Abstract
Pellicle formation and lipopeptide production was analysed in standing cultures of different Bacillus subtilis strains producing two or three families of lipopeptides. Despite its ability to produce surfactin, B. Subtilis ATCC 6633 was unable to form stable pellicle at air–water interface. For the ATTC 21332 and ATCC 9943 strains, it was shown for the first time that the lipopeptides were also produced in standing cultures at productivities similar or lower than those obtained when the culture medium is agitated. A differentiated behaviour was observed between these strains in repetitive batch cultures. B. subtilis 9943 formed a wrinkled, thinner and more resistant pellicle than B. subtilis 21332. The structure of the pellicle determined by electron microscopy observations showed that cells of B. subtilis 9943 formed microcolonies whereas those of B. subtilis 21332 rapidly died. Under these conditions, surfactin production by strain 21332 decreased after 2 days whereas it remained stable for B. subtilis 9943 during the 6 days of the cultures. These data indicate that cells of B. subtilis strains growing in pellicle can produce lipopeptides differently depending on their cellular organisation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In natural habitats like rhizosphere, micro-organisms most commonly exist as bacteria communities exhibiting a high degree of structure. Bacillus subtilis is also well described to form architecturally complex communities named biofilms when cells are associated with surfaces. In a standing culture, cells switch from a submerged, highly motile planktonic state in which the bacteria swim as single cells, to a non-motile state in which the cells migrate to the air–liquid interface and proliferate as long chains bound together tightly by an extracellular matrix containing both exopolysaccharides and proteins and form a floating biofilm named pellicle (Branda et al. 2004, 2006; Kearns et al. 2005). This structured multicellular organisation enables cells to establish long-term relationships with each other and their immediate surroundings.
Bacillus subtilis strains can synthesize up to three distinct lipopeptide families: surfactins, iturins and fengycins (Stein 2005; Ongena and Jacques 2008). In this paper, three B. subtilis strains were used producing different types of lipopeptides: B. subtilis strain ATCC 9943 which secretes molecules belonging to the surfactin, the fengycin and the iturin families, B. subtilis strain ATCC 21332 which produces the single surfactin lipopeptide and B. subtilis ATCC 6633, co-producer of surfactin and mycosubtilin, a member of iturin family (Leenders et al. 1999). Lipopeptides belonging to the three families are amphiphilic cyclic peptides composed of ten amino acids for the fengycins or seven amino acids for iturins and surfactins. This peptidyl part is linked to a β-amino– in the case of the iturins and β-hydroxy-fatty acid for the surfactins and fengycins. The length of the fatty acid chain can vary from C13 to C16, C14 to C17 or C14 to C18 for the surfactins, iturins, and fengycins, respectively, and defines the homologous compounds of a family (Schneider et al. 1999; Bonmatin et al. 2003).
The three molecules are non-ribosomally synthesized by multienzymatic complexes. These large proteins can be subdivided in modules which contain the enzyme activities necessary for the incorporation of one amino acid into the peptide chain. The operons which encode these non-ribosomal peptide synthetase (NRPS) were all described. Three large open reading frames coding for surfactin synthetases are designated as srfA-A, srfA-B and srfA-C (Peypoux et al. 1999). They present a linear array of seven modules (one module per residue), three modules are present in the products of srfA-A and srfA-B and the last one in srfA-C. The fatty acid chain is added to the amino acid activated in the first module. Similarly, fengycin or plipastatin are synthesized by NRPSs encode by an operon with five open reading frames fenA-E (or ppsA-E) (Chen et al. 1995; Steller et al. 1999). Contrary to surfactin and fengycin, iturin derivatives are synthesized by a PKS-NRPS hybrid complex (Duitman et al. 1999; Tsuge et al. 2001; Moyne et al. 2004). The operon is constituted of four ORFs called fenF, mycA, mycB and mycC or ituD, ituA, ituB and ituC for mycosubtilin or iturin, respectively.
The expression of srfA is regulated via a complex network that governs cellular differentiation including quorum sensing via the biosynthesis of two pheromones PhrC and ComX (Hamoen et al. 2003). The expression of mycosubtilin is governed by another regulatory cascade of which AbrB forms the centre. AbrB is a pleiotropic regulator and one of the main transition state regulators in B. subtilis (Duitman et al. 2007). The mechanism for fengycin regulation is completely unknown.
Depending on the peptidyl sequence, these lipopeptides display different biological activities. The lipoheptapeptide surfactin is one of the most powerful biosurfactants known to act as a detergent on biological membranes (Peypoux et al. 1999; Carillo et al. 2003). Members of the iturin family exhibit strong antifungal and haemolytic activities (Maget-Dana and Peypoux 1994). Fengycins also show antifungal properties although restricted to filamentous fungi. A recent review has highlighted the importance of the lipopeptides in biocontrol and the diversity of their mode of action (Ongena and Jacques 2008). Indeed, these families of molecules are involved in most of the mechanisms described until now to explain biocontrol effects: root colonisation, antibiosis and induction of plant defence mechanisms (Leclère et al. 2005; Ongena et al. 2005, 2007). Attachment and aggregation in colonies or biofilms is the basic mechanism for colonizing plant roots.
Some studies have shown that surfactin was involved in biofilm formation at least on solid surfaces (Branda et al. 2001; Julkowska et al. 2005; Leclère et al. 2006). Hofemeister et al. (2004) have demonstrated that surfactin production is also necessary to pellicle formation. In addition, Bais et al. (2004) have shown that B. subtilis 6051 formed a stable extensive biofilm and secreted surfactin, both acting together to protect plants against attack by pathogens.
In this article, we compared the behaviour of different B. subtilis strains in shaken and standing cultures in term of growth, pellicle formation and lipopeptide production. We also determined the production kinetics of lipopeptides in non-agitated repetitive batch cultures and continuous cultures and analysed using an electron microscope the varying evolution of the different cells inside the pellicle they formed.
Materials and methods
Strains
Bacillus subtilis ATCC 9943, ATCC 21332 and ATCC 6633 were supplied by American Type Culture Collection. The three strains were stored at −20°C.
Batch cultures
All the media were complemented by 100 mmol l−1 MOPS and pH was adjusted to 7. The five media tested for the production were E medium (Davis et al. 1999), modified Landy medium (ML) (Guez et al. 2008), ACS medium (Vanittanakom et al. 1986), B medium (Julkowska et al. 2005) and MSgg medium (Branda et al. 2001). The temperature of the cultures was maintained at 30°C (±1). A single colony cultivated on Luria Bertani medium (LB) was inoculated in 5 ml of E medium and incubated at 300 rpm for 8 h. The culture was centrifuged for 15 min at 8,000 g, washed with distilled water and the optical density at 600 nm (OD600) was estimated using a spectrophotometer (Kontron, Rotkreuz, Switzerland). An E medium flask was inoculated with the cells to obtain an OD600 between 1 and 5 after 18 h of incubation at 120 rpm. This second culture was centrifuged and washed as described above and 50 ml of the different tested culture media were inoculated at initial OD600 of 0.5. Shaken cultures were cultivated at 120 rpm. Shaken and standing cultures were stopped after 1 to 5 days. All experiments were repeated three times.
Repetitive batch cultures and continuous cultures
Repetitive batch cultures were carried out in 500 ml flasks containing 50 ml of ML medium. The incubation was made at 30°C (±1) without agitation. Every 2 days and for 6 days, the medium was carefully removed from under the cellular layer and replaced with fresh medium. The concentration of lipopeptides was determined in each sample using HPLC analysis. The experiment was made in triplicate and every 2 days, one flask was stopped to analyse the pellicle by electron microscopy.
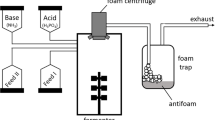
Continuous cultures were carried out using ML medium. The design of the system is shown in Fig. 1. First, flask 2 containing 400 ml of medium was inoculated with an overnight culture so as to obtain an initial OD600 equal to 0.5. The pellicle was allowed to form during 72 to 96 h at 32°C (±2). After this period of time, fresh medium was introduced through a hole in the pellicle layer. Feeding with fresh culture medium (flask 1) and removal of bioreactor content was started at a throughput of 0.15 l h−1. To avoid development of B. subtilis cells in the flask 1, the latter was put on ice and the contents were changed twice every 24 h. During incubation, 2 ml samples were taken regularly under the pellicle, after gentle agitation with a magnetic stirring bar, at different times. Each sample was subjected to HPLC analysis.
Electron microscopy
Two, 4- and 6-day-old pellicles from repetitive batch cultures were used for electron microscope observations. The culture medium was further pumped and replaced by a 1.5% agar solution. After solidification, samples were cut with a glass hollow rod. They were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 30 min, thoroughly washed and postfixed in 10 g l−1 OsO4 in the same buffer for 1 h. After another wash, the samples were ‘en bloc’ stained for 15 min with 2% aqueous uranyle acetate and dehydrated in graded acetonitrile solutions. After epoxy resin embedding, 90–100 nm sections were cut using a Reichert Ultracut E ultramicrotome and contrasted with uranyl acetate and lead citrate according to Reynolds (1963). The grids were observed with a Hitachi H600 electron microscope (Hitachi Ltd., Tokyo, Japan) at 75 kV.
Analyses of lipopeptides
For each sample, 1 ml of supernatant was purified on a C18 column (Extract-Clean SPE 500 mg, Alltech) and eluted in methanol. Analyses of purified lipopeptides were performed as described in Akpa et al. (2001).
Results
Influence of culture media on pellicle formation and lipopeptide productivity by B. subtilis ATCC 21332
Under vigourous shaking, B. subtilis ATCC 21332 produces great quantities of surfactin which is known to promote pellicle formation. This strain was chosen as a model to determine the best medium for synthesis of lipopeptides in standing cultures. Five media were compared and the productivity of surfactin was estimated at 72 h of culture, in standing or shaken culture conditions. ACS, ML and E media are known to promote surfactin production, B and MSgg media to promote biofilm formation (Branda et al. 2001, Julkowska et al. 2005). After 24 h of standing culture, the strain ATCC 21332 appeared as a thin pellicle on ML, ACS and E media whereas only small slab could be observed in MSgg and B media. At 48 h, a pellicle was present in all the media: it is thicker on ML, ACS and E media than on the other media and crests were observed on the surface.
The productivities of surfactin obtained at 72 h are shown in Fig. 2. Maximal values were obtained under shaken conditions on ML and E media (15.5 and 13.2 mg l−1 h−1, respectively). Maximal optical densities at 600 nm were comparable under both conditions (18 and 19, respectively). In these media, understanding conditions, productivities of surfactin were 9.2 and 8.8 mg l−1 h−1, respectively. The latter are greater than those obtained in ACS, B and MSgg media in shaken conditions, whereas OD600 obtained was quite high (9, 4.25 and 4.75, respectively). Under standing conditions, surfactin productivity in these three media was lower. The best surfactin productivity obtained with ML medium led us to retain it for the next experiments.
Productivity of surfactin by B. subtilis ATCC 21332 in five culture media after 72 h of culture under shaken at 140 rpm (planktonic culture) and standing (pellicle) conditions. Cultures were carried at 30°C. Mean values and standard deviations were obtained from three independent experiments: Planktonic culture (open square); Pellicle (filled square)
Analysis of pellicle formation and lipopeptide productivities by different strains
Two other surfactin producing strains, B. subtilis ATCC 6633 and B. subtilis ATCC 9943 were tested for lipopeptide production and pellicle formation in standing cultures on ML medium. B. subtilis ATCC 6633 is a co-producer of surfactin and mycosubtilin whereas B. subtilis ATCC 9943 produces the three families of lipopeptides (surfactin, iturin A and fengycin). After 72 h, no pellicle was observed for B. subtilis ATCC 6633 suggesting that surfactin production is not sufficient to ensure pellicle formation. The presence of lipopeptides was confirmed by thin layer chromatography (data not shown). For B. subtilis ATCC 9943 pellicle appeared thick, white and wrinkled. The productivities of the three lipopeptides at 72 h are shown in Fig. 3. Contrary to B subtilis 21332 where production of surfactin in the pellicle was only 59% of that obtained in shaken cultures, B subtilis 9943 showed equivalent surfactin productivity in both systems of culture. The production of the other two lipopeptides was less in standing cultures than in shaken ones (55 and 39% for fengycin and iturin, respectively).
Productivity of lipopeptides by B. subtilis ATCC 9943 after 72 h of culture at 30°C under shaken at 140 rpm (planktonic culture) and standing (pellicle) conditions. Mean values and standard deviations were obtained from three independent experiments. (open square) Planktonic culture; (filled square) Pellicle
Repetitive batch cultures
Repetitive batch cultures were performed in standing conditions with both pellicle forming strains. When the medium was pumped under the pellicle and replaced by fresh medium every 48 h, the two strains exhibited a different lipopeptide productivity calculated at each removal of medium. Surfactin productivity of B. subtilis 21332 regularly decreased every 48 h (Fig. 4a) whereas it increased with B. subtilis 9943 strain after the change of medium and remained stable from 48 h (Fig. 4b). The same effect was observed for the production of fengycins. Under these experimental conditions, the productivity of iturins was very low (under 0.42 mg l−1 h−1) and was not displayed in the figure.
Lipopeptide productivity by Bacillus subtilis during repetitive batch cultures. a Bacillus subtilis 21332. b Bacillus subtilis 9943 during semi-discontinuous culture. The culture medium is replaced every 48 h. Mean values and standard deviations were obtained from three independent experiments. Surfactin (filled square); Fengycin (open square)
In B. subtilis 21332, Yeh et al. (2005) have shown that surfactin is a growth-associated product. They also showed that its concentration decreases after depletion of glucose probably because cells assimilate it as a carbon source. This was in agreement with the high productivity observed after 48 h of culture and the surfactin decrease observed from 96 h of culture onwards with this strain. In contrast, B. subtilis 9943 did not show a surfactin and fengycin decrease but also did not produce significant quantities of iturin.
Continuous cultures
As the concentration of surfactin produced by B. subtilis 21332 in repetitive batch decreased with time, probably as the result of carbon source depletion, renewal of medium under the pellicle should improve the yield of surfactin. Continuous cultures were thus performed with this strain and with B. subtilis 9943. The productivities obtained are shown in Table 1 and compared with results in repetitive batch cultures. The productivities were calculated according the mode of culture: from pseudo fed-batch cultures, it represents the average of the productivities obtained at 48, 96 and 144 h and from continuous cultures it was calculated taking into account the dilution rate (0.15 h−1).
In our experimental conditions, for the strain 21332, the lipopeptide productivities were very low (less than 1.05 mg l−1 h−1) when the medium was renewed at 0.06 l h−1. In contrast, in the continuous condition the strain 9943 showed a relatively high surfactin productivity and a better fengycin productivity than in repetitive batch cultures. The different levels of productivity between both strains suggest that the strains behave differently for lipopeptide production.
Organization and physiological state of the cells in the pellicle
Twenty-four hours after the inoculation of both strains in the ML medium, pellicles had already formed and visual examination showed irregular ridges more marked for B subtilis 9943. The thickness of the pellicule, measured using confocal observations, developed by cells of B. subtilis 21332 (0.9–1.6 mm) was approximately 1.5 times as much as that formed by B. subtilis 9943 (0.6–1 mm). Nevertheless, the latter was more difficult to break indicating higher density and resistance.
Some differences were observed between the fine structure of the pellicles of both strains. The microscopic observations of vertical thin sections (Figs. 5, 6) showed vegetative cells dispersed in a matrix for B. subtilis 21332 at 48 h of culture whereas many empty cells envelopes and spores in the lower surface (containing the nutrient medium) were present in the pellicle formed by B. subtilis 9943. This observation could explain the high level of surfactin obtained with B. subtilis 21332 from 48 h of culture because cells were in a better physiological state to produce lipopeptides compared to those of B. subtilis 9943.
Electron micrographs of thin sections of Bacillus subtilis 21332 pellicle obtained on repetitive batch standing cultures. Each column shows consecutives views through a transversal section of a biofilm from surface to bottom. a–c 48 h of culture. The cells are evenly scattered through the biofilm. d–f 96 h of culture. Numerous empty envelopes can be seen. g–i 144 h of culture. Numerous empty envelopes can also be seen and only a small part of the biofilm contains live cells (i). Bar 10 μm
Electron micrographs of thin sections of Bacillus subtilis 9943 pellicle obtained on repetitive batch standing culture. Each column shows consecutive views through a transversal section of a biofilm from surface to bottom. a–c 48-h of culture. Arrowheads represent spores. d–f 96-h of culture. Numerous homogeneous colonies can be observed (star). g–i 144-h of culture. The colonies seem heterogeneous. Bar 10 μm
In the 96-h-old pellicle, cells of B subtilis 21332 were deformed, became longer than cells 48 h earlier (Fig. 5) and as observed in confocal imaging, they formed chains bound together (arrows in Fig. 7). Many cells became empty. This phenomenon was accentuated at 144 h. Tenerio et al. (2003) had shown with E. coli that elongated cells were less adherent and formed a fragile biofilm. This agreed with the observed case where the pellicle formed by cells of strains 21332 could be broken. Moreover at 96 h, the latter was a little thinner (1.6–4.1 mm; according to the measurements made next to or on ridges) than pellicles formed by cells of B. subtilis 9943 (1.8–4.3 mm) suggesting the use of matrix as nutrients by B. subtilis 21332. Contrary to the latter strain, from 48 h, cells of B. subtilis 9943 were not deformed into elongated cells and they aggregated to form microcolonies (Fig. 6; star in Fig. 7).
At 144 h, cells became deformed for the two strains and they emptied. From 8- to 10-day-old, pellicles formed by B. subtilis 21332 tended to disintegrate and break into fragments. This phenomenon was slowly delayed for B subtilis 9943.
Discussion
In this study, lipopeptide productivity and pellicle formation by B. subtilis ATCC 21332 were first checked in five different media. A pellicle is formed in each tested condition. However, it appeared faster on ML, ACS and E media and was thicker on the three media after 48 h of culture compared to the two other tested conditions. Comparing the composition of E and ML media with the three others showed some differences on the basis of the presence or absence of MnSO4, CuSO4 and yeast extract. The latter component was already shown to promote lipopeptide production in several studies (Jacques et al. 1999; Al-Ajlani et al. 2007; Guez 2007) and was probably responsible for the best results obtained with E and ML media which both contained yeast extract, on the contrary to the three other media.
Comparison of surfactin productivity in the different conditions highlighted the importance of the choice of culture conditions. For planktonic cultures, the productivity in ML medium was more than 40 times higher than this obtained in MSgg medium. When this production is related to optical density, the rate is still more than 20 times higher. Interestingly, while lower than in planktonic culture, lipopeptide biosynthesis is still important and similar dependence to culture media was observed. To our knowledge, this is the first time that quantitative measurements were proposed for the lipopeptide production by cells organized in a pellicle.
In our experimental conditions, we observed that two of the strains (B. subtilis ATCC 21332 and ATCC 9943) are able to form a pellicle and the latter exhibited distinctive characteristics according to the strain. In particular, the robustness of the surface and the appearance of the pellicle were different (more ridges with 9943 than with 21332). This can be assigned to a different social behaviour leading to a particular architecture as observed by microscopic observations.
Standing liquid cultures tend to deprive cells of oxygen. According to the strains the response to this stress differs. Cells of B. subtilis 21332 showed morphological changes (cells became longer) whereas B. subtilis 9943 emptied and sporulated in the area of film adjacent to the liquid. Mogilnaya et al. (2003) also observed from B. subtilis cultivated in similar conditions, that sporulation mainly occurred in the film depth. After this period of distress, B. subtilis 9943 would opt for a multicellular organization with microcolonies creating a circulation network for feed and metabolic products. B. subtilis 21332 did not show this arrangement and cells rapidly died.
The production of lipopeptides can be correlated with the specific physiological state of cells in the pellicle. B. subtilis 21332, which showed a high level of surfactin in the pellicle after 48 h of culture, produced less surfactin during the following days. Microscopic observations showed that cells began to sporulate after 48 h in the pellicle. Surfactin synthesis expression and sporulation are controlled by a mechanism linked to quorum sensing by the way of pheromones as PhrC and ComX produced and recognized by the cells. Such a mechanism is probably promoted in pellicles or biofilms and could explain the results obtained with strain 21332. The different behaviour observed with strain 9943 could be an indication of different regulation processes. The formation of microcolonies allows this latter strain to produce surfactin and fengycin lipopeptide during a longer period of time. Selection of strains able to develop such a structured multicellular organisation could be interesting in different applications such as development of bioprocesses for lipopeptide production with immobilized cells or in biocontrol of plant diseases for which cells are probably frequently organized as biofilms.
References
Al-Ajlani MM, Sheikh MA, Ahmad Z, Hasnain S (2007) Production of surfactin from Bacillus subtilis MZ-7 grown on pharmamedia commercial medium. Microb Cell Fact 6:17
Akpa E, Jacques P, Wathelet B, Paquot M, Fuchs R, Budzikiewicz H, Thonart P (2001) Influence of culture conditions on lipopeptide production by Bacillus subtilis. Appl Biochem Biotechnol 91:551–561
Bais HP, Fall R, Vivanco JM (2004) Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 134:307–319
Bonmatin JM, Laprevote O, Peypoux F (2003) Diversity among microbial cyclic lipopeptides: iturins and surfactins activity–structure relationships to design new bioactive agents. Comb Chem High Throughput Screen 6:541–556
Branda SS, Gonzalez-Pastor JE, Ben-Yehuda S, Losick R, Kolter R (2001) Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci 98:11621–11626
Branda SS, Gonzalez-Pastor JE, Dervyn E, Ehrlich SD, Losick R, Kolter R (2004) Genes involved in formation of structured multicellular communities by Bacillus subtilis. J Bacteriol 186:3970–3979
Branda SS, Chu F, Kearns DB, Losick R, Kolter R (2006) A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol 59:1229–1238
Carillo C, Teruel JA, Aranda FJ, Ortiz A (2003) Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochim Biophys Acta 1611:91–97
Chen CL, Chang LK, Chang YS, Liu ST, Tschen JSM (1995) Transposon mutagenesis and cloning of the genes encoding the enzymes of fengycin biosynthesis in Bacillus subtilis. Mol Gen Genet 248:121–125
Davis DA, Lynch HC, Varley J (1999) The production of surfactin in batch culture by Bacillus subtilis ATCC 21332 is strongly influenced by the conditions of nitrogen metabolism. Enzyme Microb Technol 25:322–329
Duitman EH, Hamoen LW, Rembold M, Venema G, Seitz H, Saenger W, Bernhard F, Reinhardt R, Schmidt M, Ullrich C, Stein T, Leenders L, Vater J (1999) The mycosubtilin synthetase of Bacillus subtilis ATCC6633: a multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. Proc Natl Acad Sci USA 96:13294–13299
Duitman EH, Wyczawski D, Boven LG, Venema G, Kuipers OP, Hamoen LW (2007) Novel methods for genetic transformation of natural Bacillus subtilis isolates used to study the regulation of the mycosubtilin and surfactin synthetases. Appl Environ Microbiol 73:3490–3496
Guez JS (2007) Etude de la productivité et de la sélectivité de la biosynthèse de mycosubtiline, un antibiotique surfactant de Bacillus subtilis. Apport du génie biochimique et de la transcriptomique. Ph.D. thesis, Université des Sciences et Technologies de Lille, France
Guez JS, Müller CH, Danze PM, Büchs J, Jacques P (2008) Respiration Activity MOnitorong System (RAMOS), an efficient tool to study the influence of oxygen transfer rate on the synthesis of lipopeptides by Bacillus subtilis ATCC 6633. J Biotechnol 134:121–126
Hamoen LW, Kausche D, Marahiel MA, van Sinderen D, Venema G, Serror P (2003) The Bacillus subtilis transition state regulator AbrB binds to the–35 promoter region of comK. FEMS Microbiol Lett 218:299–304
Hofemeister J, Conrad B, Adler B, Hofemeister B, Feesche J, Kucheryava N, Steinborn G, Franke P, Grammel N, Zwintscher A, Leenders F, Hitzeroth G, Vater J (2004) Genetic analysis of the biosynthesis of non-ribosomal peptide- and polyketide-like antibiotics, iron uptake and biofilm formation by Bacillus subtilis A1/3. Mol Genet Genomics 272:363–378
Jacques P, Hbid C, Destain J, Razafindralambo H, Paquot M, De Pauw E, Thonart P (1999) Optimization of biosurfactant lipopeptide production from Bacillus subtilis S499 by Plackett–Burman design. Appl Biochem Biotech 77:223–233
Julkowska D, Obuchowski M, Holland IB, Seror SJ (2005) Comparative analysis of the development of swarming communities of Bacillus subtilis 168 and a natural wild type: critical effects of surfactin and the composition of the medium. J Bacteriol 187:65–76
Kearns DB, Chu F, Branda SS, Kolter R, Losick R (2005) A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol 55:739–749
Leclère V, Béchet M, Adam A, Guez JS, Wathelet B, Ongena M, Thonart P, Gancel F, Chollet-Imbert M, Jacques P (2005) Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl Environ Microbiol 71:4577–4584
Leclère V, Marti R, Béchet M, Fickers P, Jacques P (2006) The lipopeptides mycosubtilin and surfactin enhance spreading of Bacillus subtilis strains by their surface-active properties. Arch Microbiol 186:475–483
Leenders F, Stein TH, Kablitz B, Franke P, Vater J (1999) Rapid typing of Bacillus subtilis strains by their secondary metabolites using matrix-assisted laser desorption/ionization mass spectrometry of intact cells. Rapid Commun Mass Spectrom 13:943–949
Maget-Dana R, Peypoux F (1994) Iturins, a special class of pore-forming lipopeptides: biological and physicochemical properties. Toxicology 87:151–174
Mogilnaya OA, Krylova TY, Popova LY (2003) Development and morphological features of biofilms formed by transgenic and wild type strains of Bacillus subtilis. Microbiol Res 158:327–335
Moyne AL, Cleveland TE, Tuzun S (2004) Molecular characterization and analysis of the operon encoding the antifungal lipopeptide bacillomycin D. FEMS Microbiol Lett 234:43–49
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Ongena M, Jacques P, Touré Y, Destain J, Jabrane A, Thonart P (2005) Involvement of fengycin-type lipopeptides in the multifaced biocontrol potential of Bacillus subtilis. Appl Microbiol Biotechnol 69:29–38
Ongena M, Jourdan E, Adam A, Paquot M, Brans A, Joris B, Arpigny JL, Thonart P (2007) Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ Microbiol 9:1084–1090
Peypoux F, Bonmatin JM, Wallach J (1999) Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol 51:553–563
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain for electron microscopy. J Cell Biol 17:208–212
Schneider J, Taraz K, Budzikiewicz H, Deleu M, Thonart P, Jacques P (1999) The structure of two fengycins from Bacillus subtilis S499. Z Naturforsch 54c:859–866
Stein T (2005) Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol 56:845–857
Steller S, Vollenbroich D, Leenders F, Stein T, Conrad B, Hofemeister J, Jacques P, Thonart P, Vater J (1999) Structural and functional organization of the fengycin synthetase multienzyme system from Bacillus subtilis b213 and A1/3. Chem Biol 6:31–41
Tenerio E, Saeki T, Fujita K, Kitakawa M, Baba T, Mori H, Isono K (2003) Systematic characterization of Escherichia coli genes/ORFs affecting biofilm formation. FEMS Microbiol Lett 225:107–114
Tsuge K, Akiyama T, Shoda M (2001) Cloning, sequencing, and characterization of the iturin A operon. J Bacteriol 183:6265–6273
Vanittanakom N, Loeffler W, Koch U, Jung G (1986) Fengycin-a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J Antibiot 39:888–901
Yeh MS, Wei YH, Chang JS (2005) Enhanced production of surfactin from Bacillus subtilis by addition of solids carriers. Biotechnol Prog 21:1329–1334
Acknowledgments
This work was supported by the Université des Sciences et Technologies de Lille, the Region Nord Pas de Calais, the Ministere de la Recherche Scientifique (ANR) and the European Funds for Regional Development. The authors thank William Everett for the re-reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
M. Chollet-Imbert and F. Gancel have contributed equally to the scientific work.
Rights and permissions
About this article
Cite this article
Chollet-Imbert, M., Gancel, F., Slomianny, C. et al. Differentiated pellicle organization and lipopeptide production in standing culture of Bacillus subtilis strains. Arch Microbiol 191, 63–71 (2009). https://doi.org/10.1007/s00203-008-0429-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-008-0429-8