Abstract
A Bacillus sp. strain producing a bacteriocin-like substance was characterized by biochemical profiling and 16S rDNA sequencing. The phylogenetic analysis indicated that this strain has low sequence similarity with most Bacillus spp., suggesting a new species was isolated. The antimicrobial activity was detected starting at the exponential growth phase, and maximum activity was observed at stationary phase. The substance was inhibitory to a broad range of indicator strains, incluing pathogenic and food spoilage bacteria such as Listeria monocytogenes, B. cereus, Aeromonas hydrophila, Erwinia carotovora, Pasteurella haemolytica, Salmonella Gallinarum, among other. The antibacterial substance was stable over a wide pH range, but it was sensitive to pronase E and lipase. The antibacterial substance was bactericidal and bacteriolytic to L. monocytogenes and B. cereus at 160 AU ml−1. The identification of a broad range bacteriocin-like inhibitory substance active against L. monocytogenes addresses an important aspect of food protection against pathogens and spoilage microorganisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimicrobial substances are widespread produced among bacteria. Bacteriocins and bacteriocin-like inhibitory substances (BLIS) are antimicrobial peptides produced by a number of different bacteria that are often effective against closely related species (Tagg et al. 1976; Riley and Wertz 2002).
Bacteriocins have received increasing attention due to their potential use as natural preservatives in food industry, as probiotics in the human health, and as therapeutic agents against pathogenic microorganisms (Riley and Wertz 2002). Althoug research efforts are mainly focused on bacteriocins produced by lactic acid bacteria, bacteriocins from a variety of Gram-positive and Gram-negative species have been characterized (Gould 1996; McAuliffe et al. 2001). They can be divided into three major classes of which class I and II are quite heat-stable. Class I contains modified bacteriocins, so-called lantibiotics, that are found amongst many different Gram-positive bacteria but have yet to be found in Gram-negative bacteria. They are divided into two subclasses (a) the linear and cationic peptide and (b) the globular peptides; the latter normally are hydrophobic but not cationic. The second major class (II) contains small heat-stable bacteriocins that lack posttranslational modifications as found in lantibiotics, and are presently clustered into at least two groups: pediocin-like bacteriocins and two-peptide bacteriocins (Diep and Nes 2002). A third class (III) of bacteriocins has been also defined. They are normally larger in size and are easily subjected to heat inactivation (Klaenhammer 1993).
The conventional wisdom about the killing range of bacteriocins from Gram-positive bacteria is that they are restricted to killing other Gram-positive bacteria. The range of susceptible strains can vary significantly, from relatively narrow as in the case of lactococcins A, B and M, which have been found to kill only Lactococcus, to extraordinarily broad (Ross et al. 1999).
Bacillus species are aerobic spore formers commonly found in soil and ground water and often encountered on plants and animals at the point of harvest or slaughter. Bacillus is a genus that have been investigated for producing so-called bacteriocin-like inhibitory substance (BLIS) and strains of B. thuringiensis, B. subtilis, B. stearothermophilus, B. licheniformis, B. megaterium and B. cereus have been reported to produce BLIS (Stein 2005; Gray et al. 2006; He et al. 2006; Lisboa et al. 2006; Sharma et al. 2006).
We recently reported antimicrobial activity among several bacteria isolated from aquatic environments of Brazilian Amazon basin (Motta et al. 2004). The bacterium P34 was isolated from the Amazonian fish Piau-com-pinta as a strain producing an antimicrobial substance that inhibits the pathogen Listeria monocytogenes. This antimicrobial substance has a molecular mass of 1,456 Da, was relatively heat stable and sensitive to proteolytic enzymes, suggesting a lipopetide molecule (Motta et al. 2007). The objective of this work was the description of a new Bacillus sp. strain P34 and to provide further characterization of its antibacterial substance.
Materials and methods
Reagents and media
Brain heart infusion (BHI) broth was from Oxoid (Basingstoke, UK). Trypticase soy broth (TSB) was from Acumedia (Baltimore, USA). Lipase (from Candida rugosa), trypsin and pronase E were from Sigma (St. Louis, USA). All other media and reagents were from Merck (Darmstadt, Germany).
Bacterial strains and culture conditions
The producer strain P34 was given by Universidade Federal do Amazonas (Manaus, Brazil). The organism was isolated from the intestinal contents of the teleost fish Piau-com-pinta (Leporinus sp.) of Amazon basin, at central Amazonia, near Manaus, Brazil (3°06′S, 60°01′W).
The indicator strains used in the study were from ATCC (American Type Culture Collection, Rockville, USA), NCTC (National Collection of Type Culture, Colendale, UK) and our own culture collection (UFRGS, Porto Alegre, Brazil) and were kept frozen at −21°C in BHI containing 20% (v/v) glycerol.
Taxonomical studies
Phenotypic characterization of the strain P34 included morphological, cultural, physiological, biochemical and antibiotic susceptibility features, listed in Table 1. All test procedures were carried out as described elsewhere (Claus and Berkeley 1986; MacFaddin 2000). B. subtilis ATCC 6633 and B. licheniformis ATCC 14580 were used as reference strains. Additionally, an API 50CHB kit was used and the data was submitted to automated interpretation using the APILAB Plus software (BioMérieux, Marcy-l’Etoile, France).
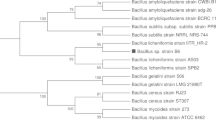
The sequence of 16S rDNA was obtained after genomic DNA extraction, PCR amplification and sequencing based on previous work (Bastos et al. 2000). The bacterial 16S rRNA sequencing primers were fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and rD1 (5′-AAGGAGGTGATCCAGCC-3′); 341–357f (5′-CCTACGGGAGGCAGCAG-3′) and 357–341r (5′-CTGCTGCCTCCCGTAGG-3′); 685–704f (5′-GTAGSGGTGAAATSCGTAGA-3′) and 704–685r (5′-CTACGSATTTCACCSCTAC-3′); 1099–1114f (5′-GCAACGAGCGCAACCC-3′) and 1114–1099r (5′-GGGTTGCGCTCGTTGC-3′). The DNA was amplified using a Geneamp PCR System 2400 (Perkin Elmer, Norwalk, USA) by denaturation at 96°C (3 min), 30 cycles consisting of 94°C (1 min), 55°C (30 s) and 72°C (2 min), and a final extension step at 72°C (7 min). The PCR-amplified 16S rDNA was sequenced by the ABI Prism 377 DNA Sequencer (Perkin Elmer) based on fluorescent-labeled dideoxynucleotide terminators. The 1,522-bp sequence was submitted to Genbank (accession number AY962472). The BLAST algorithm was used to search for homologous sequences in Genbank. The phylogenetic tree was inferred from Jukes-Cantor distances using the neighbor-joining method (software MEGA3, Kumar et al. 2004). The branching pattern was checked by 1,000 bootstrap replicates.
Transmission electron microscopy
Cells of strain P34 were harvested from BHI agar plates after 24 h of incubation at 37°C. The cells were fixed with 2.5% (v/v) glutaraldehyde, 2% (v/v) formaldehyde in 0.12 mol l−1 phosphate buffer for 10 days and then posfixed in 2% (w/v) osmium tetroxide in the same buffer for 45 min before dehydration. Dehydration was done in a graded acetone series (30–100%) and embeeding in Araldite-Durcupan for 72 h at 60°C. Thin sections were prepared with a Leica Ultracul UCT ultramicrotome (Leica, Benshein, Germany), mounted on grids, covered with collodium film, and poststained with 2% (w/v) uranyl acetate in Reynold’s lead citrate. All preparations were observed with a Philips EM 208-5 transmission electron microscope (Philips Electronic Instruments Inc., Mahwah, USA) operating at 100 kV.
Production of antimicrobial substance
For the production of antibacterial substance, the strain P34 was grown in 100 ml BHI-medium at 30°C in a rotary shaker at 180 cycles min−1 for desired times. Determination of the number of viable cells (CFU ml−1) was carried out as described elsewhere (Motta and Brandelli 2002). After cultivation for 24 h, the cells were harvested by centrifugation at 10,000g for 15 min and the culture supernatant was sterilized by filtration with 0.22 μm membranes (Millipore, Bedford, USA). The filtrate was precipitated with ammonium sulfate at 20% saturation. The precipitate was dissolved in 10 mM phosphate buffer pH 7.0. This solution was further purified by gel filtration chromatography on a Sephadex G-100 column. Fractions positive for antimicrobial activity were pooled and applied to a column of DEAE-Sepharose, eluted with the same buffer followed by a gradient from 0 to 1.5 M NaCl. The active peaks were dialyzed and rechromatographed according to the same process. Purity was checked by cappilary zone electrophoresis, performed as described elsewhere (Bastiani et al. 2002), and using a 60 cm × 50 μm capillary. Samples of 5 ml were run in 50 mM borate buffer pH 9.2, and detected by laser fluorescence.
Antimicrobial activity assay
Antimicrobial activity was determined essentially as described previously (Motta and Brandelli 2002). An aliquot of 20 μl of partially purified BLIS was applied on discs (6 mm) on BHI agar plates previously inoculated with a swab submerged in a indicator strain suspension which corresponded to a 0.5 McFarland turbidity standard solution. Plates were incubated at the optimal temperature of the test organisms (Table 2) and inhibitory zones were measured after 24 h.
The antimicrobial activity titre was determined by the serial twofold dilution method previously described by Mayr-Harting et al. (1972). Activity was defined as the reciprocal of the dilution after the last serial dilution giving an inhibition zone and expressed as arbitrary unit (AU) per mililitre. The AU ml−1 were determined against L. monocytogenes ATCC 7644 as indicator strain.
Chemical stability
Partially purifed BLIS samples were used to determine the susceptibility in different conditions essentialy as described previously (Cladera-Olivera et al. 2004). Samples (1 ml) were treated at 37°C for 60 min with 2 mg ml−1 (final concentration) of trypsin, pronase E and lipase. Samples were then boiled for 3 min for enzyme inativation.
The antimicrobial activity at different pH values was estimated by adjusting the pH of samples from pH 3.0 to 10.0. To evaluate pH stablitity, the antimicrobial substance was incubated at pH 3.0–10.0 for 30 min and the pH was neutralized to 7 before testing for antimicrobial activity.
Chemicals (working concentration in Table 3) were added to the antimicrobial substance and the samples were incubated for 60 min at 37°C before being tested for antimicrobial activity. After treatments with TCA, samples were centrifuged at 10,000g for 5 min, pellet was solubilized in 10 mM Tris pH 8.0 and the supernatant was neutralized to pH 7.0, before testing for antimicrobial activity. Chemicals diluted with 8.75 g l−1 NaCl were used as controls.
After each treatment the samples were tested for antimicrobial activity againts L. monocytogenes ATCC 7644.
Effect on L. monocytogenes and B. cereus
Overnight cultures of L. monocytogenes ATCC 7644 and B. cereus 8A were obtained by growing in TSB medium at 37°C for 18 h. A sample (1%) of those cultures were inoculated in Erlenmeyer flasks containing 50 ml TSB and incubated at 37°C. The growth was checked at 2-h intervals by O.D. 600 nm and by viable cell counts (CFU ml−1).
BLIS (final concentration 160 AU ml−1) was added separately, to culture of L. monocytogenes and B. cereus after 6 h of growth and the effect on turbidity and on the number of viable cells was determined at 2-h intervals. The colonies were counted after 24 h of incubation at 37°C.
Results
Characterization of strain P34
The morphological and physiological characteristics of the isolate are summarized in Table 1. Microscopic observation of the isolate showed a straight rod with endospores. The spores were eliptical, located at subterminal position. Morphological features were detailed by transmission electron microscopy, revealing a typical Gram-positive cell envelope profile (Fig. 1). The cytoplasmic membrane was surrounded by a thin peptidoglycan layer; an overlaid surface layer was separated from peptidoglycan by a zone of low contrast. The bacterium grew aerobically, was strongly catalase positive, presented variable Gram-stain, and was Gram-positive in the KOH test. Together with additional biochemical tests (Table 1) and the use of an API 50CHB kit, these characteristics indicated that the isolate belongs to the genus Bacillus (Claus and Berkeley 1986). The analysis with the APILAB Plus software indicated a very good identity to the genus Bacillus.
The phylogenetic analysis confirmed that the isolate was a Bacillus sp. and revealed that strain P34 was closest to B. infernus (Fig. 2). The P34 sequence shared low similarity with most Bacillus species and showed 91% similarity with B. infernus. The cluster formed by P34 and B. infernus was supported by high bootstrap values (Fig. 2).
Production of antimicrobial activity
Bacillus sp. P34 was aerobically incubated in BHI medium at 30°C. Exponential cell growth reached the stationary phase after 12 h of cultivation (Fig. 3). Remarkably, antibacterial activity can be observed in the exponential growth phase (6 h), with maximal values in the stationary phase (24–30 h). It was observed that pH values were nearly constant (pH = 7.0–7.5) during cultivation.
The antimicrobial substance obtained at 24 h of cultivation, was then purified from the culture supernatant by ammonium sulfate precipitation and liquid chromatography. The antimicrobial activity eluted in the void volume of the Sephadex G-100 column, separated of the main bulk of protein. This column was also eluted with buffer containing 1.5 M NaCl, and the activity was detected within the included volume of the column (not shown). Further purification by ion exchange chromatography yielded a single peak containing the antimicrobial activity. A single component was observed by capillary electrophoresis, confirming the homogeneity of the purified molecule. As a single antimicrobial substance was detected, subsequent experiments were carried out with the partially purified fraction from gel filtration chromatography.
Inhibitory spectrum
The antimicrobial substance was active against Gram-positive and Gram-negative bacteria, including important pathogenic and spoilage microorganisms. The results are shown in Table 2. The inhibitory activity was observed on L. monocytogenes, L. innocua, Corynebacterium fimi, B. cereus, B. subtilis, Erwinia carotovora, Aeromonas hydrophila, Pasteurella haemolytica, among other. L. monocytogenes ATCC 7644 was used as indicator strain in subsequent experiments.Fig. 4
Chemical stability
The antimicrobial substance was tested for sensitivity to enzymes and residual activity was measured by agar disc diffusion assay against L. monocytogenes ATCC 7644. The substance was sensitive to lipase, trypsin and pronase E at the concentration of 2 mg ml−1 (Table 3).
The effect of several chemicals on the antimicrobial activity was evaluated. The substance lost its activity after treatment with trichloroacetic acid (TCA) and 2-mercaptoethanol (Table 3). When treated with organic solvents and other chemicals, the antimicrobial activity was only affected by butanol, and in lesser extent by acetone and methanol (Table 3).
The antimicrobial substance was stable in all pH tested (3.0–10.0), remaining 100% its initial activity. When the activity was tested within this pH range, at least 70% of maximum activity, observed at pH 6.0–8.0, was observed.
Considering the properties of the inhibitory substance produced by Bacillus sp. strain P34, it was characterized as a bacteriocin-like compound.
Effect on L. monocytogenes and B. cereus
The effects of the BLIS on the growth of L. monocytogenes and B. cereus are shown in Fig. 4. The addition of BLIS (160 AU ml−1) to cells suspensions of L. monocytogenes or B. cereus at the exponential growth phase results in a difference in viable counts related to the controls. The addition of the antimicrobial substance inhibited the growth of the indicator strains resulting in a decrease in the number of viable cells and in optical density over a period of 24 h (Fig. 4). This indicated that BLIS has a bactericidal and bacteriolytic effect.
Discussion
A bacterium producing a BLIS was isolated from the intestinal contents of Leporinus sp., a teleost fish of Amazon basin, and was identified as Bacillus sp. by biochemical profiling and 16S rDNA sequencing. From the sequence analysis of the 16S rDNA gene, strain P34 was found to be clustered with the B. infernus, although an important difference in sequence was observed. In addition, B. infernus thrives in heat and anaerobiosis (Boone et al. 1995). Because these characteristics are not in agreement with those of strain P34, this species can be assigned to the genus level as Bacillus sp. These data suggest that our isolate is a new Bacillus species (Stackebrandt and Goebel 1994; Palys et al. 1997; Goto et al. 2000). Considering it was isolated from a fish of Brazilian Amazon basin, we propose this bacterium be classified in the genus Bacillus as “B. amazonensis”.
The antimicrobial substance was shown to be broadly active, which is also typical of Gram-positive BLIS and particularly of antimicrobial peptides produced by Bacillus species (Jack et al. 1995; Hyronimus et al. 1998; Bizani and Brandelli 2002; Risoen et al. 2004). C. fimi NCTC 7547, described as being susceptible to all bacteriocins tested (Oliveira et al. 1998), was also sensitive to the BLIS produced by Bacillus sp. P34. The antimicrobial substance was able to inhibit the growth of L. monocytogenes, a very important property in food safety. A. hydrophila, an important pathogen linked to seafood and water outbreaks (Tsai and Chen 1996), was also inhibited. Therefore, this novel BLIS may represent a relevant alternative against several food pathogenic and spoilage microorganisms.
The production of antimicrobial activity stated during the exponential growth phase, reaching maximum values at stationary phase. Production of antimicrobial peptides by strains of Bacillus is suggested to be under complex genetic regulation (Marahiel et al. 1993; Duitman et al. 1999). It has been observed that modification of the culture conditions, like nitrogen source and pH, may induce the production of different antimicrobial peptides. A single strain can produce different antimicrobial peptides simultaneously or at least at different time intervals or growth conditions (Duitman et al. 1999). Decrease of antimicrobial activity at the late stationary phase could be associated to degradation by extracellular proteases, which are often produced by Bacillus spp. (Bizani and Brandelli 2002). However, most of the known bacteriocins are highly stable against the proteases of producer strain. The activity of linocin M18, a bacteriocin that forms large proteinaceous aggregates, diminished rapidly after 1–2 days at room temperature, even in the presence of protease inhibitors (Valdes-Stauber and Scherer 1994).
Bacteria can produce a variety of inhibitory substances. In this case, organic acids can be ruled out, since the pH in the growth medium was always in the range of 7.0–7.5 during cultivation in BHI. The antimicrobial activity was sensitive to the enzymes tested, with complete inactivation by the broad range protease pronase E, indicating its proteinaceous nature. In addition, BLIS sensitivity to lipase may suggest a lipopeptide structure, in agreement to previous results of infrared spectroscopy (Motta et al. 2007). A bacteriocin produced by B. licheniformis, Lichenin A, was completely inactivated by proteinase K treatment but was resistant to trypsin (Pattnaik et al. 2001). Enzymes such as proteinase K and pronase E were shown to eliminate thuricin 439 activity, indicating a bacteriocin-like inhibitory compound (Ahern et al. 2003). The fact that the antimicrobial activity was completely lost by only few enzymes and was inactivated by 2-mercaptoethanol may suggest that the BLIS have intamolecular disulfide bonds. This agrees with the fact that the inhibitory compound was relatively heat stable and shows stability within the pH range of 3.0–10.0. A BLIS from B. amyloliquefaciens strain I3 was recently characterized (Lisboa et al. 2006), showing different pH stability and increased resistance to proteases when compared with P34.
The BLIS appeared as a single band with a molecular mass of about 6 kDa, and shown a molecular mass of 1,456 Da by mass spectroscopy (Motta et al. 2007). However, the antimicrobial activity eluted in the void volume of the Sephadex G-100 column. This indicates that native BLIS forms aggregates of high molecular masses (>150 kDa). This behavior was similar to that observed for the bacteriocin linocin M18 (Valdes-Stauber and Scherer 1994). Some bacteriocin molecules present a substantial portion of hydrophobic residues and their association into large aggregates is possibly because of the highly hydrophobic nature of the peptides.
The decline in the number of living cells of L. monocytogenes and B. cereus after the addition of BLIS suggest that the antimicrobial effect was bactericidal. The decrease in O.D. readings indicated that cells of indicator strains were lysed. It has been suggested that the antimicrobial effect can be dependent on the assay conditions, such as the amount and purity of bacteriocin, culture media, indicator strain and its cellular concentration (Motta and Brandelli 2002). The BLIS identified in this work showed bactericidal and bacteriolytic effect on L. monocytogenes and B. cereus at 160 AU ml−1.
Bacteriocins may play a defensive role to hinder the invasion of ecosystem of other strains or species into an occupied niche (Riley and Wertz 2002). Antibacterial substances produced by different bacteria seem to play an important role in the bacterial antagonism in aquatic ecosystems (Dopazo et al. 1988). In addition, it has been found that intestinal bacteria from both freshwater and marine fishes show an inhibitory effect on fish pathogenic bacteria (Olsson et al. 1992; Sugita et al. 1996). These include antimicrobial substances produced by Bacillus strains isolated from fish intestines (Sugita et al. 1998; Ghosh et al. 2003). A similar role could be assigned to the strain P34, where its BLIS may help to avoid pathogen colonization of fish intestines. The broad inhibitory spectrum of strain P34 may indicate an ecological advantage, since it would be capable to inhibit several competing bacteria.
While many studies on BLIS have shown their importance as food preservatives, few attention have been addressed to their application as antimicrobials in clinical studies. Because bacteriocin treatment is potentially effective and non-toxic to human and animals, it has been already proposed as an alternative for disease control (Oliveira et al. 1998; Twomey et al. 2000). The BLIS produced by strain P34 may represent an antimicrobial substance with potential application in the prevention and treatment of Salmonella Gallinarum, which causes severe systemic disease in domestic poultry (Johnson et al. 1977). In this regard, the lantibiotic mersacidin produced by B. subtilis has shown promising applications particularly against methicillin-resistant staphylococci (Bierbaum et al. 1995). The rapid rise and spread of multi-resistant bacterial pathogens have forced the consideration of alternative methods of combating infections. For example, several strains of L. monocytogenes have acquired resistance to conventional bacteriocins (Rasch and Knochel 1998; van Schaik et al. 1999). Thus, there is a need for new substances that exhibit efficient antimicrobial activity against such strains. Given the diversity of bacteriocins produced in nature, they can be considered as an alternative to combat infections against specific pathogens (Neu 1992; Riley and Wertz 2002). Therefore, research for new products with antimicrobial activity is a very important field. The identification and chemical characterization of bacteriocins produced by Bacillus spp., and exploration of their potential use in the control of pathogenic and spoilage microorganisms addresses this subject.
References
Ahern M, Verschueren S, van Sindersen D (2003) Isolation and characterisation of a novel bacteriocin produced by Bacillus thuringiensis strain B439. FEMS Microbiol Lett 220:127–131
Bastiani M, Hillebrand S, Horn F, Kist TBL, Guimarães JA, Termignoni C (2002) Cattle tick Boophilus microplus salivary gland contains a thiol-activated metalloendopeptidase displaying kininase activity. Insect Biochem Mol Biol 32:1439–1446
Bastos AER, Moon DH, Rossi A, Trevors JT, Tsai SM (2000) Salt-tolerant phenol-degrading microorganisms isolated from Amazonian soil samples. Arch Microbiol 174:346–352
Bierbaum G, Brotz H, Koller KP, Sahl HG (1995) Cloning, sequencing and production of the lantibiotic mersacidin. FEMS Microbiol Lett 127:121–126
Bizani D, Brandelli A (2002) Characterisation of a bacteriocin produced by a newly isolated Bacillus sp. strain 8A. J Appl Microbiol 93:512–519
Bizani D, Dominguez APM, Brandelli A (2005) Purification and partial chemical characterization of the antimicrobial peptide cerein 8A. Lett Appl Microbiol 41:269–273
Boone DR, Liu Y, Zhao ZJ, Balkwill DL, Drake GR, Stevens TO, Aldrich HC (1995) Bacillus infernus sp. nov., an Fe(III)- and Mn(IV)-reducing anaerobe from the deep terrestrial subsurface. Int J Syst Bacteriol 45:441–448
Cladera-Olivera F, Caron GR, Brandelli A (2004) Bacteriocin-like substance production by Bacillus licheniformis strain P40. Lett Appl Microbiol 38:251–256
Clauss D, Berkeley RCW (1986) Genus Bacillus Cohn 1872. In: Sneath PHA (eds) Bergey’s manual of systematic bacteriology, vol 2, 1st edn. Williams & Wilkins, Baltimore, pp 1105–1141
Diep DB, Nes IF (2002) Ribossomally synthesized antibacterial peptides in Gram-positive bacteria. Curr Drugs Target 3:107–122
Dopazo CP, Lemos ML, Lodeiros C, Bolinches J, Barja JL, Toranzo AE (1988) Inhibitory activity of antibiotic-producing marine bacteria against fish pathogens. J Appl Bacteriol 65:97–101
Duitman EH, Hamoen LW, Rembold M, Venema G, Seitz H, Saenger W, Bernhard F, Reinhardt R, Schmidt M, Ulrich C, Stein T, Leenders F, Vater J (1999) The mycosubtilin synthetase of Bacillus subtilis ATCC6633: a multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthetase. Proc Natl Acad Sci USA 96:13294–13299
Ghosh K, San SK, Ray AK (2003) Supplementation of an isolated fish gut bacterium, Bacillus circulans in formulated diet for Rohu, Labeo Rohita, Fingerlings. Isr J Aquacult 55:13–21
Goto K, Omura T, Hara Y, Sadaie Y (2000) Application of the partial 16S rDNA sequence as an index for rapid identification of species in the genus Bacillus. J Gen Appl Microbiol 46:1–8
Gould G (1996) Industry perspectives on the use of natural antimicrobials and inhibitors for food applications. J Food Prot 59:S82–S86
Gray EJ, Lee KD, Souleimanov AM, Di Falco MR, Zhou X, Ly A, Charles TC, Driscoll BT, Smith DL (2006) A novel bacteriocin, thuricin 17, produced by plant growth promoting rhizobacteria strain Bacillus thuringiensis NEB17: isolation and classification. J Appl Microbiol 100:545–554
He L, Chen W, Liu Y (2006) Production and partial characterization of bacteriocin-like peptides by Bacillus licheniformis ZJU12. Microbiol Res 161:321–326
Hyronimus B, Le Marrec C, Urdaci MC (1998) Coagulin, a bacteriocin-like inhibitory substance produced by Bacillus coaglulans I4. J Appl Microbiol 85:42–50
Jack RW, Tagg JR, Ray B (1995) Bacteriocins of gram-positive bacteria. Microbiol Rev 59:171–200
Johnson DC, Keenum KG, Perry HP (1977) A fowl typhoid outbreak in a chicken breeder flock. Avian Dis 21:716–719
Klaenhammer TR (1993) Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev 12:39–86
Kumar S, Tamura K, Nei M (2004) Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinf 5:150–163
Lisboa MP, Bonatto D, Bizani D, Henriques JA, Brandelli A (2006) Characterization of a bacteriocin-like substance produced by Bacillus amyloliquefaciens isolated from the Brazilian Atlantic forest. Int Microbiol 9:111–118
MacFaddin F (2000) Biochemical test for identification of medical bacteria, 3rd edn. Williams and Wilkins, Baltimore
Marahiel MA, Nakano MM, Zuber P (1993) Regulation of peptide antibiotic production in Bacillus. Mol Microbiol 7:631–636
Mayr-Harting A, Hedjes AJ, Berkeley CW (1972) Methods for studying bacteriocins. In: Norris JB, Ribbons D (eds) Methods in microbiology, vol 7. Academic, New York, pp 315–412
McAuliffe O, Ross RP, Hill C (2001) Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiol Rev 25:285–308
Motta AS, Brandelli A (2002) Characterization of an antibacterial peptide produced by Brevibacterium linens. J Appl Microbiol 92:63–71
Motta AS, Cladera-Olivera F, Brandelli A (2004) Screening for antimicrobial activity among bacteria isolated from Amazon basin. Braz J Microbiol 35:307–310
Motta AS, Lorenzini DM, Brandelli A (2007) Purification and partial characterization of an antimicrobial peptide produced by a novel Bacillus sp. isolated from Amazon basin. Curr Microbiol 54:282–286
Neu HC (1992) The crisis in antibiotic resistance. Science 257:1064–1073
Oliveira SS, Abrantes J, Cardoso M, Sordelli D, Bastos MCF (1998) Staphylococcal strains involved in mastitis are inhibited by Staphylococcus aureus antimicrobial peptides. Lett Appl Microbiol 27:287–291
Olsson JC, Westerdahl A, Conway PL, Kjelleberg S (1992) Intestinal colonization potential of turbot (Scophthalmus maximus) and dab (Limanda limanda) associated bacteria with inhibitory effects against Vibrio anguillarum. Appl Environ Microbiol 58:551–556
Palys T, Nakamura LK, Cohen FM (1997) Discovery and classification of ecological diversity in the bacterial world: the role of DNA sequence data. Int J Syst Bacteriol 47:1145–1156
Pattnaik P, Kaushik JK, Grover S, Batish VK (2001) Purification and characterization of a bacteriocin-like compound (Lichenin) produced anaerobically by Bacillus licheniformes isolated from water buffalo. J Appl Microbiol 91:636–645
Rasch M, Knochel S (1998) Variations in tolerance of Listeria monocytogenes to nisin, pediocin PA 1 and bavaricin A. Lett Appl Microbiol 27:275–278
Riley MA, Wertz JE (2002) Bacteriocins: evolution, ecology and application. Annu Rev Microbiol 56:117–137
Risoen PA, Ronning P, Hegna IK, Kolsto AB (2004) Characterization of a broad-range antimicrobial substance from Bacillus cereus. J Appl Microbiol 96:648–655
Ross RP, Galvin M, McAuliffe O, Morgan SM, Ryan MP (1999) Developing applications for lactococcal bacteriocins. Antonie Van Leeuwenhoek 76:337–346
Sharma N, Kapoor G, Neopaney B (2006) Characterization of a new bacteriocin produced from a novel isolated strain of Bacillus lentus NG121. Antonie Van Leeuwenhoek 89:337–343
Stein T (2005) Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol 56:845–857
Stackebrandt E, Goebel BM (1994) Taxonomic note: a place of DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol 44:846–849
Sugita H, Matsuo N, Shibuya K, Deguchi Y (1996) Production of antibacterial substances by intestinal bacteria isolated from coastal crab and fish species. J Mar Biotechnol 4:220–223
Sugita H, Hirose Y, Matsuo N, Deguchi Y (1998) Production of the antibacterial substance by Bacillus sp. strain NM12, an intestinal baterium of Japanese coastal fish. Aquaculture 165:269–280
Tagg JR, Dajani AS, Wannamaker LK (1976) Bacteriocins of gram-positive bacteria. Bacteriol Rev 40:722–756
Tsai GJ, Chen TH (1996) Incidence and toxigenicity of Aeromonas hydrophila in seafood. Int J Food Microbiol 31:121–131
Twomey DP, Wheelock AI, FlynnJ, Meaney WJ, Hill C, Ross RP (2000) Protection against Staphylococcus aureus mastitis in dairy cows using a bismuth-based teat seal containing the bacteriocin lacticin 3147. J Dairy Sci 83:1981–1988
Valdés-Stauber N, Scherer S (1994) Isolation and characterization of linocin M18, a bacteriocin produced by Brevibacterium linens. Appl Environ Microbiol 60:3809–3814
Van Schaik W, Gahan CG, Hill C (1999) Acid adapted Listeria monocytogenes displays enhanced tolerance against the lantibiotic nisin and lacticin 3147. J Food Prot 62:536–539
Acknowledgments
Authors thank Dr. Spartaco Astolfi-Filho from Universidade Federal do Amazonas for kindly provide the bacterial strain P34, Centro de Microscopia Eletronica of UFRGS and ULBRA for technical support in electron microscopy. This work received financial support from CNPq and CAPES, Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jean-Luc Pernodet.
Rights and permissions
About this article
Cite this article
Motta, A.S., Cannavan, F.S., Tsai, SM. et al. Characterization of a broad range antibacterial substance from a new Bacillus species isolated from Amazon basin. Arch Microbiol 188, 367–375 (2007). https://doi.org/10.1007/s00203-007-0257-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-007-0257-2