Abstract
The luminous marine bacterium Photobacterium mandapamensis was synonymized several years ago with Photobacterium leiognathi based on a high degree of phenotypic and genetic similarity. To test the possibility that P. leiognathi as now formulated, however, actually contains two distinct bacterial groups reflecting the earlier identification of P. mandapamensis and P. leiognathi as separate species, we compared P. leiognathi strains isolated from light-organ symbiosis with leiognathid fishes (i.e., ATCC 25521T, ATCC 25587, lequu.1.1 and lleuc.1.1) with strains from seawater originally described as P. mandapamensis and later synonymized as P. leiognathi (i.e., ATCC 27561T and ATCC 33981) and certain strains initially identified as P. leiognathi (i.e., PL-721, PL-741, 554). Analysis of the 16S rRNA and gyrB genes did not resolve distinct clades, affirming a close relationship among these strains. However, strains ATCC 27561T, ATCC 33981, PL-721, PL-741 and 554 were found to bear a luxF gene in the lux operon (luxABFE), whereas ATCC 25521T, ATCC 25587, lequu.1.1 and lleuc.1.1 lack this gene (luxABE). Phylogenetic analysis of the luxAB(F)E region confirmed this distinction. Furthermore, ATCC 27561T, ATCC 33981, PL-721, PL-741 and 554 all produced a higher level of luminescence on high-salt medium, as previously described for PL-721, whereas ATCC 25521T, ATCC 25587, lequu.1.1 and lleuc.1.1 all produced a higher level of luminescence on low-salt medium, a characteristic of P. leiognathi from leiognathid fish light organs. These results demonstrate that P. leiognathi contains two evolutionarily and phenotypically distinct clades, P. leiognathi subsp. leiognathi (strains ATCC 25521T, ATCC 25587, lequu.1.1 and lleuc.1.1), and P. leiognathi subsp. mandapamensis (strains ATCC 27561T, ATCC 33981, PL-721, PL-741 and 554).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photobacterium mandapamensis, a luminous marine bacterium, was originally described by Hendrie et al. (1970) from strains isolated from coastal seawater in the vicinity of Mandapam, southern India. In a comprehensive analysis of the taxonomy of luminous marine bacteria, Reichelt and Baumann (1973) grouped the Hendrie et al. (1970) strains together with several other phenotypically similar strains under P. mandapamensis, choosing strain 480 (ATCC 27561T) as the type strain. However, recognition that Boisvert et al. (1967) previously had described as Photobacterium leiognathi strains from the light organs of leiognathid fish that were phenotypically similar to P. mandapamensis led to reassessment of the validity of P. mandapamensis. Based on similarities between ATCC 27561T and P. leiognathi strains in mol% G+C content, flagellation, and chemotaxonomic characters, and in accord with nomenclatural priority, P. mandapamensis was synonymized with P. leiognathi, and one of the light-organ isolates of Boisvert et al. (1967), ATCC 25521T, was designated as the type (Reichelt and Baumann 1975). This synonymy was supported by DNA reassociation values of 84% for ATCC 25521T and ATCC 27561T and 78% for ATCC 25521T and ATCC 33981 (P. mandapamensis strain 391 of Hendrie et al. 1970) (Reichelt and Baumann 1975; Reichelt et al. 1976). Currently, strains originally described as P. mandapamensis (e.g., ATCC 27561T, ATCC 33981), phenotypically similar strains from the surfaces of fish and seawater (e.g., PL-721, PL-741, and 554), and strains from leiognathid light-organ symbiosis (e.g., ATCC 25521T and ATCC 25587) are placed in a single species, P. leiognathi.
Bacterial luminescence is encoded by genes of the lux operon. The lux operon gene order most common in luminous bacteria is luxCDABEG, with luxA and luxB encoding the α and β subunits of bacterial luciferase, luxC, luxD and luxE specifying the fatty acid reductase, acyl-transferase, and acyl-protein synthetase components, respectively, of the fatty acid reductase complex of the luminescence system, and luxG encoding a flavin reductase (Dunlap and Kita-Tsukamoto 2001). Certain strains of Photobacterium, however, bear an additional gene, luxF, which encodes a non-fluorescent flavoprotein, with a gene order of luxCDABFEG (Meighen and Dunlap 1993). Specifically, Photobacterium phosphoreum NCIMB 844 (Soly et al. 1988) and P. leiognathi strains PL-741 (Baldwin et al. 1989) and 554 (Illarionov et al. 1990) bear luxF (previously referred to as luxN and luxG, respectively, in the latter two studies). In contrast, P. leiognathi ATCC 25521T lacks luxF (Lee et al. 1991), as do Vibrio fischeri and all other luminous Vibrio species examined to date (Meighen and Dunlap 1993; Dunlap and Kita-Tsukamoto 2001). Sequence variation in luxA has been useful for the phylogenetic characterization and identification of luminous bacteria (Haygood 1990; Wimpee et al. 1991; Nealson et al. 1993; Makemson et al. 1997).
Previously, we reported that strain PL-721, isolated from a deep-sea evermannelid fish and identified as P. leiognathi (Nealson and Hastings 1977), differed in its growth and luminescence physiology in response to salt content of the medium compared to strains of P. leiognathi isolated from the light organs of leiognathid fishes (Dunlap 1985). These physiological differences and the different ecological origins of strains currently identified as P. leiognathi led us to test the possibility that this species contains two phylogenetically distinct groups of strains. Based on sequence analysis of regions encoding 16S rRNA, DNA gyrase subunit B (gyrB) and Lux proteins (luxAB(F)E), together with distinguishing phenotypic traits, we report here that strains originally described as P. mandapamensis and later synonymized with P. leiognathi (i.e., ATCC 27561 and ATCC 33981) and certain strains originally identified as P. leiognathi (i.e., PL-721, PL-741, 554) form a phylogenetically coherent clade that is distinct from other P. leiognathi strains and from other Photobacterium species. These distinctions demonstrate the presence of two subspecies within P. leiognathi, specifically P. leiognathi subsp. leiognathi and P. leiognathi subsp. mandapamensis.
Materials and methods
Cultivation and isolation of bacteria
Bacterial strains used in this study and their sources are listed in Table 1. For routine culture, cells were grown at 22–24°C in a seawater-based medium (Dunlap et al. 2004) containing 10 g tryptone, 5 g yeast extract, and 700 ml artificial seawater per liter (LSW-70), with 15 g agar per liter for solid medium. To test for maximum temperature of growth, strains were grown for 48 h in 3 ml of LSW-70 broth in a temperature-controlled water bath shaker with moderate aeration. To test for minimal growth temperature, strains were plated on LSW-70 agar and incubated at 6–8°C for one week; growth data for other temperatures (4 and 11°C) were taken from the literature (Table 2). Minimal medium, for testing response to salt, contained 15 mM NH4Cl, 0.2 mM α-glycerophosphate, 20 mM glycerol, 10 μg ferric ammonium citrate per milliliter, 20 mM HEPES (pH 7.25), 15 g agar per liter, and either 400 ml/l (BM-40) or 925 ml/l (BM-100) of artificial seawater. The minimal medium was supplemented with 0.03% yeast extract, inclusion of which allowed all strains to grow well and at similar rates on the low-salt and high-salt media.
To isolate luminous bacteria, seawater samples were aseptically collected from coastal seawater at Ft. Lauderdale, FL, and plated on LSW-70 or Photobacterium broth (Difco) agar. Luminous colonies arising on these plates were then picked and purified on LSW-70 agar. Luminous strains exhibiting colony characteristics on LSW-70 of the Vibrio harveyi group (large colonies, forming brownish pigmentation in older cultures, producing strong odor) or of V. fischeri (colonies forming yellow cell-associated pigmentation) (Reichelt and Baumann 1973; P.V. Dunlap, pers. obs.) were excluded from further screening.
Sequencing and phylogenetic analysis
Phylogenetic analysis involved comparisons of 16S rRNA gene sequences, gyrB sequences, organization of the luxAB(F)E genes (i.e., presence or absence of luxF), and sequences of the luxAB(F)E region. Nucleotide sequences were obtained by PCR-amplification of bacterial genomic DNA using standard methods (see Supplementary Table 1 for primer sequences). Previously reported sequences were downloaded from GenBank or were taken from the published literature, as indicated in Table 1. Sequencing of PCR products was carried out by staff of the University of Michigan Sequencing Core using dye terminator cycle sequencing. 16S rRNA gene sequences were aligned by eye, and gyrB and luxAB(F)E sequences were aligned by inferred amino acid sequence. Parsimony analyses were performed with PAUP* (Swofford 2003) using 1,000 heuristic search replicates with TBR branch swapping. Jackknife support was calculated with PAUP* using 1,000 replicates emulating Jac resampling. Bremer support was calculated with the aid of TreeRot (Sorenson 1999). Sequences of the 16S rRNA gene, gyrB and luxABE region of V. fischeri ATCC 7744T were used to root the phylogenetic hypotheses. GenBank accession numbers for sequences obtained in this study for the 16S rRNA gene, gyrB and luxAB(F)E (see Table 1) are as follows. ATCC 25915T (AY455890), ATCC 33539T (AY455889), ATCC 51760T (AY455878), ATCC 25521T (AY455879), ATCC 25587 (AY455870, AY455880, AY456750), lequu.1.1 (AY455881, AY341069), lleuc.1.1 (AY455882, AY341070), ATCC 27561T (AY341441, AY455883, AY341067), ATCC 33981 (AY341442, AY455884, AY341068), PL-721 (AY341440, AY455885, AY341066), seafl.1.1 (AY455871, AY455886, AY456751), seafl.1.3 (AY455872, AY455887, AY456752), seafl.1.4 (AY455873, AY455888, AY456753), ATCC 11040T (AY341437, AY455875, AY341063), NCIMB 844 (AY341438, AY455876, AY341064), pjapo.1.1 (AY341439, AY455877, AY341065), JCM 10084T (AY455892), SS9 (AY455891), ATCC 7744T (AY341436, AY455874, AY341062).
Results
To test the possibility that P. leiognathi as now formulated contains two distinct bacterial clades, we examined several representatives of P. leiognathi for differences in phylogenetically informative loci. Included in the analysis were the type strains for P. leiognathi, ATCC 25521T, and P. mandapamensis, ATCC 27561T, together with several additional strains.
16S rRNA gene and gyrB analysis
Comparison of the aligned 16S rRNA gene from species of Photobacterium resulted in a single phylogenetic hypothesis, with no clear distinctions among the strains identified as P. leiognathi from light organs of leiognathid fishes (ATCC 25521T, ATCC 25587, lequu.1.1, lleuc.1.1) and from other habitats (ATCC 27561T, ATCC 33981, PL-721) (Supplementary Fig. 1). Although clear distinctions were apparent for most Photobacterium species, the 16S rRNA gene sequences of the P. leiognathi strains were nearly identical, differing by 0.6% or less in pair wise comparisons. Like the 16S rRNA gene, gyrB also provided little insight into possible distinctions among P. leiognathi strains. We found that sequence differences in gyrB, while more numerous than in the 16S rRNA gene and supporting species-level distinctions among other members of Photobacterium, did not provide sufficient information for phylogenetic resolution of strains currently grouped within P. leiognathi (Supplementary Fig. 2).
Analysis of the luxAB(F)E region
To assess the possible value of the lux region for distinguishing closely related strains of P. leiognathi, we examined the gene organization and sequence of luxAB(F)E. First, to determine whether the presence of a luxF gene is characteristic of P. phosphoreum and as a consequence could provide a comparison with P. leiognathi strains lacking or bearing this gene, we examined strains ATCC 11040T and pjapo.1.1. Sequence analysis of the lux operons of both strains revealed a luxF gene between luxB and luxE (Fig. 1).
Organization of the luxCDAB(F)E genes in luminous bacteria. Designated are the oligonucleotide primers used for PCR-amplification of specific fragments of the luxAB(F)E region of V. fischeri, P. leiognathi subsp. leiognathi, P. leiognathi subsp. mandapamensis and P. phosphoreum. Each primer designation represents at least two species-specific sequences. See Supplementary Table 1 for the sequences of primers used for each species. Presence of the luxF gene in P. phosphoreum and P. leiognathi subsp. mandapamensis and its absence in V. fischeri and P. leiognathi subsp. leiognathi, based on sequence analysis (see Fig. 2 and Supplementary Fig. 3), is indicated
Next, we examined P. leiognathi strains not previously tested for the presence of luxF, including ATCC 27561T, ATCC 33981, PL-721, ATCC 25587, lequu.1.1, and lleuc.1.1. As found for the P. phosphoreum strains, sequence analysis of the lux operons of strains ATCC 27561T, ATCC 33981, and PL-721 revealed the presence of luxF, with a gene order of luxABFE. In contrast, however, luxF was not present in the lux operons of strains ATCC 25587, lequu.1.1 and lleuc.1.1 (Fig. 1), which were isolated from leiognathid fish light organs. Furthermore, we tested several additional strains of P. leiognathi isolated from leiognathid fish light organs, including strains from a recent study of genomotypic diversity in this species (Dunlap et al. 2004); all lacked a luxF gene (J.C. Ast and P.V. Dunlap, unpublished data).
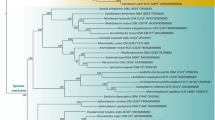
To test the phylogenetic relationships among these strains, we next analyzed the sequence of luxAB(F)E, examining individual protein coding regions (Fig. 1) in a combined analysis. Parsimony analysis resulted in a single hypothesis, which resolved P. phosphoreum and two closely related but phylogenetically distinct clades within what is currently referred to as P. leiognathi (Supplementary Fig. 3). To examine the relationship of these strains together with other Photobacterium species, some of which are non-luminous, we then carried out a combined multilocus analysis, using the 16S rRNA gene and gyrB, together with the genes and inter-genic spacers of the lux region for luminous species. The multilocus analysis resolved P. phosphoreum and two P. leiognathi clades (Fig. 2) in a manner very similar to that of luxAB(F)E alone. Analysis of 16S and gyrB, however, contributed little to the distinction between strains, as indicated in Fig. 2, whereas gene organization and sequence of the lux region diagnosed the two P. leiognathi clades. One clade contains strains originally described as P. leiognathi (ATCC 25521T and ATCC 25587) and other strains from leiognathid light-organ symbiosis (lequu.1.1 and lleuc.1.1). The other clade contains strains originally described as P. mandapamensis (ATCC 27561T and ATCC 33981) and certain strains identified after the 1975 synonymy (Reichelt and Baumann 1975) as P. leiognathi (PL-721, PL-741, and 554). The Bremer support and jackknife resampling values (Fig. 2) indicate very strong support for the two clades. These results indicate that P. leiognathi is composed of two evolutionarily distinct groups, designated here as the leiognathi clade (P. leiognathi subsp. leiognathi; Boisvert et al. 1967; strains ATCC 25521T, ATCC 25587, lequu.1.1 and lleuc.1.1) and the mandapamensis clade (P. leiognathi subsp. mandapamensis; Hendrie et al. 1970; strains ATCC 27561T, ATCC 33981, PL-721, PL-741, and 554).
Phylogram of the most parsimonious hypothesis resulting from combined multilocus analysis of 16S rRNA gene, gyrB, and luxAB(F)E sequences of Photobacterium species (1,429 informative characters, length=2,627, CI=0.76, RI=0.87). Numbers below branches are Bremer support and jackknife resampling values. Numbers above the branches indicate the quantitative contribution of each locus, 16S rRNA gene, gyrB, and luxAB(F)E, to branch length. For example, for the P. leiognathi subsp. leiognathi clade, the values of 0/5/305 represent no steps contributed by 16S rRNA gene, five steps contributed by gyrB, and 305 steps contributed by luxAB(F)E. See Table 1 for strain information
Distinguishing the leiognathi and mandapamensis clades
With this phylogenetic resolution, we were in a position to reassess the possibility that phenotypic characters might allow members of the two clades to be distinguished, despite their substantial similarity. However, a comprehensive re-examination of the published chemotaxonomic characters reported for strains of P. leiognathi and P. mandapamensis (Hendrie et al. 1970; Reichelt and Baumann 1973, 1975; Reichelt et al. 1976; Ruby and Morin 1978) failed to identify characters that would consistently distinguish members of the two clades.
We therefore sought to identify new phenotypic characters for this purpose. Initially, we examined minimal and maximal temperatures for growth. Certain strains of each clade, however, failed to grow at the low and the high temperatures examined (Table 2). Thus, minimal and maximal growth temperatures apparently are not effective for distinguishing members of the leiognathi and mandapamensis clades.
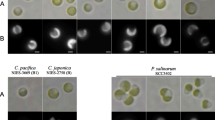
We next compared the luminescence responses of strains of the two clades grown on a minimal medium containing either a low level (BM-40) or a high level (BM-100) of seawater salts. Strains ATCC 27561T, ATCC 33981 and PL-721 produced a higher level of luminescence on BM-100 compared to BM-40. In contrast, strains ATCC 25521T, ATCC 25587, lequu.1.1 and lleuc.1.1 produced a higher level of luminescence on BM-40 compared to BM-100 (Table 2). These results indicate that comparison of relative luminescence intensity on low and high salt-containing minimal medium can distinguish members of the mandapamensis clade from those of the leiognathi clade. Furthermore, strain PL-721 (mandapamensis clade) was noted earlier to produce luminescence that is bluer in color than the blue–green luminescence of P. leiognathi from leiognathid fish light organs (Dunlap 1984). We therefore screened strains ATCC 27561T, ATCC 33981, PL-721, ATCC 25521T, ATCC 25587, lequu.1.1 and lleuc.1.1 for luminescence color. Strongly luminous strains of the mandapamensis clade were distinctly bluer in color than strongly luminous strains of the leiognathi clade (Fig. 3).
Luminescence color of members of the leiognathi and mandapamensis clades of P. leiognathi. Strains PL-721 (left: mandapamensis clade) and lequu.1.1 (right: leiognathi clade) were grown overnight on LSW medium and photographed by the light produced by the cells. The image was captured with Fujichrome Sensia 400 daylight film exposed to the plate in complete darkness at an f11 for ∼5 min using a Nikon N70 SLR camera fitted with a Nikon Nikkor AF Micro 60 mm 1:2.8D lens. The bluer color was characteristic of strains ATCC 27561T, ATCC 33981 and PL-721, whereas the more blue–green color was characteristic of strains ATCC 25521T, ATCC 25587, lequu.1.1 and lleuc.1. Distinctions between strains were made most readily on strongly luminous strains
The qualitative nature of these phenotypic differences, however, and the inter-strain variations in levels of light, which can complicate comparisons of luminescence intensity and color, led us to seek a more definite means of distinguishing these strains. To take advantage of the difference in the presence of luxF, we designed a primer pair (luxBf/luxBEr; Fig. 1 and Supplementary Table 1) that specifically amplifies the region of the lux operon spanning luxB and luxE. The expectation was that a larger PCR product, approximately 1,140 bp long, would be generated by the luxBf/luxBEr primer pair from strains bearing luxF, whereas a smaller PCR product, approximately 500 bp long, would be generated for strains lacking luxF. To test the ability of the primer pair to distinguish between strains of the two clades, we used them to direct the amplification of DNA from ATCC 27561T, ATCC 33981, PL-721, ATCC 25521T, ATCC 25587, lequu.1.1 and lleuc.1. A smaller product, approximately 500 bp in length, was recovered from ATCC 25521T, ATCC 25587, lequu.1.1 and lleuc.1, whereas a larger product, approximately 1,140 bp in length, was recovered from ATCC 27561T, ATCC 33981 and PL-721 (Fig. 4). These results establish the ability of the luxBf/luxBEr primer pair to distinguish members of the two clades.
PCR-based differentiation between members of the leiognathi and mandapamensis clades of P. leiognathi. Amplification of DNA was directed by the luxBf/luxBEr primer pair (Supplementary Table 1). The presence of luxF (strains of the mandapamensis clade) results in a larger PCR product, approximately 1,140 bp, whereas the absence of luxF (strains of the leiognathi clade) results in a smaller PCR product, approximately 500 bp. See Fig. 1 for gene organization
Identification of unknown strains
To test the effectiveness of these phenotypic screening and PCR procedures on unidentified bacteria, we examined previously uncharacterized strains of luminous bacteria recently isolated from coastal seawater in Florida. Three of the strains, seafl.1.1, seafl.1.3, and seafl.1.4 (Table 1), were found through phenotypic screening to exhibit stronger luminescence on BM-100 compared to BM-40 and to produce a bluer color of luminescence. When tested with the luxBf/luxBEr primer pair, each generated a product of approximately 1,140 bp long (data not shown), consistent with presence of a luxF gene and indicating membership in the mandapamensis clade.
To test clade membership of these strains, we analyzed their 16S rRNA gene, gyrB and luxAB(F)E sequences. The 16S rRNA gene and gyrB sequences of seafl.1.1, seafl.1.3, and seafl.1.4, as found for ATCC 27561T, ATCC 33981, PL-721, ATCC 25521T, ATCC 25587, lequu.1.1 and lleuc.1, placed them within P. leiognathi but did not resolve clade membership (Supplementary Figs. 1 and 2). Analysis of the lux region, however, confirmed the presence of luxF, and sequence analysis of the luxABFE region resolved these three strains to the mandapamensis clade (Supplementary Fig. 3), as did a multilocus phylogenetic analysis based on sequences of the 16S rRNA gene, gyrB and luxABFE (Fig. 2).
Discussion
The results of this study demonstrate the effectiveness of sequence analysis of the lux region for distinguishing closely related luminous bacteria. Two evolutionarily distinct clades of bacteria within P. leiognathi were resolved based on presence of the luxF gene and sequence of the luxAB(F)E region. One clade (mandapamensis; ATCC 27561T, ATCC 33981, PL-721, PL-741, and 554) comprises strains bearing luxF, whereas strains of the other clade (leiognathi; ATCC 25521T, ATCC 25587, lequu.1.1, lleuc.1.1) lack luxF (Baldwin et al. 1989; Illarionov et al. 1990; Lee et al. 1991; this study). A PCR method based on presence or absence of luxF was developed and its efficacy, in combination with phenotypic differences, for provisional clade assignment of strains was demonstrated with previously unstudied luminous bacteria. Clade membership was then confirmed with sequence analysis of 16S rRNA gene, gyrB and lux. DNA reassociation values of 78 and 84% between members of the leiognathi and mandapamensis clades previously placed these bacteria within a single species, P. leiognathi (Reichelt and Baumann 1975; Reichelt et al. 1976). However, the phylogenetic differences described here demonstrate the presence of evolutionarily distinct lineages consistent with two subspecies, P. leiognathi subsp. leiognathi and P. leiognathi subsp. mandapamensis. Further study might reveal these differences to be indicative of a species-level divergence.
Sequence divergence in luxA was used previously to assess the phylogenetic relationships between uncultured luminous bacteria symbiotic with anomalopid fish and known luminous bacteria (Haygood 1990) and as the basis for a hybridization method for distinguishing different species of luminous bacteria (Wimpee et al. 1991; Nealson et al. 1993). More recently, the sequence of luxA has been analyzed in the context of the description of a new species of luminous bacterium, Shewanella woodyi (Makemson et al. 1997), and in that report differences were noted in the luxA sequences of strains characterized more fully here at the sequence level, ATCC 25521T and PL-721. In the present study, we used the sequence of the luxAB(F)E region, which in contrast to sequences of 16S rRNA gene and gyrB provided substantial phylogenetic insight, to distinguish two evolutionarily distinct clades within the species P. leiognathi. The similarity of the combined 16S rRNA gene, gyrB and lux analysis to that of lux alone demonstrates the phylogenetic resolving power of luxAB(F)E, a locus that appears to be very effective for examining fine-scale within-species and between-species divergence among luminous bacteria.
Recently, the gyrB locus, which specifies the B subunit of gyrase, an enzyme essential for DNA replication, has been found to be more effective for resolving relationships among various bacteria than the 16S rRNA gene (Yamamoto et al. 2000; Dauga 2002; Yáñez et al. 2003). Consistent with these reports, gyrB provides good species resolution in Photobacterium (Supplementary Fig. 2), diagnosing most of the currently recognized species in this genus. However, we demonstrate here that gyrB, like the 16S rRNA gene, does not distinguish members of the leiognathi and mandapamensis clades. The inability of both 16S rRNA and gyrB to distinguish between members of these clades affirms a close evolutionary relationship. Analysis of gyrB therefore may prove most effective for resolving more distantly related species.
The results of this study have implications for the phylogeny of other species of Photobacterium. Based on analysis of the 16S rRNA gene, Urakawa et al. (1999) moved Photobacterium iliopiscarium from Vibrio to Photobacterium. In the present study, using multiple strains of P. phosphoreum and a combined 16S rRNA gene/gyrB analysis, we find that P. iliopiscarium is nested among, rather than sister to, P. phosphoreum. We find also that Photobacterium damselae is sister to all other Photobacterium species, as previously reported (Dunlap and Kita-Tsukamoto 2001), rather than sister to P. leiognathi (e.g., Nogi et al. 1998; Urakawa et al. 1999). It is likely, however, that with additional study, especially of more strains of each species, the view presented here of evolutionary relationships among members of the Photobacterium clade (Fig. 2) will require further modification.
Presence of luxF in the three examined strains of P. phosphoreum, NCIMB 844 (Soly et al. 1988), ATCC 11040T and pjapo.1.1 (this study), suggests that luxF is characteristic of P. phosphoreum. We were interested therefore in the possibility that the presence of luxF provides insight into speciation in luminous bacteria. The luxF gene is thought to have arisen in the ancestor of Photobacterium through a luxB gene duplication event (Meighen and Dunlap 1993). Based on the results presented here, we propose that following this duplication, luxF was subsequently lost from strains that became P. leiognathi subsp. leiognathi while being retained by strains that became P. phosphoreum and P. leiognathi subsp. mandapamensis (Fig. 5). This proposed loss of luxF therefore would mark a major evolutionary divergence in the Photobacterium lineage. An alternative possibility is that luxF arose independently in P. phosphoreum and P. leiognathi subsp. mandapamensis. This alternative scenario, however, requires two separate gene acquisition events and is not well supported at the sequence level. Specifically, the non-coding region 3′ of luxB in P. leiognathi subsp. leiognathi has regions identical to the non-coding regions in P. leiognathi subsp. mandapamensis between luxB and luxF and between luxF and luxE (Fig. 5). These sequence identities indicate a closer relationship between members of the leiognathi and mandapamensis clades than between P. leiognathi subsp. mandapamensis and P. phosphoreum. It is interesting to speculate that the absence of luxF in members of the leiognathi clade, and the resulting possible functional differences in its luminescence system, relate in some way to a difference in the native ecology of this bacterium compared to members of the mandapamensis clade.
Organization of the luxB(F)E region, including inter-genic spacer regions in V. fischeri and Photobacterium species. Identical sequences of DNA in the spacer regions (drawn to scale) are indicated by colored rectangles; the gray shaded region shared by P. leiognathi subsp. mandapamensis and P. leiognathi subsp. leiognathi indicates an area that, while not identical in sequence, is readily alignable, whereas the unshaded white regions indicate areas that could not be aligned. The cladogram maps characters onto the hypotheses presented in Fig. 2 and shows the hypothesized sequence of events in Photobacterium evolution: luxF and the green and black regions are acquired before the divergence of P. phosphoreum and the ancestor of P. leiognathi subsp. leiognathi and P. leiognathi subsp. mandapamensis; the red and blue regions are acquired after the divergence of P. phosphoreum but before the divergence of P. leiognathi subsp. leiognathi and P. leiognathi subsp. mandapamensis; finally, luxF is lost in P. leiognathi subsp. leiognathi after its divergence from P. leiognathi subsp. mandapamensis
An inference arising from this study is that members of the leiognathi and mandapamensis clades may be ecologically distinct. While P. leiognathi (leiognathi and mandapamensis clades) can be isolated from seawater (Baumann and Baumann 1981; Hastings and Nealson 1981; Dunlap and Kita-Tsukamoto 2001), strains examined in this study lacking luxF (leiognathi clade) all were isolated from the light organs of leiognathid fishes, whereas the strains bearing luxF (mandapamensis clade) were isolated from seawater and the surface of marine fish, but not from light organs of leiognathid fishes (Table 1). Whether members of the mandapamensis clade enter into a bioluminescent symbiosis is not known at this time, but the more blue color of luminescence produced by members of this clade suggests that such an association might be found with animals occurring at greater depths in the ocean than with the shallow-dwelling leiognathid fishes. Assessment of previous reports of the incidence of these two bacteria (e.g., Baumann and Baumann 1981) is complicated, however, by their prior synonymy and by the previous inability to distinguish them phenotypically. Establishment of P. leiognathi subsp. mandapamensis as a subspecies phylogenetically distinct from P. leiognathi subsp. leiognathi and application of the methods described here for differentiating between strains of the mandapamensis and leiognathi clades, using previously isolated strains and new isolates, should allow rapid progress now to be made in understanding the extent to which the ecologies of these two closely related luminous bacteria differ. Specifically, for example, we can state with reasonable confidence based on the results presented here that P. leiognathi subsp. mandapamensis is geographically widespread, with phylogenetically very similar strains occurring in seawater from locations as distant as southern India (Hendrie et al. 1970) and southern Florida (this study). P. leiognathi subsp. mandapamensis therefore may be cosmopolitan in its distribution.
The inability of methods considered standard for bacterial species identification (i.e., mol% G+C, DNA reassociation, 16S rRNA gene sequence, chemotaxonomic characters) to distinguish members of the leiognathi and mandapamensis clades (Reichelt and Baumann 1973, 1975; this study) is intriguing in light of the distinct separation provided by luxAB(F)E analysis. The shortcomings of the standard methods have been noted previously, and there is growing recognition of the importance of multilocus sequence analysis, as used here, in bacterial systematics for species identification (e.g., Palys et al. 1997; Stackebrandt et al. 2002). Based on the example presented here, our view is that analysis of inherited characters holds great promise for providing the information, sensitivity and precision necessary to capture an accurate picture of bacterial species diversity. Analysis of the lux operon, a locus that has diverged more rapidly than the 16S rRNA gene or gyrB, may have special value for resolving questions of species identity, clade membership and geographic distribution in luminous bacteria.
References
Baldwin TO, Devine JH, Heckel RC, Lin J-W, Shadel GS (1989) The complete nucleotide sequence of Vibrio fischeri and the luxABN region of Photobacterium leiognathi and the mechanism of control of bacterial bioluminescence. J Biolum Chemilum 4:326–341
Baumann P, Baumann L (1981) The marine gram-negative eubacteria: genera Photobacterium, Beneckea, Alteromonas, Pseudomonas, and Alcaligenes. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes: a handbook on habitats, isolation, and identification of bacteria. Springer, Berlin Heidelberg New York, pp 1302–1331
Boisvert H, Chatelain R, Bassot J-M (1967) Étude d’un Photobacterium isolé de l’organe lumineux de poissons Leiognathidae. Ann Inst Pasteur Paris 112:520–524
Dauga C (2002) Evolution of the gyrB gene and the molecular phylogeny of Enterobacteriaceae: a model molecule for molecular systematics studies. Int J Syst Evol Microbiol 52:531–547
Dunlap PV (1984) The ecology and physiology of the light-organ symbiosis between Photobacterium leiognathi and ponyfishes. PhD dissertation. University of California, Los Angeles, p 290
Dunlap PV (1985) Osmotic control of luminescence and growth in Photobacterium leiognathi from light organs of ponyfish. Arch Microbiol 141:44–50
Dunlap PV, Kita-Tsukamoto K (2001) Luminous bacteria, Ch. 329. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes, an evolving electronic resource for the microbiological community. Academic, New York
Dunlap PV, Jiemjit A, Ast JC, Pearce MM, Marques RR, Lavilla-Pitogo CR (2004) Genomic polymorphism in symbiotic populations of Photobacterium leiognathi. Environ Microbiol 6:145–158
Hastings JW, Nealson KH (1981) The luminous bacteria. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes: a handbook on habitats, isolation, and identification of bacteria. Springer, Berlin Heidelberg New York, pp 1332–1345
Haygood MG (1990) Relationship of the luminous bacterial symbiont on the Caribbean flashlight fish, Kryptophaneron alfredi (family Anomalopidae) to other luminous bacteria based on bacterial luciferase (luxA) genes. Arch Microbiol 154:496–503
Hendrie MS, Hodgkiss W, Shewan JM (1970) The identification, taxonomy and classification of luminous bacteria. J Gen Microbiol 64:151–169
Illarionov BA, Blinov VM, Donchenko AP, Protopopova MV, Karginov VA, Mertvetsov NP, Gitelson JI (1990) Isolation of bioluminescent functions from Photobacterium leiognathi: analysis of luxA, luxB, luxG and neighboring genes. Gene 86:89–94
Lee CY, Szittner RB, Meighen EA (1991) The lux genes of the luminous bacterial symbiont, Photobacterium leiognathi, of the ponyfish. Eur J Biochem 201:161–167
Makemson JC, Fulayfil NR, Landry W, Van Ert LM, Wimpee CF, Widder EA, Case JF (1997) Shewanella woodyi sp. nov., an exclusively respiratory luminous bacterium isolated from the Alboran Sea. Int J Syst Bacteriol 47:1034–1039
Meighen EA, Dunlap PV (1993) Physiological, biochemical and genetic control of bacterial bioluminescence. Adv Microbial Physiol 34:1–67
Nealson KH, Hastings JW (1977) Low oxygen is optimal for luciferase synthesis in some bacteria. Arch Microbiol 112:9–16
Nealson KH, Wimpee B, Wimpee C (1993) Identification of Vibrio splendidus as a member of the planktonic luminous bacteria from the Persian Gulf and Kuwait Region with luxA probes. Appl Environ Microbiol 59:2684–2689
Nogi Y, Masui N, Kato C (1998) Photobacterium profundum sp. nov., a new, moderately barophilic bacterial species isolated from a deep-sea sediment. Extemophiles 2:1–7
Palys T, Nakamura LK, Cohan FM (1997) Discovery and classification of ecological diversity in the bacterial world: the role of DNA sequence data. Int J Syst Bacteriol 47:1145–1156
Reichelt JL, Baumann P (1973) Taxonomy of the marine, luminous bacteria. Arch Mikrobiol 94:283–330
Reichelt JL, Baumann P (1975) Photobacterium mandapamensis Hendrie et al. a later subjective synonym of Photobacterium leiognathi Boisvert et al. Int J Syst Bacteriol 25:208–209
Reichelt JL, Baumann P, Baumann L (1976) Study of genetic relationships among marine species of the general Beneckea and Photobacterium by means of in vitro DNA/DNA hybridization. Arch Microbiol 110:101–120
Reichelt JL, Nealson KH, Hastings JW (1977) The specificity of symbiosis: pony fish and luminescent bacteria. Arch Microbiol 112:157–161
Ruby EG, Morin JG (1978) Specificity of symbiosis between deep-sea fish and psychrotrophic luminous bacteria. Deep-Sea Res 25:161–171
Soly RR, Mancini JA, Ferri SR, Boylan M, Meighen EA (1988) A new lux gene in bioluminescent bacteria codes for a protein homologous to the bacterial luciferase subunits. Biochem Biophys Res Commun 155:351–358
Sorenson MD (1999) TreeRot Version 2a. Boston University
Stackebrandt E, Frederiksen W, Garrity GM, Grimont PAD, Kämpfer P, Maiden MCJ, Nesme X, Rosselló-Mora R, Swings J, Trüper HG, Vauterin L, Ward AC, Whitman WB (2002) Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int J Syst Evol Microbiol 52:1043–1047
Swofford DL (2003) PAUP* Phylogenetic Analysis Using Parsimony (*and Other Methods) Version 4.0b10. Sinauer Associates, Sunderland
Urakawa H, Kita-Tsukamoto K, Ohwada K (1999) Reassessment of the taxonomic position of Vibrio iliopiscarius (Onarheim et al. 1994) and proposal for Photobacterium iliopiscarium comb. nov. Int J Syst Bacteriol 49:257–260
Wimpee CF, Nadeau T-L, Nealson KH (1991) Development of species-specific hybridization probes for marine luminous bacteria using in vitro DNA amplification. Appl Environ Microbiol 57:1319–1324
Yamamoto S, Kasai H, Arnold DL, Jackson RW, Vivian A, Harayama S (2000) Phylogeny of the genus Pseudomonas: intrageneric structure reconstructed from the nucleotides sequences of gyrB and rpoD genes. Microbiology 146:2385–2395
Yáñez MA, Catalán V, Apráiz D, Figueras MJ, Martínez-Murcia AJ (2003) Phylogenetic analysis of members of the genus Aeromonas based on gyrB gene sequences. Int J Syst Evol Microbiol 53:875–883
Acknowledgments
We thank M. Pearce for technical assistance, D. Bartlett for the gift of strains JCM 10084T (DSJ4) and SS9, C. Miyamoto and E. Meighen for the gift of strain NCIMB 844, and K. Nealson for the gift of strain PL-721. A. Gorog kindly provided helpful comments on the manuscript. DNA sequencing was carried out by staff of the University of Michigan Sequencing Core. Support was provided by the University of Michigan Center for Japanese Studies.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Ast, J.C., Dunlap, P.V. Phylogenetic analysis of the lux operon distinguishes two evolutionarily distinct clades of Photobacterium leiognathi . Arch Microbiol 181, 352–361 (2004). https://doi.org/10.1007/s00203-004-0663-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-004-0663-7