding gene was found in the complete genome sequence of several Xanthomonas species, such as X. albilineans (Pieretti et al. 2009), X. axonopodis pv. citri (synonyms, X. citri pv. citri, and X. citri subsp. citri) (da Silva et al. 2002), X. axonopodis pv. citrumelo (Jalan et al. 2011), X. campestris pv. campestris (da Silva et al. 2002; Qian et al. 2005; Vorholter et al. 2008), X. campestris pv. raphani (Bogdanove et al. 2011), and X. campestris pv. vesicatoria (Thieme et al. 2005), X. oryzae pv. oryzae (Lee et al. 2005; Salzberg et al. 2008), and X. oryzae pv. oryzicola (Bogdanove et al. 2011). To date, only three reports were found in the literature regarding Xanthomonas GalU. First, it was indicated that the gene encoding X. campestris pv. campestris GalU could restore a non-mucoid mutant isolated from X. campestris pv. campestris by Tn5 mutagenesis to mucoid phenotype (Wei et al. 1996). Second, the GalU encoding sequence in X. campestris pv. campestris and X. axonopodis pv. citri was cloned and overexpressed in Escherichia coli, and the recombinant GalU proteins were purified and characterized (Bosco et al. 2009). Third, the role of the galU gene in the virulence of X. axonopodis pv. citri was evaluated, and it was found that the galU gene is required for EPS production and pathogenicity in X. axonopodis pv. citri (Guo et al. 2010).
X. campestris pv. campestris is the causative agent of black rot in crucifers, a disease that causes tremendous agricultural losses (Williams 1980). The virulence of X. campestris pv. campestris toward plants depends on a number of factors, including the ability to produce EPS and LPS, secrete several extracellular enzymes (such as cellulase, and mannanase), and cell motility (Dow and Daniels 1994; Dow et al. 1995; Chan and Goodwin 1999; Dow et al. 2003; McCarthy et al. 2008; Buttner and Bonas 2010). Although a previous study indicated that inactivation of galU by transposon mutagenesis revealed a non-mucoid phenotype and the cloned galU gene responsible for the mucoid phenotype restoration (Wei et al. 1996), no other biological functions were examined. The aim of the current work was to further characterize galU and to gain insights into its additional biological functions in X. campestris pv. campestris. The presented data indicated that GalU plays a role in bacterial attachment, cell motility, EPS and LPS synthesis, pathogenicity, and stress tolerance.
Materials and methods
Bacterial strains, plasmids, media, and culture conditions
E. coli DH5α (Hanahan 1983) served as the host for DNA cloning. X. campestris pv. campestris strain Xcc17 was a virulent wild-type strain isolated in Taiwan (Yang and Tseng 1988). The wzt mutant was Xcc17-derived mutant with EZ-Tn5 inserted in wzt gene collected in our laboratory. The putative function of wzt gene product is the ATP-binding component of an ABC-transporter that specifically exports surface polysaccharides (Vorholter et al. 2001). Luria-Bertani (LB) medium (Miller 1972) was the general-purpose medium for cultivating E. coli and X. campestris pv. campestris at 37 and 28 °C, respectively. The compositions of XOLN and XVM2 media are given elsewhere (Fu and Tseng 1990; Wengelnik et al. 1996). The following antibiotics were added when necessary: ampicillin (50 μg/ml), gentamycin (15 μg/ml), and tetracycline (15 μg/ml). Liquid cultures were shaken at 220 rpm. Solid media contained 1.5 % agar.
DNA techniques
Enzymes were purchased from Promega and Roche. Standard protocols have been described elsewhere (Sambrook et al. 1989). Polymerase chain reaction (PCR) was carried out as previously described (Hsiao et al. 2005). DNA sequences were determined by Mission Biotech Co., Ltd. (Taipei, Taiwan). Transformation of E. coli was performed by the standard method (Sambrook et al. 1989) and that of X. campestris pv. campestris by electroporation (Wang and Tseng 1992).
Construction of galU mutant
A galU mutant was constructed by insertional mutagenesis of Xcc17 and designated as SXG17. For the insertion, the 438-bp fragment internal to the Xcc17 galU gene was PCR amplified using primers MTF (5′-CGAGCGCGCAGGCAAGCTCG-3′) and MTR (5′-GCGCCCGTGGACTCCAGGTACT-3′) and cloned into pUC19G (Yen et al. 2002). The resultant plasmid, pUCgalU, was electroporated into Xcc17 allowing for homologous recombination through the identical regions in the chromosome and the plasmid by a single crossover. Insertion of pUCgalU into galU was confirmed by PCR.
Complementation of galU mutant
The 982-bp PstI-XbaI fragment encompassing the upstream 87-bp fragment plus the entire coding region of the Xcc17 galU was PCR amplified using primers CMF (5′-CTGCAGCCGGATTTTGCGGCTGCT-3′; PstI site underlined) and CMR (5′-TCTAGACTCAGCCGCGTGCGTCGG-3′; XbaI site underlined) and cloned into the PstI-XbaI sites of the broad-host-range vector pRK415 (Keen et al. 1988), generating pRKgalU. This construct contained the galU gene in the downstream and orientated in the same direction as the lac promoter. For complementation of galU mutant, plasmid pRKgalU was electroporated into the mutant SXG17.
Pathogenicity test
The virulence of X. campestris pv. campestris in cabbage was estimated after bacteria were introduced into the leaves by the leaf-clipping method (Hsiao et al. 2011). Lesion lengths were measured 14 days post-inoculation. Three independent experiments with six replicates each were carried out.
EPS production assay and LPS analysis
The levels of EPS were measured as described previously (Hsiao et al. 2011) with a minor modification. Briefly, bacterial cells from an overnight culture were diluted 50-fold into fresh LB broth and grown at 28 °C for 24 h. The EPS in the supernatant was precipitated by addition of two volumes of ethanol (95 %), and the mixtures were kept at −20 °C for 1 h. The precipitated EPS was centrifuged at 12,000 g for 10 min and dried at 65 °C in an oven overnight before determination of dry weights. LPS was isolated according to a previously described method (Nesper et al. 2000) with minor modifications. Bacteria were grown overnight in LB medium at 28 °C. Five-milliliter overnight cultures were centrifuged at 12,000 g at 4 °C for 2 min. The collected cells were washed in 1 ml of TNE (10 mM Tris–HCl, pH 8, 10 mM NaCl, 10 mM EDTA) and resuspended in 540 μl of TNEX (TNE–1 % Triton X-100). Fifteen microliter of lysozyme (20 mg/ml) was added, and the mixture was incubated for 20 min at 37 °C. Following lysozyme digestion, the mixture was treated with 60 μl protease K (10 mg/ml) overnight at 55 °C to obtain the LPS samples. The isolated LPS was resolved by Tricine sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Lesse et al. 1990) and visualized by silver stain following the manufacturer’s instructions (Bio-Rad). Standard LPS from Salmonella enterica serovar Typhimurium was purchased from Sigma.
Plate assay for extracellular enzyme activity
Assays of the activities of two extracellular enzymes (cellulase and mannanase) were performed on agar plates containing the appropriate substrate according to published methods (Hsiao et al. 2011) with minor modifications. Briefly, 3 μl of overnight culture (OD550 = 1) was deposited onto the surface of LB plates containing carboxymethyl cellulose (0.5 %) or locust bean gum (0.2 %). After 2 days of incubation, enzyme activity was measured as described previously (Hsiao et al. 2011).
Motility and attachment assay
To test the motility, 3 μl of overnight culture (OD550 = 1) was deposited onto the surface of XOLN plates (0.3 % agar). The diameter of the migration ring was measured after 2 days of incubation at 28 °C. Bacterial adhesion under static conditions was quantified by a crystal violet incorporation assay with polystyrene plates according to a previously described method (Hsiao et al. 2012). Briefly, overnight culture of each strain was diluted with XOLN medium containing 2 % glucose. Then, 150 μl of this diluted culture (OD550 = 0.1) was pipetted into the wells of polystyrene 96-well flat-bottom microtiter plates (Nunc), which were then allowed to stand for 24 h at 28 °C. Cells that attached to the surface of the wells were measured by removing the medium, rinsing the wells with 180 μl distilled water (three times), and staining attached bacteria with 180 μl 0.1 % (w/v) crystal violet at room temperature for 5 min. Then, free dye was removed, and the wells were rinsed three times with distilled water. The dye incorporated in attached cells was solubilized in 200 μl ethanol (95 %) and the OD595 was determined.
Stress tolerance assays
Strains were grown in XVM2 media (OD550 = 0.35) in the presence or absence of one type of stress condition, and growth of each strain was evaluated by measuring OD550 after growth for 24 h at 28 °C with shaking at 200 rpm. The stress conditions were as follows: for copper sulfate treatment, 0.05 mM CuSO4 was added; for oxidative stress, 0.003 % H2O2 was added; for high osmolarity, 0.3 M NaCl was added; for SDS stress, 0.01 % SDS was added.
Promoter activity assay
Reporter constructs containing the upstream region of flagellar gene fliC and attachment-related genes xadA, fhaC, and yapH were obtained as previously described (Hsiao et al. 2012; Liu et al. 2013). Briefly, the upstream regions of these genes were PCR amplified and cloned into the upstream of the promoter-less lacZ gene in the promoter-probing vector pFY13–9 (Lee et al. 2001), giving pFYfliC, pFYxadA, pFYfhaC, and pFYyapH. These reporter constructs were electroporated separately into X. campestris pv. campestris wild-type Xcc17 and galU mutant SXG17. X. campestris pv. campestris strains harboring these constructs were grown overnight and inoculated into fresh media to obtain an initial OD550 of 0.35, after which growth was allowed to continue. Samples were taken in triplicate at 6 h, and the β-galactosidase activity was assayed as previously described, with the enzyme activity expressed in Miller units (Miller 1972).
Sequence alignment
Multiple sequence alignment was generated by use of the clustalx package, with the default protein weight matrix (standard point accepted mutation series).
Statistical analysis
Values are the means of three technical replicates per experiment, and each experiment was performed at least three times. A nonparametric Kruskal–Wallis test followed by a Tukey HSD test was used to determine the statistical significance of differences between means. All statistical analyses were performed using the SPSS statistical software program (version 17.0; SPSS, Inc., Chicago, IL).
Results and discussion
Characteristics of the X. campestris pv. campestris galU gene
To date, complete genomic sequences of three X. campestris pv. campestris strains (ATCC33913, 8004, and B100) have been deposited in a public database (da Silva et al. 2002; Qian et al. 2005; Vorholter et al. 2008). A survey of the genome sequence data of the X. campestris pv. campestris revealed that one galU gene is annotated in the fully sequenced genomes of these three different X. campestris pv. campestris strains. The open reading frame number of galU gene in strains ATCC33913, 8004, and B100 is XCC2188, XC_1930, and xccb100_1992, respectively (Table S1) (da Silva et al. 2002; Qian et al. 2005; Vorholter et al. 2008). There is also a homologue, XC861, in the genome sequence of strain Xcc17 (a draft genome) (http://xcc.life.nthu.edu.tw). The Xcc17 GalU shared 99.7, 99.7, and 100 % identity with homologues from ATCC33913, 8004, and B100, respectively. A database search revealed that GalU is highly conserved in several sequenced Xanthomonas species, such as X. axonopodis, X. campestris, and X. oryzae, with >80 % amino acid identity (Table S1).
From database searches, the deduced amino acid sequence of X. campestris pv. campestris GalU was found to be, among structurally characterized proteins, the most similar to GalU from Sphingomonas elodea (PDB code 2UX8) (Aragao et al. 2007), with 53 % identity and 68 % similarity. In addition, it also shared over 40 % identity with several crystalized GalU proteins from other bacteria, such as Helicobacter pylori (PDB code 3JUJ) (Kim et al. 2010), E. coli (PDB code 2E3D) (Thoden and Holden 2007b), and Corynebacterium glutamicum (PDB code 2PA4) (Thoden and Holden 2007a). Multiple sequence alignment revealed that several residues involved in enzyme activity described by crystallographic or mutagenetic data are well conserved in X. campestris pv. campestris GalU (Figure S1). When compared with the crystallographic data from S. elodea GalU, it is revealed that: (1) Asp135, Gly174, Glu193, and Val206 form hydrogen bonds with glucose-1-phosphate; (2) Ala13, Gly14, Gln 106, Gly111, and Asp 134 form hydrogen bonds with nucleotide; and (3) Leu112, Leu132, Tyr210, and Leu233 constitute a hydrophobic cap to the base of the sugar ring at the catalytic cavity. Catalytic residue Arg15 (numbering as in the H. pylori GalU) has been confirmed to be essential for enzyme activity by mutagenetic analysis (Kim et al. 2010). It is also conserved in the sequence of GalU proteins aligned and is situated at Arg18 in X. campestris pv. campestris GalU (Figure S1).
The galU gene is essential for full virulence of X. campestris pv. campestris
Although bioinformatics analysis revealed several orthologous galU genes in Xanthomonas species, only the galU genes from X. axonopodis pv. citri and X. campestris pv. campestris have been biochemically characterized to encode a functional UTP-glucose-1-phosphate uridylyltransferase (Bosco et al. 2009). Recently, the galU gene from X. axonopodis pv. citri has been functionally evaluated (Guo et al. 2010). In X. campestris pv. campestris, it is only known that a recombinant clone, pEK135, has an insert of 3.0-kb KpnI-EcoRI fragment harboring a complete galU gene and was able to restore the non-mucoid mutant G76E (Wei et al. 1996). To explore the physiological role of galU in X. campestris pv. campestris, a galU mutant was constructed by mutagenizing the wild-type strain Xcc17 using homologous recombination (see Materials and methods section for details). The obtained mutants were designated as SXG17. A complemented strain, named SXG17(pRKgalU), was simultaneously created by introducing the galU-expression plasmid pRKgalU into the mutant strain SXG17.
In many animal-pathogenic bacteria, such as A. pleuropneumoniae (Rioux et al. 1999), A. hydrophila (Vilches et al. 2007), K. pneumonia (Chang et al. 1996), P. mirabilis (Jiang et al. 2010), P. aeruginosa (Priebe et al. 2004), and V. cholerae (Nesper et al. 2001), mutation in galU caused attenuated virulence. Comparatively little is known regarding the role of the galU gene in the virulence of plant-pathogenic bacteria. Only galU genes from P. syringae (a necrotizing plant pathogen) and X. axonopodis pv. citri (a causal agent of citrus canker) have been reported to play a role in pathogenicity (Deng et al. 2010; Guo et al. 2010). Nothing is known about the role of galU in X. campestris pv. campestris virulence. To investigate the association between galU and pathogenicity of X. campestris pv. campestris, the virulence of the mutant was tested on host plant cabbage by the leaf-clipping method (Hsiao et al. 2011). Fourteen days post-inoculation, no obvious black rot symptoms were observed on the leaves inoculated with galU mutant SXG17, while typical “V”-shape black rot symptoms were observed on leaves inoculated with wild-type Xcc17 (Fig. 1). Introduction of the cloned gene into the galU mutant restored virulence to wild-type level (Fig. 1). The mean lesion lengths were 1.90 ± 0.13 and 1.94 ± 0.17 cm, respectively, for Xcc17 (wild type) and SXG17(pRKgalU) (complemented) at 14 days after inoculation. The mean lesion lengths caused by the complemented strain and the wild-type strain were not significantly different (P = 0.673). These results demonstrated that galU is required for virulence of X. campestris pv. campestris. X. campestris pv. campestris is a vascular pathogen mainly gains entry into plants via hydathodes at the leaf margins or through wounds. Leaf-clipping method introduces the bacteria directly into the vascular system. Whether different inoculation techniques possess different lesion lengths remain to be evaluated.
Effect of mutation of galU on virulence of X. campestris pv. campestris to cabbage. X. campestris pv. campestris wild type carrying empty broad-host-range vector pRK415 and the complemented galU mutant strains caused black rot symptoms when the host plant cabbage was inoculated, while the galU mutant evoked no obvious disease symptoms. Photographs were taken on day 14 post-inoculation
The lack of pathogenicity of the galU mutant might result from its inability to grow in planta. It has been reported that the growth of galU mutant of X. axonopodis pv. citri was significantly reduced in grapefruit leaves (Guo et al. 2010) and mutation in galU of P. syringae reduces bacterial multiplication in susceptible bean plants (Deng et al. 2010). Whether similar situation exists in X. campestris pv. campestris galU mutant remains to be determined.
The galU gene is involved in polysaccharide biosynthesis in X. campestris pv. campestris
In Xanthomonas species, it is indicated that successful infection and bacterial multiplication in the host tissue depend on a number of virulence factors, such as EPS, LPS, and extracellular degradative enzymes (Buttner and Bonas 2010; Ryan et al. 2011). The results showing the galU gene is essential for pathogenicity suggested that the ability of SXG17 to produce these virulence factors might have been impaired.
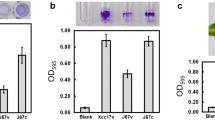
Compared to the wild type, the colony of SXG17 showed a much drier surface, indicating that the EPS synthesis was decreased (Fig. 2a). After EPS quantification, the result clearly showed that the EPS productivity was about 1.57 mg/ml for Xcc17 and 0.87 mg/ml for SXG17 grown in LB media (Fig. 2a) (P < 0.001). Complementation of SXG17 with plasmid to express full-length GalU restored the mucoid phenotype to that of the wild type and restored the EPS production to a near-wild-type level (1.54 mg/ml) (Fig. 2a) (P = 0.847). Similar situations are observed in X. axonopodis pv. citri, in which the galU gene is involved in EPS biosynthesis and the galU mutant is less viscous (Guo et al. 2010). The galU mutant of K. pneumonia also revealed a nonmucoid colony morphology (Chang et al. 1996).
Effect of mutation of galU on polysaccharide synthesis. a EPS production in LB medium by wild-type Xcc17 and its derivatives. Values presented are the means ± standard deviations from three repeats. Asterisk indicates significance at P < 0.001. b Analysis of LPS extracted from X. campestris wild-type and mutant strains. The LPS produced by wild-type Xcc17 and its derivatives were extracted, subjected to Tricine SDS-PAGE analysis, and visualized by silver staining. LPS of Salmonella enterica serovar Typhimurium was used as a standard. Bands I and II represent O-antigen containing LPS and core oligosaccharide, respectively. The arrows denote new bands in the mutants. The experiments were repeated three times with similar results, and the results of only one experiment are presented
The effect of a galU mutation on the production of extracellular degradative enzymes was evaluated by a substrate-supplementary plate assay. The diameters of the colonies formed by different cells on the same plate were similar. No significant differences in the activities of extracellular enzymes, including cellulase and mannanase were observed between Xcc17 and SXG17 (data not shown). In X. axonopodis pv. citri, three genes encoding cell wall degrading enzymes (XAC0028, pelB, and XAC0165) were significantly downregulated at the transcriptional level in galU mutant compared to the wild-type strain (Guo et al. 2010). The encoding enzyme products are cellulase for XAC0028, pectate lyase II for pelB, and arabinosidase for XAC0165 (da Silva et al. 2002). In X. campestris pv. campestris genome, such homologues are present with gene numbers XCC0026, XCC2815, and XCC0149 in strain ATCC33913 (da Silva et al. 2002). The involvement in virulence of these genes is still unknown. Whether a galU mutation has any impact on the expression of these genes requires further study.
To investigate the role of X. campestris pv. campestris galU gene in LPS production, LPS was isolated, analyzed by Tricine SDS-PAGE followed by silver staining, and was compared with LPS from X. campestris pv. campestris wild-type Xcc17, as well as with LPS from a wzt mutant in which the biosynthesis of LPS was impaired (Vorholter et al. 2001). As shown in Fig. 2b, (1) the LPS of the galU mutant migrated similarly to that of wzt mutant and showed an altered LPS pattern compared with Xcc17; (2) the LPS production of the complemented strain was similar to that of the wild-type strain. These results indicated that galU is involved in LPS synthesis of X. campestris pv. campestris. In previous studies, disruption of the galU gene of several bacteria affected the LPS profile compared with that of the wild type, such as A. pleuropneumoniae (Rioux et al. 1999), A. hydrophila (Vilches et al. 2007), E. coli (Genevaux et al. 1999), K. pneumonia (Chang et al. 1996), P. mirabilis (Jiang et al. 2010), P. aeruginosa (Priebe et al. 2004), P. syringae (Deng et al. 2010), and V. cholerae (Nesper et al. 2001). However, this is not the case in X. axonopodis pv. citri, in which the LPS pattern of the galU mutant strain was indistinguishable from that of the wild-type strain (Guo et al. 2010), suggesting that the function of GalU may differ between these two Xanthomonas species.
Cell motility is reduced after galU mutation
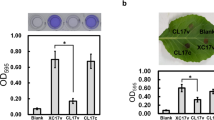
The galU mutation also drastically affected the cell motility of several bacteria, such as E. coli (Komeda et al. 1977; Genevaux et al. 1999), P. mirabilis (Jiang et al. 2010), and P. syringae (Deng et al. 2010). To test whether a mutation in galU has any effect on the cell motility of X. campestris pv. campestris, the galU mutant was evaluated for the mobile ability on 0.3 % agar plate. The results showed that the motility zone diameters of SXG17(pRK415) exhibited a significant decrease (0.74 cm) when compared with Xcc17(pRK415) (1.27 cm) (Fig. 3a) (P < 0.001). In SXG17 with cloned galU, SXG17(pRKgalU), wild-type-level motility (1.33 cm) was restored (Fig. 3a). These results indicated that the GalU of X. campestris pv. campestris is implicated in bacterial motility.
Effect of mutation of galU on cell motility (a) and bacteria attachment (b). a The cell motility was evaluated using XOLN medium supplemented with 0.3 % agar plate. Scale bars = 1 cm. Similar results were obtained at least three times. The diameter of the motility zone of each strain was measured after 2 days of incubation at 28 °C. Values presented are the means ± standard deviations from three repeats. Asterisk indicates significance at P < 0.001. b Cells were grown in XOLN medium supplemented with glucose in 96-well polystyrene microtiter plates and incubated at 28 °C for 24 h. After crystal violet staining, attached cells were quantified by solubilizing the dye in ethanol and measuring the absorbance at 595 nm. Values presented are the means ± standard deviations from three repeats. Asterisk indicates significance at P < 0.001
Mutation of galU affects the attachment of X. campestris pv. campestris to polystyrene
The galU gene is involved in bacterial attachment in several bacteria, such as E. coli (Genevaux et al. 1999), Haemophilus parasuis (Zou et al. 2013), P. syringae (Deng et al. 2010), V. cholerae (Nesper et al. 2001), and X. axonopodis pv. citri (Guo et al. 2010). To test the impact of galU mutation in X. campestris pv. campestris adhesion, bacterial attachment was examined in minimal XOLN medium plus 2 % glucose and analyzed by crystal violet staining. The galU mutant SXG17(pRK415) exhibited significant reduction in bacterial attachment on a polystyrene surface compared with that of the wild type, where the level of adherence was approximately 75 % of the wild-type level (Fig. 3b) (P < 0.001). The complementary strain SXG17(pRKgalU) was restored to levels similar to those of the wild-type strain (Fig. 3b). These findings indicated that the galU gene is involved in bacterial attachment in X. campestris pv. campestris.
The galU gene is involved in the tolerance of X. campestris pv. campestris to various stresses
Experimental evidence suggests that EPS not only acts as a virulence factor but also suppresses basal plant defense responses and protect bacteria against environmental stress (Buttner and Bonas 2010). Furthermore, mutations in LPS gene clusters render the bacteria more susceptible against harsh environmental conditions (Buttner and Bonas 2010). The observations that mutation of galU resulted in reduced EPS synthesis and altered LPS pattern suggested that this gene might play a role in environmental stress tolerance. To verify whether galU plays a role in environmental stress tolerance of X. campestris pv. campestris, the growth of wild-type Xcc17(pRK415), galU mutant SXG17(pRK415), and complementary strain SXG17(pRKgalU) under different stress conditions was evaluated. As shown in Fig. 4, the galU mutant revealed a remarkable growth reduction with 30.0, 49.6, 79.5, and 35.9 % of growth retained in XVM2 supplemented with CuSO4, H2O2, NaCl, and SDS, respectively, compared to wild type under the same condition. These phenotypic changes were restored in the complemented strain (Fig. 4). These data indicated that the galU mutant was more sensitive to these stresses than the wild type. The involvement of galU in stress tolerance has been studied in P. mirabilis (Jiang et al. 2010), P. syringae (Deng et al. 2010), and V. cholerae (Nesper et al. 2001). The P. mirabilis and V. cholerae galU mutant is more sensitive to SDS compared to the wild-type strain (Nesper et al. 2001; Jiang et al. 2010). The P. syringae galU mutant is more sensitive to H2O2 than the wild type (Deng et al. 2010).
Mutation of galU in X. campestris pv. campestris changes the transcriptional expression of certain motility and attachment-related genes
Reduction in cell motility and attachment could result from downregulation of genes related to these phenotypes. In X. campestris pv. campestris, flagellin gene fliC is essential for motility and flagellar biogenesis (Lee et al. 2003). The adhesion ability of X. campestris pv. campestris to surfaces has not been previously studied. In other Xanthomonas species, mutations in genes encoding XadA, YapH, and FhaC show altered adherence abilities (Darsonval et al. 2009; Das et al. 2009; Gottig et al. 2009). In X. campestris pv. campestris genomes, such homologues are present. To test the involvement of galU in the expression of these genes in Xcc17, a reporter assay was performed to analyze their expression in SXG17 using Xcc17 for comparison. The results showed that the promoter activity of fliC, xadA, fhaC, and yapH in SXG17 was decreased to 74, 29, 91, and 52 %, respectively, of that in Xcc17 (Table 1). These results suggested that the transcription level of the genes encoding flagellin (fliC), the outer membrane adhesin (xadA), the outer membrane hemolysin activator protein (fhaC), and the YapH protein (yapH) was reduced after galU mutation.
Although the galU gene has a role in cell motility in several bacteria, the effect of galU mutation on flagellin synthesis is different (Komeda et al. 1977; Deng et al. 2010; Jiang et al. 2010). In E. coli, it is indicated that the galU mutant is nonmotile and has much reduced amounts of flagellin and flagellin-specific mRNA (Komeda et al. 1977). In P. mirabilis, the galU mutant is defective in swarming motility and synthesized lower level of flagellin than did the wild-type strain, and synthesized a smaller amount of mRNA of flhDC, which is a master regulator controlling the expression of flagellum genes (Jiang et al. 2010). However, the P. syringae galU mutant has marked reduction in motility, whereas the amount of flagellins in the total flagellin fractions of both wild-type and mutant strains was equal (Deng et al. 2010). The observation showing fliC expression is reduced after galU mutation in this study is similar to the case of E. coli. In addition to fliC, the genes rpoN2, fleQ, fliA, flhA, flhB and flgM are essential for motility and normal flagellar biogenesis in X. campestris pv. campestris (Liu et al. 2013). Whether GalU has a role in FliC protein synthesis and in the expression of other genes involved in flagellar synthesis remains to be evaluated.
While galU mutants in several bacteria reduces bacteria attachment (Genevaux et al. 1999; Deng et al. 2010; Guo et al. 2010), no information is available regarding the role of galU in the expression of attachment-related genes. Here, the expression of three potential attachment-related genes was reduced in the X. campestris pv. campestris galU mutant. Although the involvement of virulence and adhesion in X. campestris pv. campestris is not yet recognized, these results extend our insight into the physiological role of GalU.
Conclusion
To date, only two reports have documented galU in X. campestris pv. campestris. One described the role of galU in EPS synthesis and revealed that a DNA fragment carrying galU gene can restore the non-mucoid phenotype (Wei et al. 1996), and the other described the coding product of galU and demonstrated that the coding product possesses UTP-glucose-1-phosphate uridylyltransferase activity (Bosco et al. 2009). In this study, we characterized galU in strain Xcc17 of X. campestris pv. campestris to gain more insights into the physiological role of the X. campestris pv. campestris GalU. To this end, we set out to construct a galU mutant and subjected it to phenotypic evaluation. There were several important findings: (1) deletion of galU has an impact on pathogenicity; (2) GalU is involved in polysaccharide production, including EPS and LPS; (3) GalU is required for cell motility and bacterial attachment; (4) GalU is implicated in environmental stress tolerance; (5) GalU has a role in the expression of flagellin gene fliC as well as the attachment-related genes xadA, fhaC, and yapH. The findings presented here showing GalU plays an essential role in X. campestris pv. campestris pathogenicity, in virulence determinant production and environmental response suggest that GalU is a potential target for antibacterial agent screening and may help to develop new strategies for the control of the black rot disease.
References
Aragao D, Fialho AM, Marques AR, Mitchell EP, Sa-Correia I, Frazao C (2007) The complex of Sphingomonas elodea ATCC 31461 glucose-1-phosphate uridylyltransferase with glucose-1-phosphate reveals a novel quaternary structure, unique among nucleoside diphosphate-sugar pyrophosphorylase members. J Bacteriol 189:4520–4528. doi:10.1128/JB.00277-07
Bogdanove AJ et al (2011) Two new complete genome sequences offer insight into host and tissue specificity of plant pathogenic Xanthomonas spp. J Bacteriol 193:5450–5464. doi:10.1128/JB.05262-11
Bosco MB, Machtey M, Iglesias AA, Aleanzi M (2009) UDPglucose pyrophosphorylase from Xanthomonas spp. Characterization of the enzyme kinetics, structure and inactivation related to oligomeric dissociation. Biochimie 91:204–213. doi:10.1016/j.biochi.2008.09.001
Buttner D, Bonas U (2010) Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol Rev 34:107–133. doi:10.1111/j.1574-6976.2009.00192.x
Chan JW, Goodwin PH (1999) The molecular genetics of virulence of Xanthomonas campestris. Biotechnol Adv 17:489–508
Chang HY, Lee JH, Deng WL, Fu TF, Peng HL (1996) Virulence and outer membrane properties of a galU mutant of Klebsiella pneumoniae CG43. Microb Pathog 20:255–261. doi:10.1006/mpat.1996.0024
da Silva AC et al (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417:459–463
Darsonval A, Darrasse A, Durand K, Bureau C, Cesbron S, Jacques MA (2009) Adhesion and fitness in the bean phyllosphere and transmission to seed of Xanthomonas fuscans subsp. fuscans. Mol Plant Microbe Interact 22:747–757. doi:10.1094/MPMI-22-6-0747
Das A, Rangaraj N, Sonti RV (2009) Multiple adhesin-like functions of Xanthomonas oryzae pv. oryzae are involved in promoting leaf attachment, entry, and virulence on rice. Mol Plant Microbe Interact 22:73–85. doi:10.1094/MPMI-22-1-0073
Deng WL et al (2010) Effects of galU mutation on Pseudomonas syringae-plant interactions. Mol Plant Microbe Interact 23:1184–1196. doi:10.1094/MPMI-23-9-1184
Dow JM, Daniels MJ (1994) Pathogenicity determinants and global regulation of pathogenicity of Xanthomonas campestris pv. campestris. Curr Top Microbiol Immunol 192:29–41
Dow JM, Osbourn AE, Wilson TJ, Daniels MJ (1995) A locus determining pathogenicity of Xanthomonas campestris is involved in lipopolysaccharide biosynthesis. Mol Plant Microbe Interact 8:768–777
Dow JM, Crossman L, Findlay K, He YQ, Feng JX, Tang JL (2003) Biofilm dispersal in Xanthomonas campestris is controlled by cell–cell signaling and is required for full virulence to plants. Proc Natl Acad Sci U S A 100:10995–11000. doi:10.1073/pnas.1833360100
Fu JF, Tseng YH (1990) Construction of lactose-utilizing Xanthomonas campestris and production of xanthan gum from whey. Appl Environ Microbiol 56:919–923
Genevaux P, Bauda P, DuBow MS, Oudega B (1999) Identification of Tn10 insertions in the rfaG, rfaP, and galU genes involved in lipopolysaccharide core biosynthesis that affect Escherichia coli adhesion. Arch Microbiol 172:1–8
Gottig N, Garavaglia BS, Garofalo CG, Orellano EG, Ottado J (2009) A filamentous hemagglutinin-like protein of Xanthomonas axonopodis pv. citri, the phytopathogen responsible for citrus canker, is involved in bacterial virulence. PLoS One 4:e4358. doi:10.1371/journal.pone.0004358
Guo Y, Sagaram US, Kim JS, Wang N (2010) Requirement of the galU gene for polysaccharide production by and pathogenicity and growth in planta of Xanthomonas citri subsp. citri. Appl Environ Microbiol 76:2234–2242. doi:10.1128/AEM.02897-09
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hsiao YM, Liao HY, Lee MC, Yang TC, Tseng YH (2005) Clp upregulates transcription of engA gene encoding a virulence factor in Xanthomonas campestris by direct binding to the upstream tandem Clp sites. FEBS Lett 579:3525–3533
Hsiao YM, Liu YF, Fang MC, Song WL (2011) XCC2731, a GGDEF domain protein in Xanthomonas campestris, is involved in bacterial attachment and is positively regulated by Clp. Microbiol Res 166:548–565. doi:10.1016/j.micres.2010.11.003
Hsiao YM, Song WL, Liao CT, Lin IH, Pan MY, Lin CF (2012) Transcriptional analysis and functional characterization of XCC1294 gene encoding a GGDEF domain protein in Xanthomonas campestris pv. campestris. Arch Microbiol 194:293–304. doi:10.1007/s00203-011-0760-3
Jalan N et al (2011) Comparative genomic analysis of Xanthomonas axonopodis pv. citrumelo F1, which causes citrus bacterial spot disease, and related strains provides insights into virulence and host specificity. J Bacteriol 193:6342–6357. doi:10.1128/JB.05777-11
Jiang SS, Lin TY, Wang WB, Liu MC, Hsueh PR, Liaw SJ (2010) Characterization of UDP-glucose dehydrogenase and UDP-glucose pyrophosphorylase mutants of Proteus mirabilis: defectiveness in polymyxin B resistance, swarming, and virulence. Antimicrob Agents Chemother 54:2000–2009. doi:10.1128/AAC.01384-09
Keen NT, Tamaki S, Kobayashi D, Trollinger D (1988) Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191–197
Kim H et al (2010) Structural basis for the reaction mechanism of UDP-glucose pyrophosphorylase. Mol Cells 29:397–405. doi:10.1007/s10059-010-0047-6
Komeda Y, Icho T, Iino T (1977) Effects of galU mutation on flagellar formation in Escherichia coli. J Bacteriol 129:908–915
Lee TC et al (2001) The early stages of filamentous phage ϕLf infection require the host transcription factor, Clp. J Mol Microbiol Biotechnol 3:471–481
Lee MC, Weng SF, Tseng YH (2003) Flagellin gene fliC of Xanthomonas campestris is upregulated by transcription factor Clp. Biochem Biophys Res Commun 307:647–652
Lee BM et al (2005) The genome sequence of Xanthomonas oryzae pathovar oryzae KACC10331, the bacterial blight pathogen of rice. Nucleic Acids Res 33:577–586
Lesse AJ, Campagnari AA, Bittner WE, Apicella MA (1990) Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods 126:109–117
Liu YF et al (2013) GsmR, a response regulator with an HD-related output domain in Xanthomonas campestris, is positively controlled by Clp and is involved in the expression of genes responsible for flagellum synthesis. FEBS J 280:199–213. doi:10.1111/febs.12061
McCarthy Y et al (2008) The role of PilZ domain proteins in the virulence of Xanthomonas campestris pv. campestris. Mol Plant Pathol 9:819–824. doi:10.1111/j.1364-3703.2008.00495.x
Miller JH (1972) Experiments in molecular genetics. Cold Spring Habor Laboratory, Cold Spring Harbor, NY
Nesper J, Kapfhammer D, Klose KE, Merkert H, Reidl J (2000) Characterization of Vibrio cholerae O1 antigen as the bacteriophage K139 receptor and identification of IS1004 insertions aborting O1 antigen biosynthesis. J Bacteriol 182:5097–5104
Nesper J, Lauriano CM, Klose KE, Kapfhammer D, Kraiss A, Reidl J (2001) Characterization of Vibrio cholerae O1 El tor galU and galE mutants: influence on lipopolysaccharide structure, colonization, and biofilm formation. Infect Immun 69:435–445. doi:10.1128/IAI.69.1.435-445.2001
Pieretti I et al (2009) The complete genome sequence of Xanthomonas albilineans provides new insights into the reductive genome evolution of the xylem-limited Xanthomonadaceae. BMC Genomics 10:616
Priebe GP et al (2004) The galU gene of Pseudomonas aeruginosa is required for corneal infection and efficient systemic spread following pneumonia but not for infection confined to the lung. Infect Immun 72:4224–4232. doi:10.1128/IAI.72.7.4224-4232.2004
Qian W et al (2005) Comparative and functional genomic analyses of the pathogenicity of phytopathogen Xanthomonas campestris pv. campestris. Genome Res 15:757–767
Rioux S et al (1999) Isolation and characterization of mini-Tn10 lipopolysaccharide mutants of Actinobacillus pleuropneumoniae serotype 1. Can J Microbiol 45:1017–1026
Ryan RP et al (2011) Pathogenomics of Xanthomonas: understanding bacterium-plant interactions. Nat Rev Microbiol 9:344–355. doi:10.1038/nrmicro2558
Salzberg SL et al (2008) Genome sequence and rapid evolution of the rice pathogen Xanthomonas oryzae pv. oryzae PXO99A. BMC Genomics 9:204. doi:10.1186/1471-2164-9-204
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd. In. Cold Spring Habor Press, Cold Spring Harbor, NY
Thieme F et al (2005) Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J Bacteriol 187:7254–7266
Thoden JB, Holden HM (2007a) Active site geometry of glucose-1-phosphate uridylyltransferase. Protein Sci 16:1379–1388. doi:10.1110/ps.072864707
Thoden JB, Holden HM (2007b) The molecular architecture of glucose-1-phosphate uridylyltransferase. Protein Sci 16:432–440. doi:10.1110/ps.062626007
Vilches S et al (2007) Mesophilic Aeromonas UDP-glucose pyrophosphorylase (GalU) mutants show two types of lipopolysaccharide structures and reduced virulence. Microbiology 153:2393–2404. doi:10.1099/mic.0.2007/006437-0
Vorholter FJ, Niehaus K, Puhler A (2001) Lipopolysaccharide biosynthesis in Xanthomonas campestris pv. campestris: a cluster of 15 genes is involved in the biosynthesis of the LPS O-antigen and the LPS core. Mol Genet Genomics 266:79–95
Vorholter FJ et al (2008) The genome of Xanthomonas campestris pv. campestris B100 and its use for the reconstruction of metabolic pathways involved in xanthan biosynthesis. J Biotechnol 134:33–45
Wang TW, Tseng YH (1992) Electrotransformation of Xanthomonas campestris by RF DNA of filamentous phage ϕLf. Lett Appl Microbiol 14:65–68
Wei CL, Lin NT, Weng SF, Tseng YH (1996) The gene encoding UDP-glucose pyrophosphorylase is required for the synthesis of xanthan gum in Xanthomonas campestris. Biochem Biophys Res Commun 226:607–612. doi:10.1006/bbrc.1996.1403
Wengelnik K, Marie C, Russel M, Bonas U (1996) Expression and localization of HrpA1, a protein of Xanthomonas campestris pv. vesicatoria essential for pathogenicity and induction of the hypersensitive reaction. J Bacteriol 178:1061–1069
Williams PH (1980) Black rot: a continuing threat to world crucifers. Plant Dis 64:736–742
Yang BY, Tseng YH (1988) Production of exopolysaccharide and levels of protease and pectinase activity in pathogenic and non-pathogenic strains of Xanthomonas campestris pv. campestris. Bot Bull Acad Sin 29:93–99
Yen MR, Lin NT, Hung CH, Choy KT, Weng SF, Tseng YH (2002) oriC region and replication termination site, dif, of the Xanthomonas campestris pv. campestris 17 chromosome. Appl Environ Microbiol 68:2924–2933
Zou Y et al (2013) The role of galU and galE of Haemophilus parasuis SC096 in serum resistance and biofilm formation. Vet Microbiol 162:278–284. doi:10.1016/j.vetmic.2012.08.006
Acknowledgments
This work was supported by the National Science Council of Taiwan Grant No. NSC 101-2313-B-166-001-MY3 to Yi-Min Hsiao, and the Central Taiwan University of Science and Technology Grant No. CTU100-PC-010 to Chao-Tsai Liao.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liao, CT., Du, SC., Lo, HH. et al. The galU gene of Xanthomonas campestris pv. campestris is involved in bacterial attachment, cell motility, polysaccharide synthesis, virulence, and tolerance to various stresses. Arch Microbiol 196, 729–738 (2014). https://doi.org/10.1007/s00203-014-1012-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-014-1012-0