Abstract
Aerobic anoxygenic phototrophs (AAPs) are prokaryotic microorganisms capable of harvesting light using bacteriochlorophyll-based reaction centres. Marine AAP communities are generally dominated by species belonging to the Roseobacter clade. For this reason, we used marine Roseobacter-related strain COL2P as a model organism to characterize its photosynthetic apparatus, level of pigmentation and expression of photosynthetic complexes. This strain contained functional photosynthetic reaction centres with bacteriochlorophyll a and spheroidenone as the main light-harvesting pigments, but the expression of the photosynthetic apparatus was significantly reduced when compared to truly photoautotrophic species. Moreover, the absence of peripheral light-harvesting complexes largely reduced its light-harvesting capacity. The size of the photosynthetic unit was limited to 35.4 ± 1.0 BChl a molecules supplemented by the same number of spheroidenone molecules. The contribution of oxidative phosphorylation and photophosphorylation was analysed by respiration and fluorometric measurements. Our results indicate that even with a such reduced photosynthetic apparatus, photophosphorylation provides up to three times higher electron fluxes than aerobic respiration. These results suggest that light-derived energy can provide a substantial fraction of COL2P metabolic needs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacteriochlorophyll a-containing bacteria were recently found to account for a significant fraction of the microbial community in the upper ocean (Kolber et al. 2000, 2001). These first field observations were later confirmed by independent studies using epifluorescence microscopy (Cottrell et al. 2006; Sieracki et al. 2006; Jiao et al. 2007), infrared fluorometry (Koblížek et al. 2006, 2007) and genetic analyses (Béjà et al. 2002; Yutin et al. 2007). Laboratory-based studies of Rsb. denitrificans (originally denoted as Erythrobacter OCh 114), conducted by Shiba, Harashima and co-workers in 1980s (Harashima et al. 1989) provided the basic understanding of their metabolic properties. These organisms, called aerobic anoxygenic phototrophs (AAPs), are obligate aerobes utilizing dissolved organic matter as a source of organic carbon for their metabolism and growth. In addition, they harvest light using bacteriochlorophyll a (BChl a) –based pigments and reaction centres (Yurkov and Csotonyi 2009) to satisfy portion of their energy requirements.
Although AAP bacteria represent only a functional group composed of different Proteobacterial taxa, members of Roseobacter clade (Wagner-Döbler and Biebl 2006, Brinkhoff et al. 2008) frequently dominate the marine AAP communities. The Roseobacter-related species were shown to dominate AAP communities in the Red and Mediterranean Seas (Oz et al. 2005), as well as in the open ocean (Yutin et al. 2007). Representatives of the Rsb. clade also form a large part of AAP communities in eutrophic environments such as the Baltic Sea (Salka et al. 2008).
Besides expressing phototrophy, AAPs differ in other characteristics from typical heterotrophic bacteria. They are on average two times bigger (Sieracki et al. 2006; Lami et al. 2007), moreover, they appear to grow faster than their heterotrophic counterparts (Koblížek et al. 2007). Both these facts indicate that AAPs may contribute significantly to the marine organic carbon fluxes. The quantification of this contribution requires a more detailed knowledge of AAP physiology, metabolism and their photosynthetic properties. Despite the growing body of data regarding their abundance and diversity, little is known about the metabolism and phototrophic properties of AAPs. For this reason, we used the marine AAP isolate COL2P belonging to the Roseobacter clade to characterize the architecture of its photosynthetic apparatus, level of pigment expression and activity of photosynthetic electron transfer in order to quantify the contribution of light-derived energy to its cellular metabolism. We believe that information from laboratory-based studies is crucial to understand the role the AAP bacteria play in the marine environment and to properly evaluate their contribution to ocean carbon cycle.
Materials and methods
Laboratory cultures
The chemostat cultures of COL2P were grown at 23°C in a glass vessel (volume ~ 750 mL). The growth media were based on an artificial sea water medium described earlier (Koblížek et al. 2003) supplemented with 10−6 M nicotinic acid. The medium contained 0.3 mM NaH2PO4 and 3 mM glutamic acid as a sole source of organic carbon. For pigment induction experiment, the glutamic acid was replaced with 4 mM fumarate. To induce phosphorus-limited conditions, the culture medium was enriched with 10 mM glutamic acid and 10 μM NaH2PO4. The cell suspension was thoroughly stirred and bubbled with air. The chemostat growth mode was maintained by pumping the growth media through the vessel at 30-min intervals, with dilution rate controlled between 0.1 and 1.5 day−1. The illumination was provided by a bank of luminescent tubes at irradiance level of 150 μmol quanta m−2 s−1 in 12:12 h light–dark cycle, unless stated otherwise. The Rhodobacter sphaeroides cultures were grown as described earlier (Koblížek et al. 2005). For the analyses, the cultures were harvested and characterized regarding their pigment and carbon content, respiration activity and fluorescence parameters. Cells were counted using epifluorescence microscopy following 4′,6-diamidino-2-phenylindole (DAPI) staining, which made it possible to express most of the parameters on per carbon and on per cell basis.
Analytical procedures
Absorption spectra were recorded on isolated membranes. The cells were collected by centrifugation at 10,000×g, resuspended in the breaking buffer (containing 50 mM Tris pH 7.5, 50 mM NaCl and 10 mM MgCl2) and disrupted by the French press. The cell debris was removed by second centrifugation at 10,000×g for 10 min, and the released membranes were sedimented by 30-min centrifugation at 60,000×g. Finally, the collected membranes were resuspended in the breaking buffer, and their absorption spectrum was recorded using the Shimadzu UV 3000 spectrophotometer operating in a dual beam mode, at a resolution of 0.5 nm using a 2-nm slit width. Respiration was measured with the Clark oxygen electrode placed in a temperature-stabilized measuring chamber at 23°C. The electrode signal was recorded by a custom-built potentiostat and a nanoamperometer. For organic carbon and nitrogen analyses, cells were collected by centrifugation, washed with deionized water, placed in tin capsules, dried at 70°C and stored in a desiccator. Elemental composition was determined with an automated CN elemental analyzer within 3 months after the sample collection.
Pigment analyses
For pigment analyses, about 5–10 × 1010 cells were collected by centrifugation at 10,000×g for 10 min, transferred to 1.5-mL Eppendorf tube, spun down and resuspended thoroughly in 50 μL of sea water. The suspension was extracted with 1 mL of acetone:methanol:Tris pH 7.0 mixture 7:2:1 for 15 min. Following clarification by centrifugation, BChl a was determined spectroscopically at 770 nm using a newly determined extinction coefficient (see results). Spheroidenone content was measured at 482 nm and calculated using an extinction coefficient of 123.6 mM−1 cm−1 (Shneour 1962). In some cases, the extracts were further analysed by the high-performance liquid chromatography (HPLC) using the Agilent 1100 Series system (Agilent Technologies Inc., Palo Alto, CA, USA). The instrument was equipped with the UV–VIS diode-array detector (Agilent DAD 61315B) and an in-line Agilent 1100 Series LC/MSD Trap mass spectrometer with APCI chemical ionization module (nebulizer gas 50 psi, nebulizer temperature 350°C, capillary potential 4,000 V, corona 4 A, vaporizer temperature 400°C, freq. amplitude 1.5 V). Pigments were separated using a modified method of Van Heukelem and Thomas (2001) on the heated (35°C) Phenomenex Luna 3μ C8(2) 100Å column with binary solvent system (0 min 100% A, 20 min 100% B, 25 min 100% B, 27 min 100% A, 30 min 100% A; A: 70% methanol + 28 mM ammonium acetate, B: methanol). The solvent flow rate was 0.8 mL min−1. The peak assignment was based on acquired absorption spectra and confirmed by in-line mass spectrometry.
Fluorometry
The BChl a fluorescence was recorded using a modified PSI laboratory fluorometer (FL200/PS, Photon Systems Instruments Ltd., Czechia). The cell suspension (3 mL) was placed in a glass tube within the sample chamber and illuminated by two flashing units, each populated with four green Luxeon diodes (LXHL PB09, 505 nm). The signal was detected by a standard silicon PIN photodiode-based detector (SN-SL 103, PSI Ltd., Czechia) protected by an infrared 850-nm-long pass filter (Oriel 51352, USA). The fluorescence transients were induced by 100-ms-long train of subsaturanting 10-μs-long flashes spaced at 300 μs intervals. Fluorescence relaxation kinetics was followed with 50 flashes with exponentially increasing time interval over a period of 1 s. F 0 and F M fluorescence levels were recorded at the beginning (0.1 ms) and at end of the rapid induction kinetics (100 ms), respectively. The electron transport turnover was calculated from the single exponential decay regression analysis of the relaxation kinetics.
Results
The general characteristics
The strain COL2P has been isolated from western Mediterranean coastal waters (Collioure, France, 42°30′N, 3°01′E) by agar plating technique as described earlier (Koblížek et al. 2006). Based on the 16S rRNA sequence (GenBank accession No. DQ659415), the strain belongs to the Roseobacter clade (Rhodobacterales, Alphaproteobacteria) displaying 95% identity to Maritimibacter alkaliphilus strain HTCC2654 and 92% homology to Rsb. litoralis and Rsb. denitrificans.
Similarly to other AAP species, COL2P grows aerobically and requires the presence of organic carbon sources. It grew well on glutamate, leucine, fumarate and pyruvate. Acetate, butyrate, citrate, formate, lactate and succinate were less efficient carbon sources. COL2P did not grow in the absence of oxygen. Aerobically grown cultures of strain COL2P were pink. The absorption spectrum of their membrane fraction displayed two major BChl a bands: the UV-A Soret (BY) band at 374 nm and the infrared Q Y band of light-harvesting 1 (LH1) antenna centred at 874 nm. Peripheral light-harvesting complex (B806), described in Rsb. denitrificans and Rsb. litoralis, was absent (see Fig. 1). Carotenoids were responsible for most of the light absorption between 400 and 600 nm, with a broad in vivo maximum between 480 and 520 nm. The Q X absorption bands of BChl a overlapped with carotenoid absorption peaking at 590–591 nm. A small shoulder around 413 nm originated from the γ (Soret) band of cytochrome c.
Absorption spectrum of COL2P membranes with marked positions of major BChl a (374, 591 and 874 nm), carotenoid (484 nm) and cytochrome (413 nm) absorption bands. The dashed line shows for comparison the infrared part of the absorption spectrum of Rsb. denitrificans (vertically shifted). The labels depict the position of inner (LH1) and peripheral (B806) light-harvesting complex in this species (absent in COL2P)
The functionality of photosynthetic reaction centres was verified by kinetic fluorescence measurements. The aerobic cultures of COL2P strain displayed clear induction of bacteriochlorophyll fluorescence with F V/F M ~ 0.7. The removal of oxygen from COL2P cultures by nitrogen bubbling resulted in very strong reduction of the variable signal (F V/F M ~ 0.1), indicating that anaerobic conditions inhibited the primary photochemistry in this organism (see Fig. 2).
Bacteriochlorophyll fluorescence induction in COL2P cells kept under oxic (air saturated) and anoxic conditions. The fluorescence transient was induced by a rapid sequence of light pulses spaced at 300 μs intervals. Then, the fluorescence relaxation was followed for 900 ms. Minimum fluorescence F 0 and maximum fluorescence F M levels were recorded at the beginning (0.1 ms) and at end of the fluorescence induction kinetics (100 ms), respectively
Pigment composition and stoichiometry
Pigment composition of the strain COL2P was further analysed by HPLC, and individual pigments were identified based on the absorption spectra and molecular masses (see Fig. 3). The COL2P isolate contained BChl a, with spheroidenone as the major carotenoid. BChl a exhibited three major peaks at 366, 607 and 771 nm, and its identity was confirmed by the observation of molecular ion m/z 911.4. Spheroidenone was identified by its typical broad absorption maximum 485 nm and major molecular ion m/z 583.5. Among other minor pigments, we have detected bacteriopheophytin a (BPhe a) with m/z 889.4, which constitutes a primary electron acceptor of the bacterial reaction centre (RC).
HPLC analysis of pigments extracted from strain COL2P. Chromatograms were recorded at 485 and 771 nm (note that trace at 770 nm is vertically shifted). Numbers above peaks show pigment masses determined by mass spectrometry: m/z 911.4 bacteriochlorophyll, m/z 583.5 spheroidenone and m/z 889.4 bacteriophaeophytin a. The insert shows the on line absorption spectra of BChl a and spheroidenone recorded by a diode-array detector
To determine the pigment stoichiometry in bacterial light-harvesting complexes, we have assessed the amounts of BChl a and spheroidenone spectrophotometrically in 7:2 acetone/methanol extracts. Unfortunately, recent reassessment of BChl a extinction coefficients by Permentier et al. (2000) do not provide the extinction coefficient for acetone/methanol solvent mixture (which turned out to be most effective in pigment extraction, 100% methanol does not extract well spheroidenone) and one has still to rely on the old coefficient of 76 mM−1 cm−1 provided by Clayton (1966). Hence, we decided to determine the appropriate extinction coefficient by ourselves. First, we determined BChl a content spectrophotometrically in 100% methanol pigment extracts (n = 5) using the coefficient provided by Permentier et al. (2000). These pigment extracts were used to calibrate our HPLC system. Then, 7:2:1 acetone:methanol:water extracts were prepared (n = 7), and their absorbance at 770 nm determined. The BChl a content in these extracts was determined by the HPLC and, finally, back calculated the appropriate extinction coefficient at 770 nm. The recalculated BChl a extinction coefficient in 7:2:1 acetone:methanol:water mixture was ε770 = 69.3 ± 3.3 mM−1 cm−1. It is about 10% lower than the one reported by Clayton, which means that the pigment concentrations determined using the old coefficient are about 10% underestimated.
We used this value to determine the pigment stoichiometry in bacterial light-harvesting complexes. We measured the amounts of BChl a and spheroidenone spectrophotometrically in COL2P cultures grown under varying nutrients and physiological conditions, under light and dark cycle. Despite of different nutrients conditions, these pigments displayed a constant spheroidenone to BChl a ratio of 1.00 ± 0.02 (n = 21, R 2 = 0.991), suggesting a fixed 1:1 stoichiometry in the pigment-protein complexes. Interestingly, somewhat higher (1.21 ± 0.04) spheroidenone to BChl a pigment ratios were observed in the dark-grown cultures.
To determine the number of BChl a molecules bound to the photosynthetic complexes (the photosynthetic unit size), the whole cell pigment extract was analysed by the HPLC and the BPhe a peak area recorded. Then, the same extract (1 mL volume) was acidified with 3 μL of 37% HCl and reanalysed by chromatography. Acidification converted all BChl a into BPhe a. From the ratio of BPhe a peak areas after (A ACIDIF), and before acidification (A NORM), we calculated the ratio of BChl a and BPhe a in the complexes as A ACIDIF/A NORM−1. Assuming two bacteriophaeophytin a molecules per RC (based on analogy with known bacterial RC structures), we determined that strain COL2P contains 35.4 ± 1.0 BChl a molecules per RC (mean ± SE, n = 3). The advantage of this method is that it does not require an a priori knowledge of any extinction coefficients. We have used the same approach to determine the size of light-harvesting system in semiaerobically grown (~2% oxygen) cultures of Rba. sphaeroides. We found the size of the photosynthetic units varied from 128.1 to 156.0 BChl a molecules per RC, about four times larger than in the strain COL2P.
Pigmentation and cell quotas
Pigment expression was investigated in continuous cultures of Rsb. strain COL2P. To better understand physiological responses to varying environmental conditions, the strain was grown with organic carbon or phosphorus as a limiting nutrient. These two nutrient regimes are the most frequently experienced by natural bacterioplankton in field conditions. Growth rates were stabilized at one division per day, corresponding to previously observed in natural AAP communities at sea (Koblížek et al. 2007), by controlling dilution rates in the chemostats. Interestingly, the difference in the limiting nutrient strongly affected cell sizes. The phosphorus-limited (P-limited) cells were about two times larger than the cells grown under carbon limitation (C-limited), resulting in almost eightfold difference in cell volumes (0.53 μm3 vs. 0.07 μm3, see Table 1). The large P-limited cells displayed proportionally higher pigment and carbon content, as well as enhanced dark respiration activity. Phototrophic light utilization was confirmed in both cultures by ~70% reduction of the respiration activity by light. Interestingly, the pigment content calculated on carbon basis gave similar results (1.3 ± 0.2 and 1.5 ± 0.2 × 10−3 w:w) for both cultures. We have observed similar value of 1.2–1.6 × 10−3 in our previous study with Erythrobacter sp. strain NAP1 (Koblížek et al. 2003) grown on complex media. Such low pigmentation appears to be typical for photoheterotropic organisms; the closely related photoautotrophic Rba. sphaeroides (Rhodobacterales) displayed approximately ten times higher bacteriochlorophyll content (14–28 × 10−3 BChl a per carbon w:w, see Fig. 4).
To investigate variability of the BChl a content, we grew COL2P cultures at various growth rates. Reducing growth rates from 1.4 to 0.55 to 0.17 day−1 in carbon-limited cultures increased the pigment/carbon ratios from 1.5 ± 0.2 to 2.9 ± 0.1 to 3.0 ± 0.3 × 10−3 BChl a per carbon (w:w), respectively. The cellular pigment content also increased proportionally (from 46 ± 7 to 96 ± 11 ag BChl a), whereas the carbon content remained the same (data not shown). The effect of light was even more profound. The cultures grown in constant darkness displayed five to six times smaller pigment content than cultures grown in light/dark regime, while the cultures grown in continuous light displayed almost no pigmentation (<10−4 BChl a per carbon w:w, see Fig. 4). There was no significant effect of different carbon substrates on BChl a expression; chemostat cultures grown on leucine, fumarate and pyruvate, as well as on complex media did not differ significantly from the glutamate grown cultures. Also, semiaerobic conditions (~2% oxygen) did not significantly alter the level of pigment expression (data not shown).
Photosynthetic apparatus induction
The inhibition of pigment synthesis by light offers a simple procedure for investigating the pigment expression dynamics in AAPs. The carbon-limited COL2P culture was cultivated for 1 week under continuous illumination at growth rates of ~1 day−1. Under such conditions, the cells were producing only minimal amount of pigments (<10−4 BChl a per carbon w:w). Then, the light was switched off, and the induction of photosynthetic apparatus was followed by pigment analyses and kinetic fluorometry. After a short lag-phase of about 60 min, the assembly of the photosynthetic apparatus resumed as indicated by the increase in BChl a content and fluorescence (F M, F V/F M) parameters. The BChl a levels have risen from ~5 nM to approximately 200 nM within the first 13 h (Fig. 5a). At the beginning of the pigment induction, the spheroidenone content was about four times higher than BChl a, but after 3.5 h, both pigments reached the 1:1 ratio as observed previously in the light/dark-grown cultures. The F V/F M ratio increased from 0 to 0.72 ± 0.01 during the first 7 h of the experiment, indicating the presence of fully functional RC. The turnover time of electron transport was calculated from the fluorescence relaxation kinetics after the 100-ms-long multiple turnover excitation pulse. The observed turnover time increased from relatively rapid rates of ~2 ms in the initial phases of the pigment induction to about 8 ms after 12 h of the experiment. Interestingly, the electron transport turnover time was linearly proportional to BChl a concentration, with the Y intercept at 2 ms (Fig. 5b). This value may represent a minimum turnover time for the bacterial reaction centre. Most likely, the electron transport rates slow down with increasing number of reaction centres due to limitation of electron transport capacity. Similar turnover times (0.8–1.6 ms) were observed also in the greening experiments with Rba. sphaeroides (Koblížek et al. 2005).
The induction of photosynthetic apparatus in strain COL2P following the shift from light to dark. The cells were grown in C-limited chemostat (4 mM fumarate) at rate of ~1 day−1. The upper panel reports changes of BChl a and minimum fluorescence (F 0) levels, yields of primary photochemistry F V/F M and electron transport rates during the time-course of the induction. The lower panel depicts the linear relationship between the BChl a content and the electron transport turnover and the relationship between the BChl a content and the spheroidenone (SO) to BChl a ratio
Discussion
The Rsb. strain COL2P represents a typical photoheterotrophic bacterium. Based on the absorption spectrum and fluorescence measurements, it contains functional bacterial reaction centres, but requires the organic carbon sources for growth. The size of light-harvesting complexes in strain COL2P (35.4 ± 1.0 BChl a per RC) is significantly smaller than in Rhodobacter sphaeroides. This size corresponds well with values usually reported for bacterial core (reaction centre + LH1) complexes (Bullough et al. 2009), which is consistent with the absence of the peripheral light-harvesting complexes in this organisms.
Unlike earlier described Erythrobacter species (Koblížek et al. 2003), COL2P contains only one major carotenoid, the spheroidenone, which serve as an auxiliary pigment transferring the excitation energy to BChl a molecules. The in vivo and in vitro absorption spectra (see Figs. 1, 3) indicate that spheroidenone is responsible for harvesting the majority of the solar radiation present in the water column, typically confined to blue and green light. The presence of spheroidenone was also found in Rsb. denitrificans and Rsb. litoralis (Shiba 1991) as well as in other AAP species belonging to Rsb. clade (Wagner-Döbler and Biebl 2006) which indicate that this pigment is a universal marker of this group (Koblížek et al. 2006).
The BChl a fluorescence measurements clearly demonstrated a fully functional photosynthetic apparatus under aerobic conditions, whereas the oxygen removal completely inhibited the primary photochemistry. The linear relationship between pigment content and electron transport turnover observed during the pigment induction experiment deserves further attention. As the electron transport turnover time increases, electron transport rate decreases in proportion to the pigment content, providing a simple mechanism of light adaptation. Under low light conditions, conducive to increasing pigment content (~number of reaction centres), the electron transport rates slow down without any adverse effect on the total photosynthetic fluxes. Under high light conditions, when electron transport rates become limiting, reduction in the number of RC leads to a proportional increase in the electron transport capacity.
Laboratory experiments under precisely controlled conditions, with well-characterized culture strains, provide invaluable information about characteristics and physiology of marine organisms. This information is generally inaccessible in the natural mixed microbial communities. The results of such experiments can supplement and aid in interpretation of field observations. Critical to this approach is the selection of an appropriate model organism. Strain COL2P is a convenient and easy to grow organism, suitable for laboratory work. Roseobacter-related species were frequently observed to constitute a major component of natural AAP communities (Oz et al. 2005; Yutin et al. 2007; Salka et al. 2008). Unlike Rsb. type species Rsb. litoralis and Rsb. denitrificans, our studied strain COL2P does not contain peripheral high harvesting complexes (Fig. 1). This, according to our earlier observations, represents one of the main characteristics of open sea AAP communities (Koblížek et al. 2006; Koblížek unpublished). Performing these experiments in carbon (C-) or phosphorus (P-) limited chemostats simulates the natural environmental conditions much better than the standard batch cultures.
The relevance of our experimental data to the natural field conditions is supported by the published field data. In Table 2, we review the published BChl a cell quotas determined for various natural AAP communities. The cellular pigment content determined in our C-limited culture represents the lower limit of field data, and resembles mostly the situation in the open ocean conditions. On the other hand, the large cells typical of P-limited conditions display pigment content close to the upper limit of the observed field values, characteristic of more productive areas. Our experimental regimes also resemble field conditions with regard to cellular volumes. Cell volumes observed in our experiments, 0.07 ± 0.01 (C-limitation) and 0.53 ± 0.06 (P-limitation), correspond well with that of 0.14 ± 0.03 μm3 determined by Sieracki et al. (2006) in the North Atlantic and Sargasso Sea and of 0.47 ± 0.36 μm3 from the South Pacific by Lami et al. (2007). All this suggests that our laboratory cultures respond to varying environmental conditions in a way similar to natural populations. Therefore, we postulate that the physiological characteristics such as carbon quotas, respiration and pigmentation observed under laboratory conditions are representative of field conditions. Indeed, the relatively well-constrained pigment-to-carbon ratios observed in this study could potentially allow to estimate AAP biomass from the BChl a concentration measured in the environment. This approach, however, has to be taken with care as the pigment content in AAPs generally changes in response to varying physiological conditions, and pigment synthesis is inhibited by light (Harashima et al. 1980; Iba and Takamiya 1989; this study). Our numbers are comparable to pigment levels reported for Dinoroseobacter shibae by Biebl and Wagner-Döbler (2006), but significantly higher than the values reported in the same study for poorly pigmented organisms Stappia alexandrii and Hoeflea phototrophica.
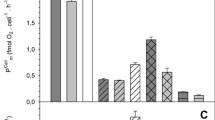
Most of earlier studies demonstrated light utilization in AAP species by documenting enhanced biomass accumulation in the presence of light when compared to cells grown in the dark (Yurkov and Van Gemerden 1993. Biebl and Wagner-Döbler 2006). Using data collected in this study, we attempted to quantify the contribution of light-derived energy to the cell metabolisms and its energy fluxes. From the cell size and the electron transport turnover, we have calculate the photosynthetic electron fluxes per unit of BChl a. Assuming the maximal electron turnover of 8 ms for fully pigmented cells, we estimate the maximum electron flux at 125 electrons per second or 4.5 × 105 electrons per hour. Using the determined value of 35.4 BChl a per RC, this corresponds to 13.95 mol electrons per g BChl a per hour. To assess the energetic contribution of photophosphorylation to cellular metabolism, we assume between 855 and 3,880 RC per cell, based on pigment cell quotas in C-limited and P-limited culture. On this basis, we estimate the maximum electron fluxes at 0.64 an 2.92 fmol electrons h−1 in C- and P-limited cultures, respectively. This is about two to three times higher than the respiratory electron flow (0.22 and 1.22 fmol electrons h−1) observed in these two growth regimes. We recognize that in field conditions, the AAP bacteria operate at less than optimal light levels during the day and no light during the night. Nevertheless, the light-driven photophosphorylation is likely to contribute a significant portion of the metabolic energy, comparable with the energy provided by oxidative phosphorylation (respiration). Therefore, the light utilization by AAP bacteria replaces significant amount of organic carbon which otherwise would have been respired.
In summary, the AAP strain COL2P appears to be a good model organism for the natural AAP communities based on the comparison with the field Roseobacter populations. The lower levels of pigmentation and small size of the light-harvesting system indicate that COL2P is a typical photoheterotroph, relying primarily on respiratory metabolism. However, under favourable light conditions, photophosphorylation might provide a significant amount of energy, which otherwise would have to be supplied by oxidative phosphorylation.
Abbreviations
- AAPs:
-
Aerobic anoxygenic phototrophs
- BChl a :
-
Bacteriochlorophyll a
- BPhe a :
-
Bacteriophaeophytine a
- FM :
-
Maximum fluorescence
- FV :
-
Variable fluorescence
- RC:
-
Reaction centre
- Rsb.:
-
Roseobacter
- Rba. :
-
Rhodobacter
References
Béjà O, Suzuki MT, Heidelberg JF, Nelson WC, Preston CM, Hamada T, Eisen JA, Fraser CM, DeLong EF (2002) Unsuspected diversity among marine aerobic anoxygenic phototrophs. Nature 415:630–633
Biebl H, Wagner-Döbler I (2006) Growth and bacteriochlorophyll a formation in taxonomically diverse aerobic anoxygenic phototrophic bacteria in chemostat culture: Influence of light regimen and starvation. Process Biochem 41:2153–2159
Brinkhoff T, Giebel H-A, Simon M (2008) Diversity, ecology, and genomics of the Roseobacter clade: a short overview. Arch Microbiol 189:531–539
Bullough PA, Qian P, Hunter CN (2009) Reaction center-light-harvesting core omplex of purple bacteria. In: Hunter CN, Daldal F, Thurnauer MC, Beaty JT (eds) The purple phototrophic bacteria. Springer, Berlin, pp 31–55
Clayton RK (1966) Spectroscopic analysis of bacteriochlorophylls in vitro and in vivo. Photochem Photobiol 5:669–677
Cottrell MT, Mannino A, Kirchman DL (2006) Aerobic anoxygenic phototrophic bacteria in the Mid-Atlantic Bight and the North Pacific Gyre. Appl Env Microbiol 72:557–564
Harashima K, Hayasaki J, Ikari T, Shiba T (1980) O2-stimulated synthesis of bacteriochlorophyll and carotenoids in marine bacteria. Plant Cell Physiol 21:1283–1294
Harashima K, Shiba T, Murata N (eds) (1989) Aerobic photosynthetic bacteria. Japan Scientific Societies Press, Tokyo
Iba K, Takamiya K (1989) Action spectra for light-inhibition of bacteriochlorophyll and carotenoid accumulation during aerobic growth of photosynthetic bacteria. Plant Cell Physiol 30:471–477
Jiao N, Zhang Y, Zeng Y, Hong N, Liu R, Chen F, Wang P (2007) Distinct distribution pattern of abundance and diversity of aerobic anoxygenic phototrophic bacteria in the global ocean. Environ Microbiol 9:3091–3099
Koblížek M, Béjà O, Bidigare RR, Christensen S, Benetiz-Nelson B, Vetriani C, Kolber MK, Falkowski PG, Kolber ZS (2003) Isolation and characterization of Erythrobacter sp. strains from the upper ocean. Arch Microbiol 180:327–338
Koblížek M, Shih JD, Breitbart SI, Ratcliffe EC, Kolber ZS, Hunter CN, Niederman RA (2005) Sequential assembly of photosynthetic units in Rhodobacter sphaeroides as revealed by fast repetition rate analysis of variable bacteriochlorophyll a fluorescence. Biochim Biophys Acta 1706:220–231
Koblížek M, Falkowski PG, Kolber ZS (2006) Diversity and distribution of photosynthetic bacteria in the Black Sea. Deep-Sea Res II 53:1934–1944
Koblížek M, Mašín M, Ras J, Poulton AJ, Prášil O (2007) Rapid growth rates of aerobic anoxygenic phototrophs in the ocean. Envir Microbiol 9:2401–2406
Kolber ZS, Van Dover CL, Niederman RA, Falkowski PG (2000) Bacterial photosynthesis in surface waters of the open ocean. Nature 407:177–179
Kolber ZS, Plumley FG, Lang AS, Beatty JT, Blankenship RE, VanDover CL, Vetriani C, Koblizek M, Rathgeber C, Falkowski PG (2001) Contribution of aerobic photoheterotrophic bacteria to the carbon cycle in the ocean. Science 292:2492–2495
Lami R, Cottrell MT, Ras J, Ulloa O, Obernosterer I, Claustre H, Lebaron P (2007) High abundances of aerobic anoxygenic photosynthetic bacteria in the South Pacific Ocean. Appl Environ Microbiol 73:4198–4205
Lami R, Čuperová Z, Ras J, Lebaron P, Koblížek M (2009) Distribution of free-living and particle-attached aerobic anoxygenic phototrophic bacteria in marine environments. Aquat Microbial Ecol. 55:31–38
Oz A, Sabehi G, Koblížek M, Massana R, Béjà O (2005) Roseobacter-like bacteria in Red and Mediterranean Sea aerobic anoxygenic photosynthetic populations. Appl Environ Microbiol 71:344–353
Permentier HP, Schmidt KA, Kobayashi M, Akiyama M, Hager-Braun C, Neerken S, Miller M, Amesz J (2000) Composition and optical properties of reaction centre core complexes from the green sulfur bacteria Prosthecochloris aestuarii and Chlorobium tepidum. Photosynth Res 64:27–39
Salka I, Moulisova V, Koblížek M, Jost G, Jürgens K, Labrenz M (2008) Abundance, depth distribution, and composition of aerobic bacteriochlorophyll a-producing bacteria in four deeps of the central Baltic Sea. Appl Environ Microbiol 74:4398–4404
Shiba T (1991) Roseobacter litoralis gen. nov., sp. nov. and Roseobacter denitrificans sp. nov., aerobic pink-pigmented bacteria which contain bacteriochlorophyll a. Syst Appl Microbiol 14:140–145
Shneour EA (1962) Carotenoid pigment conversion in Rhodopseudomonas spheroides. Biochim Biophys Acta 62:534–540
Sieracki ME, Gilg IC, Thier EC, Poulton NJ, Goericke R (2006) Distribution of planktonic aerobic anoxygenic photoheterotrophic bacteria in the northwest Atlantic. Limnol Oceanogr 51:38–46
Van Heukelem L, Thomas CS (2001) Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J Chromatogr A 910:31–49
Wagner-Döbler I, Biebl H (2006) Environmental Biology of the Marine Roseobacter Lineage. Annu Rev Microbiol 60:255–280
Yurkov VV, Csotonyi JT (2009) New light on aerobic anoxygenic phototrophs. In: Hunter CN, Daldal F, Thurnauer MC, Beaty JT (eds) The purple phototrophic bacteria. Springer, Berlin, pp 31–55
Yurkov VV, van Gemerden H (1993) Impact of light/dark regimen on growth rate, biomass formation and bacteriochlorophyll synthesis in Erythromicrobium hydrolyticum. Arch Microbiol 159:84–89
Yutin N, Suzuki MT, Teeling H, Weber M, Venter JC, Rusch DB, Béjà O (2007) Assessing diversity and biogeography of aerobic anoxygenic phototrophic bacteria in surface waters of the Atlantic and Pacific Oceans using the Global Ocean Sampling expedition metagenomes. Environ Microbiol 9:1464–1475
Acknowledgments
This research was supported by the Czech Grant Agency project 206/07/0241, GAAV project IAA608170603 and the Inst. research concepts MSM6007665808 and AV0Z50200510. The authors thank Bc. Martin Marin for his help with the maintenance of the cultures, Dr. Dagmara Sirová for the microscopic analyses and Dr. Vladimíra Moulisová for providing the 16S rRNA sequence.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
About this article
Cite this article
Koblížek, M., Mlčoušková, J., Kolber, Z. et al. On the photosynthetic properties of marine bacterium COL2P belonging to Roseobacter clade. Arch Microbiol 192, 41–49 (2010). https://doi.org/10.1007/s00203-009-0529-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-009-0529-0