Abstract
An unusual propionigenic bacterium was isolated from the intestinal tract of the soil-feeding termite Thoracotermes macrothorax. Strain TmPN3 is a motile, long rod that stains gram-positive, but reacts gram-negative in the KOH test. It forms terminal endospores and ferments lactate, glucose, lactose, fructose, and pyruvate to propionate and acetate via the methyl-malonyl-CoA pathway. Propionate and acetate are formed at a ratio of 2:1, typical of most propionigenic bacteria. Under a H2/CO2 atmosphere, the fermentation product pattern of glucose, fructose, and pyruvate shifts towards propionate formation at the expense of acetate. Cell suspensions reduce oxygen with lactate, glucose, glycerol, or hydrogen as electron donor. In the presence of oxygen, the product pattern of lactate fermentation shifts from propionate to acetate production. 16S rRNA gene sequence analysis showed that strain TmPN3 is a firmicute that clusters among the Acidaminococcaceae, a subgroup of the Clostridiales comprising obligately anaerobic, often endospore-forming bacteria that possess an outer membrane. Based on phenotypic differences and less than 92% sequence similarity to the 16S rRNA gene sequence of its closest relative, the termite hindgut isolate Acetonema longum, strain TmPN3T is proposed as the type species of a new genus, Sporotalea propionica gen. nov. sp. nov. (DSM 13327T, ATCC BAA-626T).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Little is known about the gut microbiota of soil-feeding termites (Brune 2005). The intestinal tract contains a dense and diverse community of prokaryotes (Bignell et al. 1980; Friedrich et al. 2001; Schmitt-Wagner et al. 2003a, b), and high concentrations of microbial fermentation products in the individual gut compartments indicate that the gut microbiota actively participates in digestion (Tholen 1999).
Lactic acid bacteria form a large fraction of the cultivable hindgut microbiota of both wood- and soil-feeding termites (Schultz and Breznak 1978; Tholen et al. 1997; Bauer et al. 2000). However, lactate usually does not accumulate in the hindgut fluid because the intestinal lactate pool is rapidly turned over. Microinjection of radiolabeled substrates into intact guts of the wood-feeding termite Reticulitermes flavipes revealed that about one-third of the intestinal carbon flux in this termite proceeds via lactate as an intermediate (Tholen and Brune 2000). In intact guts incubated under oxic conditions, almost all lactate is routed towards acetate production. However, when guts are incubated under a N2 atmosphere, labeled propionate and acetate is formed at a 2:1 ratio typical for propionigenic bacteria, suggesting that the lactate-fermenting bacteria shift their fermentation pattern when oxygen is present (Tholen and Brune 2000).
Also the guts of soil-feeding termites experience significant oxygen fluxes (Kappler and Brune 1999; Schmitt-Wagner and Brune 1999). At the same time, the mixed segment and P3 gut sections are significant sources of hydrogen (Schmitt-Wagner and Brune 1999; Kappler and Brune 2002). Earlier studies with Propionibacterium spp. have shown that oxygen causes a shift in the fermentation product pattern towards acetate at the expense of propionate (Pritchard et al. 1977). On the other hand, the fermentation product patterns of Propionispira arboris (Thompson et al. 1984) and Anaeromusa (formerly Selenomonas) acidaminophila (Nanninga et al. 1987) are shifted towards propionate by exogenous hydrogen.
It is not yet clear which bacteria are responsible for lactate turnover in the hindgut of soil-feeding termites. The only bacteria fermenting lactate to propionate ever isolated from termite guts, identified as Bacteroides sp., were from a wood-feeding termite (Schultz and Breznak 1978), but they were not deposited in a culture collection. In this study, we isolated an unusual propionigenic bacterium from the intestinal tract of the soil-feeding termite Thoracotermes macrothorax. The bacterium represents a new genus of the Clostridiales. In addition to generally characterizing the physiology and phylogeny of the isolate, we also tested the ability of the isolate to reduce molecular oxygen and examined the potential impact of oxygen and hydrogen on the fermentation product patterns.
Materials and methods
Isolation, cultivation, and characterization
Gut homogenates from different gut sections of T. macrothorax Sjöstedt were prepared as previously described (Tholen and Brune 1999). Anoxic, bicarbonate-buffered mineral medium (AM-5; Boga and Brune 2003) supplemented with yeast extract and casamino acids (each 0.1%, w/v) and containing l-lactate (10 mM) and dithiothreitol (DTT; 1 mM) as a reducing agent was used for serial dilutions and enrichment cultures. Pure cultures of propionigenic bacteria were isolated in agar-dilution series (Pfennig and Trüper 1981) using the same medium. Cultures were routinely grown in DTT-reduced mineral medium (AM5) without yeast extract and casamino acids but containing l-lactate as substrate, and incubated in the dark at 30°C.
The morphology of the cells was analyzed using phase-contrast microscopy. Flagella were stained as described by Blenden and Goldberg (1965). The gram type was determined by gram staining (Süßmuth et al. 1987) and by the KOH method (Gregersen 1978); Bacillus megaterium (DSM 32) and Escherichia coli (DSM 498) were used as controls.
Growth was followed directly within the culture tubes (13 mm I. D.), measuring the optical density (OD) at 578 nm with a Spectronic 20+ spectrophotometer (Thermo Electron, Waltham, MA, USA). Growth yields were estimated using an OD-to-cell-mass conversion factor determined with 1-l cultures grown on lactate. To test pH dependence of growth, the pH of the medium was adjusted by adding sterile solutions of 1 M Na2CO3 or 1 M HCl.
Fermentation balances were determined using cells grown in basal medium under an atmosphere of either H2/CO2 or N2/CO2 (80:20, v/v, 150 kPa). After 5 days of incubation on a rotary shaker at 100 rpm, liquid samples (0.5 ml) were withdrawn and acidified by the addition of H2SO4 (20 μl 5 M). Substrate utilization and product formation were assayed by high performance liquid chromatography (HPLC) using an ion-exclusion column and a refractive index detector (Tholen et al. 1997). Aromatic metabolites were quantified by reversed-phase HPLC as described previously (Brune and Schink 1992). For computation of electron balances, all metabolites were formally oxidized to CO2, and the electrons theoretically released from the respective amounts of products were compared with those of the amount of substrate consumed. Expressed on a percent basis, this calculation yielded the electron recovery as previously described (Tholen et al. 1997).
Radiotracer studies
Radiotracer studies were conducted in 5 ml AM5 medium, without yeast extract and casamino acids but containing ul-14C-glucose (5 mM, 0.62 MBq mmol–1), or unlabeled glucose (5 mM) together with 14C-labeled CO2 (30 mM, 0.43 MBq mmol–1; final values including bicarbonate buffer) under an atmosphere of H2/CO2 or N2/CO2. After 5 days of incubation, 0.5 ml NaOH (1 M) was added to the culture tubes, which were then incubated for another 4 h to trap all CO2 as carbonate in the liquid phase. The cultures were centrifuged (10,000g, 5 min), and an aliquot (0.1 ml) of the supernatant was analyzed for total radioactivity by liquid scintillation counting (LSC) as described previously (Ji et al. 2000). Another aliquot (1 ml) received 50 μl CaCl2 (2 M) to convert all CO2 to insoluble calcium carbonate, which was removed by centrifugation (20,000g, 10 min). The remaining total activity in the supernatant was assayed by LSC as described above; the radioactivity in the individual fermentation products was assayed by HPLC using a flow scintillation analyzer (Tholen and Brune 1999).
Oxygen uptake by dense cell suspensions
Cultures in the late exponential phase of growth were harvested by centrifugation (10,000g, 20 min). Cells were washed and resuspended in anoxic (N2-sparged) but non-reduced potassium phosphate buffer (0.1 M, pH 7). The cell suspensions were kept on ice and used on the day they were harvested. Oxygen uptake rates were measured using a Clark-type oxygen meter as described previously (Boga and Brune 2003). To determine the influence of oxygen on the fermentation patterns, cell suspensions (1.1 mg cells dry mass ml–1; final concentration) were incubated in assay buffer containing either lactate (2 mM) or glucose (1.5 mM) under a nitrogen headspace at 22 ± 1°C with constant stirring. Various concentrations of oxygen were added to the headspace, and after 4 h, liquid samples were assayed for fermentation products by gas chromatography (Platen and Schink 1987) and HPLC (Tholen et al. 1997).
Enzyme activities in cell-free extracts
Cell-free extracts were prepared by repeatedly passing a cell suspension through a French pressure cell at 138 MPa and subsequent centrifugation (30,000g, 20 min) to remove cell debris. NADH oxidase, catalase, and superoxide dismutase activities were assayed as described elsewhere (Boga and Brune 2003). Pyruvate oxidase activities were measured as described (Bauer et al. 2000) using 2,6-dichlorophenol indophenol (DCPIP) as electron acceptor. Carbon monoxide dehydrogenase and hydrogenase activities were assayed by following the reduction of benzyl viologen as described (Boga and Brune 2003). All photometric assays contained 50–150 μg protein ml–1; a linear relationship between enzyme activity and protein concentration was granted in all cases. Heat-inactivated cell extracts were used as controls. All activities were determined at 25°C.
Redox difference spectra
Cell-free extracts were fractionated into a membrane fraction and a soluble fraction by ultracentrifugation (126,000g, 1 h). Both fractions were assayed for the presence of cytochromes by recording difference spectra for N2S2O4-reduced minus air-oxidized samples, as described previously (Breznak et al. 1988). All procedures were carried out in potassium phosphate buffer (0.1 M, pH 7).
16S rRNA gene sequence analysis
rRNA genes were amplified in vitro and sequenced as described earlier (Springer et al. 1992). Sequence data were analyzed with the ARB software package (Ludwig et al. 2004). The new sequences were added to the ARB database and aligned with the Fast Aligner tool. Alignments were checked and corrected manually where necessary. Framework trees were calculated with fastDNAmL, a maximum-likelihood method implemented in ARB, with only almost-full-length sequences (1,400 bases). The stability of the branching pattern was tested with the neighbor-joining and maximum-parsimony (DNAPARS) methods included in the PHYLIP package implemented in ARB. The EMBL accession numbers for the 16S rRNA gene sequences are AM258974 for strain TmPM3 and AM258975 for strain TmPN3.
Results
Isolation and morphological characterization
Propionate and acetate were the major fermentation products from lactate in anoxic serial dilutions of gut homogenates prepared from the different gut sections of T. macrothorax. The highest dilutions positive for propionate formation were transferred into fresh medium, and subsequent agar dilution series yielded mostly brown, irregularly shaped colonies, from which several pure cultures were obtained. Phase-contrast microscopy showed long, straight or slightly curved rods, occurring singly or in pairs, which were morphologically indistinguishable. Strains TmPM3 and TmPN3, which stemmed from homogenates of midgut/mixed segment and the first proctodeal segment (Schmitt-Wagner et al. 2003a), respectively, were selected for further characterization. In both cases, the dilution steps indicated an original population of approximately 103–104 cells per gut section.
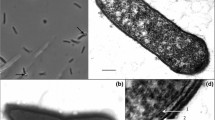
Cells measured 2.2–12 μm in length and 0.5–0.7 μm in diameter (Fig. 1a) and were motile by means of peritrichous flagella (Fig. 1b, c). They stained gram-positive but reacted gram-negative in the KOH test. In older cultures, only few cells formed refractile terminal endospores (Fig. 1a); stationary-phase cultures remained viable after pasteurization (80°C, 10 min). Since the morphological features of the two isolates were indistinguishable and the results of a phylogenetic analysis (see below) indicated that the two strains were very closely related, only strain TmPN3 was further physiologically characterized.
Growth and nutrition
Strain TmPN3 required an oxygen-free, reduced medium for growth. It grew best in medium reduced with DTT (1 mM) or cysteine (2 mM), but grew well also in medium incubated under a H2/CO2 atmosphere when a palladium catalyst was added (Tholen et al. 1997). Casamino acids and yeast extract, which were included in the enrichment medium, were fermented to acetate and propionate, but since they were not essential for growth on lactate or other substrates, they were omitted during further characterization.
Strain TmPN3 grew also on d-glucose, d-fructose, d-cellobiose, d-lactose, or d-glycerol (each 5 mM) and on pyruvate, oxaloacetate, d-citrate, l-alanine, or l-glutamate (each 10 mM); all these substrates were fermented to propionate, acetate, and CO2. By contrast, fumarate and l-aspartate (each 10 mM) were fermented to succinate and acetate, while acetate, succinate, and propionate were formed from l-malate. Fermentation balances for selected substrates are given in Table 1. Trehalose, methanol, ethanol (each 5 mM), succinate, malonate, propionate, formate, acrylate, glyoxylate, and acetate (10 mM each) were not utilized. Cells did not grow on H2 and CO2. Vanillate, syringate, and 3,4,5-trimethoxybenzoate (each 2 mM) were not demethylated. Nitrate and sulfate (each 10 mM) were not reduced with lactate as a substrate.
On basal medium with lactate, the cells grew within a pH range of 6.2–8.2 and a temperature range of 19–35 °C, but not at 4 or 40°C. Optimal growth was obtained at 30°C and pH 7.0 (Fig. 2). Under these conditions, cultures growing on lactate or fructose had doubling times of 8.4 and 10 h, respectively. Growth yields on glucose were threefold higher than on lactate, but only twofold higher on fructose (Table 1).
Influence of hydrogen on fermentation stoichiometries
Cultures of strain TmPN3 growing under a headspace of N2/CO2 fermented glucose to propionate and acetate at a 2:1 ratio. However, when growing under a H2/CO2 atmosphere, the product pattern changed towards propionate formation at the expense of acetate production, which shifted the propionate-to-acetate ratio to more than 6:1 (Table 1). Also the fermentation balances on fructose and pyruvate were shifted towards propionate at the expense of acetate in the presence of H2, and more succinate and less acetate were formed from fumarate when H2 was present. Cell yields in cultures grown under a H2/CO2 atmosphere did not differ significantly from those grown under a N2/CO2 atmosphere (Table 1). Curiously, the fermentation balance of lactate was not influenced by the presence of H2 in the headspace.
Since it is not trivial to measure CO2 formation in a bicarbonate-buffered system, the effect of hydrogen on the fermentation products was tested also in radiotracer experiments. In cultures incubated with ul-14C-glucose (Table 2), the effects of the headspace gas on the propionate-to-acetate ratio were similar to those in Table 1. The amount of 14CO2 formed per glucose was only slightly higher than the theoretical value of 1.33 expected for the methyl-malonyl-CoA pathway, and also the slight decrease in cultures incubated under a H2/CO2 atmosphere is in agreement with the increased propionate formation. Also in cultures grown on non-labeled glucose in the presence of labeled 14CO2, the amount of CO2 incorporated into propionate (0.67 for H2/CO2 and 0.71 for N2/CO2 atmosphere) did not significantly exceed the theoretical ratio of 0.5 expected for the methyl-malonyl-CoA pathway. Again, the results were not significantly affected by the presence of H2, which indicated the absence of reductive propionigenesis from CO2.
Oxygen uptake by cell suspensions
Cultures with glucose or lactate did not initiate growth in non-reduced medium. However, dense cell suspensions of strain TmPN3 grown on glucose consumed oxygen when glucose, lactate, or glycerol was added as electron donor (Table 3). Oxygen consumption by lactate-grown cells was slightly but not significantly higher than that of glucose-grown cells; the basal rates were significantly higher (t test). Rates of hydrogen-dependent oxygen reduction by lactate-grown cells were significantly lower than those obtained with lactate. In no case did the addition of acetate, pyruvate, propionate, or oxaloacetate increase oxygen consumption above the basal rates obtained prior to the addition of external substrates. Heat-inactivated cell suspensions did not consume oxygen. Oxygen consumption by cells grown on either substrate was not influenced by the addition of KCN (up to 10 mM) to the assay.
The influence of oxygen on the fermentation product pattern was tested with cell suspensions (cells grown on lactate). Oxygen-free controls (headspace N2/CO2) fermented lactate to propionate and acetate with a stoichiometry of almost 2:1. With increasing oxygen concentrations in the headspace, the product pattern shifted towards acetate formation at the expense of propionate production (Fig. 3). At an oxygen partial pressure of 1.5 kPa, acetate was the only product. The recovery of electrons in the fermentation products decreased with increasing oxygen partial pressure, which indicated that oxygen served as electron acceptor. Similar results were obtained with glucose as substrate (cells grown on glucose; details not shown) (Fig. 4).
Enzyme activities and cytochromes
Cell-free extracts of lactate-grown cells showed low activities of NADH oxidase and pyruvate oxidase [3.85 and 0.39 nmol min–1 (mg protein)–1, respectively]; catalase and superoxide dismutase were not detected. Cell-free extracts of lactate-grown cells also had low activities of hydrogenase and CO dehydrogenase [602 ± 18 and 37 ± 3 nmol (mg protein)−1, respectively].
Redox difference spectra of cell extracts of lactate-grown cells showed absorption maxima at 427, 544, and 561 nm, which indicates the presence of b-type cytochrome(s) (Dickerson and Timkovich 1975). Absorption maxima were observed only in the membrane fraction. There was no indication of the presence of a- or c-type cytochromes.
16s rRNA gene sequence analysis
Comparative 16S rRNA gene sequence analysis revealed that strains TmPN3 and TmPM3 are firmicutes phylogenetically affiliated with the Acidaminococcaceae in the order Clostridiales (Fig. 5). However, the strains were only moderately related to any of the validly described taxa of this subgroup. The closest relative of the new isolates was Acetonema longum (91.4% sequence similarity) and members of the genus Sporomusa (90.4–90.7%), followed by Anaerosinus (formerly Anaerovibrio) glycerini, Dendrosporobacter quercicolus, Propionispora vibrioides, and A. acidaminophila; an exact branching order could not be established. Sequence similarities with representatives of the other genera of the Acidaminococcaceae, i.e., Acidaminococcus, Desulfotomaculum, Dialister, Megasphaera, Pectinatus, Phascolarctobacterium, Quinella, Schwartzia, Selenomonas, Succinispira, Succiniclasticum, Veillonella, Allisonia, Zymophilus, and Propionispira, were even lower.
Phylogenetic relationship of Sporotalea propionica strain TmPN3 and other closely related gram-positive bacteria. The tree is based upon a maximum-likelihood analysis of the 16S rRNA sequences of all type strains in the Acidaminococcaceae, including Sporomusa aerivorans (AJ506191, DSM 13326T), S. malonica (AJ279799, DSM5090t), S. sphaeroides (AJ279801, DSM2875t) and S. acidovorans (AJ278798, DSM3132t). The dataset comprised 1405 homologous alignment positions that were invariant among at least 50% of the sequences. The tree topology was evaluated and corrected according to the results of distance-matrix analysis and maximum-parsimony analysis; multifurcations indicate that the branching order could not be unambiguously determined or was not supported by the results of the alternative treeing procedures. The bar indicates 5% sequence divergence
Discussion
The diversity, physiology, and possible role of the microbiota resident in the guts of termites are subjects of continuing interest. Data on molecular microbial diversity of termite gut microbiota has continued to accumulate in recent years, but physiological studies involving isolates from termite guts are scanty. Lactate has been identified as a key metabolite in the hindgut of the wood-feeding Reticulitermes flavipes (Tholen and Brune 2000) and is an intermediate also in soil-feeding termites (Tholen 1999). The strains isolated in this study are the first propionigenic bacteria obtained from soil-feeding termites. Their ability to oxidize hydrogen and their strong metabolic response to oxygen contributes important clues to understanding carbon and electron flow in the gut microenvironment.
Bacteria form propionate from a variety of substrates including glucose, lactate, dicarboxylic acids, and certain amino acids, using various metabolic pathways. The spectrum of substrates used by strain TmPN3 indicates that propionate is formed via the methyl-malonyl-CoA pathway, which is in agreement with its substrate spectrum, the presence of a b-type cytochrome (Diekert and Wohlfarth 1994), and the cumulative evidence for the occurrence of this pathway also among other Acidaminococcaceae (e.g., Sporomusa, Breznak et al. 1988, Dehning et al. 1989; Anaeromusa, Nanninga et al. 1987; and Propionispira, Schink et al. 1982).
The Acidaminococcaceae (Garrity and Holt 2001) are a heterogeneous assemblage of taxa commonly exhibiting an ultrastructure and biochemistry (presence of an outer membrane containing lipopolysaccharides; e.g., Breznak 2001; Haikara and Helander 2001) of their cell envelope otherwise typical of gram-negative bacteria. The majority of species in this family react gram-negative in the classical staining procedure, but since the peptidoglycan layer of Acidaminococcaceae appears to be thicker than that of gram-negative bacteria (Helander et al. 2004), some stain gram-positive despite the presence of an outer membrane (e.g., S. silvacetica; Kuhner et al. 1997). Also strain TmNP3 stains gram-positive, but reacts gram-negative in the KOH test, a control often used to verify the results of the notoriously unreliable gram-staining procedure (Gregersen 1978; Halebian et al. 1981). Ultrastructural information is not available, but it is unlikely that strain TmPN3 differs from its closest relatives with respect to the general structure of its cell envelope.
The next relative of strain TmPN3, A. longum, was isolated from the hindgut of Pterotermes occidentis (Kane and Breznak 1991), and also several members of the closely related genus Sporomusa (S. termitida, Breznak et al. 1988; S. aerivorans, Boga et al. 2003) were isolated from the guts of termites. Strain TmPN3, which resembles A. longum in its morphology (straight rods with terminal endospore), although it is shorter and stouter, differs strongly from its closest relatives in its general metabolic features (Table 4). It ferments glucose and other hexoses exclusively to propionate and acetate, whereas A. longum forms butyrate and H2 as major products. All Sporomusa species are unable to ferment glucose; those strains that grow on fructose form acetate as the major product. Members of both genera differ from strain TmPN3 in their capacity of reductive acetogenesis from H2 and CO2. Sporomusa species ferment lactate homoacetogenically, and A. longum does not grow on lactate.
Also the next closest relatives have different physiological traits (Table 4). P. vibrioides ferments fructose to propionate and acetate, but it does not grow on glucose, pyruvate, lactate, fumarate, malate, succinate, or alanine (Biebl et al. 2000). D. quercicolus, a species only poorly characterized with respect to growth substrates and fermentation products, is reported to form, in addition to acetate and propionate, substantial amounts of H2 and propanol from a medium containing fructose, yeast extract, and peptone (Strömpl et al. 2000). Anaerosinus (formerly Anaerovibrio) glycerini shares with strain TmPN3 the ability to ferment glycerol to propionate, but does not ferment carbohydrates, lactate, or dicarboxylic acids (Schauder and Schink 1989; Strömpl et al. 1999). Anaeromusa (formerly Selenomonas) acidaminophila grows on amino acids, but does not use any carbohydrates (Nanninga et al. 1987). Propionispira arboris (Schink et al. 1982), which resembles strain TmPN3 in several physiological traits, is phylogenetically far removed (<88% sequence similarity). Taken together, the low similarities in 16S rRNA gene sequence (< 92.0%) and the distinct morphological and physiological differences to its closest relatives among the Acidaminococcaceae require placement of strain TmPN3 into a new genus (see below).
The oxidation–reduction balance in the fermentation of glucose, lactate, or other isoelectronic compounds dictates the formation of propionate and acetate at a fixed 2:1 ratio. Only a few bacteria form more propionate at the expense of acetate in the presence of exogenous hydrogen. Strain TmPN3 shares this unusual trait with P. arboris (Thompson et al. 1984) and A. acidaminophila (Nanninga et al. 1987), but unlike P. arboris, not when grown on lactate. Thompson et al. (1984) proposed that the hydrogenase activity detected in P. arboris might function in hydrogen uptake, forming the key electron-donating reaction in the presence of hydrogen, leading to hydrogen-dependent homopropionate fermentation from glucose. The authors postulated that hydrogen dramatically alters carbon and electron flow during glucose and lactate fermentation by preventing pyruvate transformation to acetate, CO2, and H2. Although cultures of strain TmPN3 grown on glucose, fructose, or pyruvate did show a slightly increased electron recovery under a H2/CO2 atmosphere, growth yields were not affected by the presence of hydrogen with any of the substrates tested (Table 1), indicating that the shift in the fermentation products was not accompanied by an increased ATP yield of the underlying metabolism. This is consistent with observations made with P. arboris (Thompson et al. 1984).
Although strain TmPN3 possesses hydrogenase and CO dehydrogenase, the activities are more than three orders of magnitude lower than those measured in cell-free extracts of the closely related, homoacetogenic S. aerivorans (Boga and Brune 2003). Moreover, the results of the labeling experiments do not indicate reductive propionate formation from H2 and CO2 via the acetyl-CoA pathway. Rather, the incorporation of 14CO2 into propionate can be explained by heterotrophic CO2 fixation in a Wood–Werkman reaction (i.e., the carboxylation of pyruvate or phosphoenolpyruvate (PEP); Kresge et al. 2005). Hydrogenase activity may be involved in growth on substrates that are more reduced than propionate and cell mass because it would allow the release of reducing equivalents as H2, as demonstrated for A. glycerini and P. vibrioides growing on sugar alcohols (Schauder and Schink 1989; Biebl et al. 2000).
Although strain TmPN3 forms propionate from most substrates, the reductive branch of its metabolism stops at succinate when growing by disproportionation of fumarate or aspartate. Succinate is a major product also on malate, whereas propionate is the only reduced product in the fermentation of oxaloacetate, citrate, and glutamate. It has been shown that P. freudenreichii, which is incapable of net decarboxylation of C4-dicarboxylic acids, requires pyruvate or propionate as CO2 acceptor for transcarboxylation of methyl-malonyl-CoA or oxaloacetate when growing on aspartate or fumarate (Rosner and Schink 1990). Likewise, strain TmPN3, if lacking methyl-malonyl-CoA decarboxylase, would form propionate only if substrate degradation yields enough pyruvate to allow transcarboxylation. This is not a problem when growing on oxaloacetate or glutamate (if degraded via the methylaspartate pathway; Buckel 2001), but apparently becomes limiting already when growing on malate.
Also S. silvacetica, which is unable to decarboxylate succinate to propionate, forms succinate when growing on fumarate (Kuhner et al. 1997). The same applies to A. acidaminophila, which forms succinate from aspartate and propionate from glutamate (Nanninga et al. 1987). By contrast, S. malonica and S. aerivorans, which are capable of succinate decarboxylation, form propionate from fumarate and malate (Dehning et al. 1989; Boga et al. 2003). An exception is A. longum, which forms propionate from fumarate although it cannot grow by succinate decarboxylation (Kane and Breznak 1991).
There is increasing evidence that the capacity to reduce oxygen is widespread among obligately anaerobic microorganisms (Cypionka 2000; Küsel et al. 2001; Karnholz et al. 2002; Baughn and Malamy 2004; Seedorf et al. 2004). Strain TmPN3 shares the ability to reduce oxygen in cell suspensions with its closest relatives, A. longum and Sporomusa species (Boga and Brune 2003). However, the mechanism of oxygen reduction in Acidaminococcaceae is not clear. In the strictly anaerobic bacteria Desulfovibrio gigas (Lemos et al. 2001), B. fragilis (Baughn and Malamy 2004), and M. thermoacetica (Das et al. 2005), membrane-bound cytochrome bd oxidases have been implicated in quinone-dependent, cyanide-sensitive electron transport from NADH to oxygen. While oxygen reduction by the closely related S. termitida, S. aerivorans, and A. longum was inhibited by cyanide (Boga and Brune 2003), indicating the participation of a cytochrome oxidase, oxygen reduction by strain TmPN3 was not affected (this study). Therefore, another mechanism may be responsible for this activity.
Many strict anaerobes, including M. thermoacetica, possess high-molecular-weight rubredoxins (Hrb), A-type flavoproteins (FprA), superoxide reductase, and ruberythrin, which are involved in reduction of molecular oxygen and its toxic derivatives, i.e., superoxide and H2O2 (see Das et al. 2001; Seedorf et al. 2004; and references therein). Interestingly, the Hrb:dioxygen oxidoreductase of D. gigas (Roo) is not inhibited by cyanide (Chen et al. 1993). The same is true for its homolog (FprA) in Methanobrevibacter arboriphilus, which catalyzes the F420H2-dependent reduction of molecular oxygen (Seedorf et al. 2004). The presence of a cyanide-insensitive, oxygen-reducing activity in strain TmPN3 may indicate the involvement of an FprA-type oxidase in oxygen reduction.
The ability of strain TmPN3 to ferment amino acids could be an important trait for the colonization of the intestinal tract of soil-feeding termites, where the peptidic fraction of humic acids is preferentially mineralized, involving proteinases and amino-acid-degrading microbiota (Ji and Brune 2006). The ability of strain TmPN3 to reduce oxygen may be of importance for its survival under the microoxic conditions in the midgut and in the periphery of the anterior hindgut (Schmitt-Wagner and Brune 1999). The presence of microorganism like strain TmPN3 that reduce the oxygen continuously flowing through the epithelium into the gut would explain the metabolic shift from propionate to acetate observed in the wood-feeding Reticulitermes flavipes (Tholen and Brune 2000), and also the much lower propionate concentrations in termite guts than in the largely anoxic rumen (Odelson and Breznak 1983, Tholen 1999, Tholen and Brune 2000).
The terminal spores formed by strain TmPN3 strongly resemble those of A. longum and P. vibrioides both in their morphology and their position within the sporangium (Kane and Breznak 1991; Biebl et al. 2000). Since sporulated cultures of strain TmPN3 survived pasteurization and the presence of dipicolinic acid has been documented for their closest relatives, A. longum (Kane and Breznak 1991) and S. termitida (Breznak et al. 1988), it is safe to assume that they represent true endospores.
Strain TmPN3 was recovered from both the midgut and the anterior hindgut of T. macrothorax, where it and its relatives seem to be present only in small numbers (103–104 propagules per section). Taking into account the difference in volume of the respective sections (0.1 vs. 0.8 μl), average cell densities in the neutral midgut seem to be higher than in the alkaline anterior hindgut. Moreover, the neutrophilic character of strain TmPN3 makes it rather improbable that vegetative cells are metabolically active in the extremely alkaline P1 section (pH 11.9; Brune and Kühl 1996); the propagules giving rise to those enrichments were probably mostly spores.
In view of the small population sizes estimated for the Acidaminococcaceae isolated from termite guts in this and previous studies (Kane and Breznak 1991; Boga et al. 2003), it is not astonishing that their 16S rRNA genes—and those of other, uncultivated members of this family—are not represented among the almost 3000 bacterial sequences obtained from termites (http://www.ncbi.nlm.nih.gov; nucleotide database searched for “termite*+16S + bacteria”). The general undersampling bias inherent to environmental clone libraries generated with universal primers makes it difficult to detect small populations. New approaches using group-specific primers, quantitative PCR, and fluorescent in situ hybridization techniques might help establishing the numerical significance of Acidaminococcaceae and their potential function in the termite gut ecosystem.
Description of the genus Sporotalea gen. nov.
Sporotalea gen. nov. (Spo.ro.ta'le.a, Gr. n. σπορά, a spore; L. n. talea, a thin rod or stick; N. L. fem. n. Sporotalea, a spore-forming, stick-shaped bacterium).
Chemoorganotrophic bacteria with fermentative metabolism. Cell wall with outer membrane. May form heat-resistant endospores. Obligate anaerobes (grow only under anoxic conditions). Cells possess b-type cytochromes. Propionate is a major product from most substrates.
Types species: Sporotalea propionica.
Description of Sporotalea propionica sp. nov.
Sporotalea propionica sp. nov. (pro.pi.o’ni.ca, M.L. n. acidum propionicum, propionic acid; fem. adj. propionica, pertaining to propionic acid, which the organism produces from various substrates).
Long, rod-shaped bacterium, measuring 0.5–0.7 μm in length and 2.2–12 μm in diameter. Cells occur singly or in pairs. Forms heat-resistant endospores. Motile by peritrichous flagella. Stains gram-positive but reacts gram-negative in the KOH test. Catalase negative. Ferments cellobiose, glucose, fructose, lactose, citrate, lactate, mannitol, oxaloacetate, glutamate, and glycerol to propionate, acetate, and CO2. l-Malate is fermented to acetate, succinate and propionate. Fumarate and aspartate are fermented to succinate and acetate. Does not utilize trehalose, methanol, ethanol, succinate, malonate, propionate, formate, acrylate, glyoxylate, acetate, vanillate, syringate, or 3,4,5-trimethoxybenzoate. Cells possess b-type cytochromes. Does not form acetate from H2 and CO2. Nitrate and sulfate are not reduced. Resting cells reduce oxygen. Temperature range of growth 19–35°C, optimum at 30°C. No growth at 4°C and 40°C. pH range of growth 6.2–8.2, optimum at pH 7.0.
Source: the intestinal tract of the termite T. macrothorax.
Type strain: TmPN3 (= DSM 13327T = ATCC BAA-626T)
References
Baena S, Fardeau M-L, Woo THS, Ollivier B, Labat M, Patel BKC (1999) Phylogenetic relationships of three amino-acid-utilizing anaerobes, Selenomonas acidaminovorans, Selenomonas acidaminophila and Eubacterium acidaminophilum, as inferred from partial 16S rDNA nucleotide sequences and proposal of Thermanaerovibrio acidaminovorans gen. nov., comb. nov. and Anaeromusa acidaminophila gen. nov., comb. nov. Int J Syst Bacteriol 49:969–974
Bauer S, Tholen A, Overmann J, Brune A (2000) Characterization of abundance and diversity of wood- and soil-feeding termites by molecular and culture-dependent techniques. Arch Microbiol 173:126–137
Baughn AD, Malamy MH (2004) The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature 427:441–444
Biebl H, Schwab-Hanisch H, Spröer C, Lünsdorf H (2000) Propionispora vibrioides nov. gen., nov. sp., a new gram-negative, spore-forming anaerobe that ferments sugar alcohols. Arch Microbiol 174:239–247
Bignell DE, Oskarsson H, Anderson JM (1980) Distribution and abundance of bacteria in the gut of a soil-feeding termite Procubitermes aburiensis (Termitidae, Termitinae). J Gen Microbiol 117:393–403
Blenden DC, Goldberg HS (1965) Silver impregnation stain for Leptospira and flagella. J Bacteriol 89:899–900
Boga H, Brune A (2003) Hydrogen-dependent oxygen reduction by homoacetogenic bacteria isolated from termite guts. Appl Environ Microbiol 69:779–786
Boga HI, Ludwig W, Brune A (2003) Sporomusa aerivorans sp. nov., an oxygen-reducing homoacetogenic bacterium from the gut of a soil-feeding termite. Int J Syst Evol Microbiol 53:1397–1404
Breznak JA (2001) The genus Sporomusa. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds), The prokaryotes: an online electronic resource for the microbiological community, 3rd edn, release 3.5. Springer, Berlin Heidelberg New York. http://www.141.150.157.117:8080/prokPUB/index.htm
Breznak JA, Switzer JM, Seitz H-J (1988) Sporomusa termitida sp. nov., an H2/CO2-utilizing acetogen isolated from termites. Arch Microbiol 150:282–288
Brune A (2005) Symbiotic associations between termites and prokaryotes. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds), The prokaryotes: an online electronic resource for the microbiological community, 3rd edn, release 3.20. Springer, Berlin Heidelberg New York. http://141.150.157.117:8080/prokPUB/index.htm
Brune A, Kühl M (1996) pH profiles of the extremely alkaline hindguts of soil-feeding termites (Isoptera: Termitidae) determined with microelectrodes. J Insect Physiol 42:1121-1127
Brune A, Schink B (1992) Phloroglucinol pathway in the strictly anaerobic Pelobacter acidigallici: fermentation of trihydroxybenzenes to acetate via triacetic acid. Arch Microbiol 157:417–424
Buckel W (2001) Unusual enzymes involved in five pathways of glutamate fermentation. Appl Microbiol Biotechnol 57: 263–273
Chen L, Liu M-Y, LeGall J, Fareleira P, Santos H, Xavier AV (1993) Rubredoxin oxidase, a new flavo-hemo-protein, is the site of oxygen reduction to water by the “strict anaerobe” Desulfovibrio gigas. Biochem Biophys Res Commun 193:100–105
Cypionka H (2000) Oxygen respiration by Desulfovibrio species. Annu Rev Microbiol 54:827–848
Das A, Coulter ED, Kurtz DM Jr, Ljungdahl LG (2001) Five-gene cluster in Clostridium thermoaceticum consisting of two divergent operons encoding rubredoxin oxidoreductase-rubredoxin and rubrerythrin—type A flavoprotein—high-molecular-weight rubredoxin. J Bacteriol 183:1560–1567
Das A, Silaghi-Dumitrescu R, Ljungdahl LG, Kurtz DM Jr (2005) Cytochrome bd oxidase, oxidative stress, and dioxygen tolerance of the strictly anaerobic bacterium Moorella thermoacetica. J Bacteriol 187:2020–2029
Dehning I, Stieb M, Schink B (1989) Sporomusa malonica sp. nov., a homoacetogenic bacterium growing by decarboxylation of malonate or succinate. Arch Microbiol 151:421–426
Dickerson RE, Timkovich R (1975) Cytochromes c. In: Boyer PD (ed) The enzymes, vol XI, part A. Academic, New York, pp 397–547
Diekert G, Wohlfarth G (1994) Metabolism of homoacetogens. Antonie v. Leeuwenhoek 66:209–221
Friedrich MW, Schmitt-Wagner D, Lueders T, Brune A (2001) Axial differences in community structure of Crenarchaeota and Euryarchaeota in the highly compartmentalized gut of the soil-feeding termite Cubitermes orthognathus. Appl Environ Microbiol 67:4880–4890
Garrity GM, Holt JG (2001) Taxonomic outline of the Archaea and bacteria, 2nd edn. In: Boone DR, Castenholz RW (eds), Bergey’s manual of systematic bacteriology, vol 1. Springer, Berlin Heidelberg New York, pp 161–162
Gregerson T (1978) Rapid method for distinction of Gram-negative from Gram-positive bacteria. Eur J Appl Microbiol Biotechnol 5:123–127
Haikara A, Helander I (2001) Pectinatus, Megasphaera and Zymophilus. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds), The prokaryotes: an online electronic resource for the microbiological community, 3rd edn, release 3.5. Springer, Berlin Heidelberg New York. http://141.150.157.117:8080/prokPUB/index.htm
Halebian S, Harris B, Finegold SM, Rolfe RD (1981) Rapid method that aids in distinguishing Gram-positive from Gram-negative anaerobic bacteria. J Clin Microbiol 13:444–448
Helander IM, Haikara A, Sadovskaya I, Vinogradov E, Salkinoja-Salonen MS (2004) Lipopolysaccharides of anaerobic beer spoilage bacteria of the genus Pectinatus—lipopolysaccharides of a Gram-positive genus. FEMS Microbiol Rev 28:543–552
Ji R, Brune A (2006) Nitrogen mineralization, ammonia accumulation, and emission of gaseous NH3 by soil-feeding termites. Biogeochemistry 78:267–283
Ji R, Kappler A, Brune A (2000) Transformation and mineralization of synthetic 14C-labeled humic model compounds by soil-feeding termites. Soil Biol Biochem 32:1281–1291
Kane MD, Breznak JA (1991) Acetonema longum gen. nov. sp. nov. an H2/CO2 acetogenic bacterium from the termite, Pterotermes occidentis. Arch Microbiol 156:91–98
Kappler A, Brune A (1999) Influence of gut alkalinity and oxygen status on mobilization and size-class distribution of humic acids in the hindgut of soil-feeding termites. Appl Soil Ecol 13:219–229
Kappler A, Brune A (2002) Dynamics of redox potential and changes in redox state of iron and humic acids during gut passage in soil-feeding termites (Cubitermes spp.). Soil Biol Biochem 34:221–227
Karnholz A, Küsel K, Gößner A, Schramm A, Drake HL (2002) Tolerance and metabolic response of acetogenic bacteria toward oxygen. Appl Environ Microbiol 68:1005–1009
Kresge N, Simoni RD, Hill RL (2005) The discovery of heterotrophic carbon dioxide fixation by Harland G. Wood J Biol Chem 280:155–157
Kuhner CH, Frank C, Grießhammer A, Schmittroth M, Acker G, Gößner A, Drake HL (1997) Sporomusa silvacetica sp. nov., an acetogenic bacterium isolated from aggregated forest soil. Int J Syst Bacteriol 47:352–358
Küsel K, Karnholz A, Trinkwalter T, Devereux R, Acker G, Drake HL (2001) Physiological ecology of Clostridium glycolicum RD-1, an aerotolerant acetogen isolated from sea grass roots. Appl Environ Microbiol 67:4734–4741
Lemos RS, Gomes CM, Santana M, LeGall J, Xavier AV, Teixeira M (2001) The ‘strict’ anaerobe Desulfovibrio gigas contains a membrane-bound oxygen-reducing respiratory chain. FEBS Lett 496:40–43
Ludwig W, Strunk O, Westram R, Richter L, Meier H, 27 other authors (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371
Nanninga HJ, Drent WJ, Gottschal JC (1987) Fermentation of glutamate by Selenomonas acidaminophila sp. nov. Arch Microbiol 147:152–157
Odelson DA, Breznak JA (1983) Volatile fatty acid production by the hindgut microbiota of xylophagous termites. Appl Environ Microbiol 45:1602–1613
Pfennig N, Trüper HG (1981) Isolation of members of the families Chromatiaceae and Chlorobiaceae. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes. Springer, Berlin Heidelberg New York, pp 229–289
Platen H, Schink B (1987) Methanogenic degradation of acetone by an enrichment culture. Arch Microbiol 149:136–141
Pritchard GG, Wimpenny JW, Morris HA, Lewis MW, Hughes DE (1977) Effects of oxygen on Propionibacterium shermanii grown in continuous culture. J Gen Microbiol 102:223–233
Rosner B, Schink B (1990) Propionate acts as carboxylic group acceptor in aspartate fermentation by Propionibacterium freudenreichii. Arch Microbiol 155:46–51
Schauder R, Schink B (1989) Anaerovibrio glycerini sp. nov., an anaerobic bacterium fermenting glycerol to propionate, cell matter, and hydrogen. Arch Microbiol 152:473–478
Schink B, Thompson ET, Zeikus JG (1982) Characterization of Propionispira arboris gen. nov. sp. nov., a nitrogen fixing anaerobe common to wetwood of living trees. J Gen Microbiol 128:2771–2779
Schmitt-Wagner D, Brune A (1999) Hydrogen profiles and localization of methanogenic activities in the highly compartmentalized hindgut of soil-feeding higher termites (Cubitermes spp.). Appl Environ Microbiol 65:4490–4496
Schmitt-Wagner D, Friedrich MW, Wagner B, Brune A (2003a) Phylogenetic diversity, abundance, and axial distribution of bacteria in the intestinal tract of two soil-feeding termites (Cubitermes spp.). Appl Environ Microbiol 69:6007–6017
Schmitt-Wagner D, Friedrich MW, Wagner B, Brune A (2003b) Axial dynamics, stability, and interspecies similarity of bacterial community structure in the highly compartmentalized gut of soil-feeding termites (Cubitermes spp.). Appl Environ Microbiol 69:6018–6024
Schultz JE, Breznak JA (1978) Heterotrophic bacteria present in hindguts of wood-eating termites Reticulitermes flavipes (Kollar). Appl Environ Microbiol 35:930–936
Seedorf H, Dreisbach A, Hedderich R, Shima S, Thauer RK (2004) F420H2 oxidase (FprA) from Methanobrevibacter arboriphilus, a coenzyme F420-dependent enzyme involved in O2 detoxification. Arch Microbiol 182:126–137
Springer N, Ludwig W, Drozanski V, Amann R, Schleifer K-H (1992) The phylogenetic status of Sarcobium lyticum, an obligate intracellular parasite of small amoebae. FEMS Microbiol Lett 96:199–202
Strömpl C, Tindall BJ, Jarvis GN, Lünsdorf H, Moore ERB, Hippe H (1999) A re-evaluation of the taxonomy of the genus Anaerovibrio, with the reclassification of Anaerovibrio glycerini as Anaerosinus glycerini gen. nov., comb. nov., and Anaerovibrio burkinabensis as Anaeroarcus burkinensis [corrig.] gen. nov., comb. nov. Int J Syst Bacteriol 49:1861–1872
Strömpl C, Tindall BJ, Lünsdorf H, Wong T-Y, Moore ERB, Hippe H (2000) Reclassification of Clostridium quercicolum as Dendrosporobacter quercicolus gen. nov., comb. nov. Int J Syst Evol Microbiol 50:101–106
Süßmuth R, Eberspächer J, Haag R, Springer W (1987) Biochemisch-mikrobiologisches Praktikum. Thieme Verlag, Stuttgart
Tholen A (1999) Der Termitendarm als strukturiertes Ökosystem: Untersuchung der Mikrobiota und der Stoffflüsse im Darm von Reticulitermes flavipes und Cubitermes spp. PhD Thesis, University of Konstanz, Konstanz
Tholen A, Brune A (1999) Localization and in situ activities of homoacetogenic bacteria in the highly compartmentalized hindgut of soil-feeding higher termites (Cubitermes spp.). Appl Environ Microbiol 65:4497–4505
Tholen A, Brune A (2000) The impact of oxygen on metabolic fluxes and in situ rates of reductive acetogenesis in the hindgut of the wood-feeding termite Reticulitermes flavipes, determined by microinjection of radiotracers. Environ Microbiol 2:436–449
Tholen A, Schink B, Brune A (1997) The gut microflora of Reticulitermes flavipes, its relation to oxygen, and evidence for oxygen-dependent acetogenesis by the most abundant Enterococcus sp. FEMS Microbiol Ecol 24:137–149
Thompson TE, Conrad R, Zeikus JG (1984) Regulation of carbon and electron flow in Propionispira arboris: physiological function of hydrogenase and its role in homopropionate formation. FEMS Microbiol Lett 22:265–271
Acknowledgments
This study was supported by a research grant of “Deutsche Forschungsgemeinschaft” (DFG). H.I.B. was supported by a scholarship from the “Deutscher Akademischer Austauschdienst” (DAAD). We thank Edouard Miambi for collecting termites, and Bernhard Schink for continued support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boga, H.I., Ji, R., Ludwig, W. et al. Sporotalea propionica gen. nov. sp. nov., a hydrogen-oxidizing, oxygen-reducing, propionigenic firmicute from the intestinal tract of a soil-feeding termite. Arch Microbiol 187, 15–27 (2007). https://doi.org/10.1007/s00203-006-0168-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-006-0168-7