Abstract
Summary
Our systematic review and meta-analysis indicated that pro-inflammatory diets, as measured by higher Dietary Inflammatory Index scores, are significantly associated with lower BMD of lumbar spine and total hip as well as elevated risk of osteoporosis and fractures. These findings may contribute to the development of public health strategies.
Introduction
Inflammatory Index (DII) is a method to assess the inflammatory potential of diets; it has been reported to be associated with several diseases. However, the relation between DII and bone health remains controversial for the inconsistent findings from previous studies. This systematic review and meta-analysis aimed to ascertain the underlying relationships between DII and bone mineral density (BMD), osteoporosis risk, and fracture risk.
Methods
We systematically searched PubMed and Web of Science for all relevant epidemiological studies published up to May 1, 2020. Fixed-effects model or random-effects model was employed to pool the study-specific effect sizes (ESs) and 95% confidence intervals (CIs).
Results
Eleven studies with a total of 127,769 participants were included. We found that continuous DII was negatively associated with BMD of lumbar spine (odds ratios [OR]: 0.990; 95% CI: 0.984, 0.995) and total hip (OR: 0.995; 95% CI: 0.990, 0.999), but not femoral neck (OR: 0.998; 95% CI: 0.994, 1.002). Moreover, the highest category of DII displayed significantly associations to increased risk of osteoporosis (ES: 1.31; 95% CI: 1.16, 1.48) and fractures (ES: 1.28; 95% CI: 1.03, 1.59) compared with the lowest category of DII, respectively.
Conclusion
Our analysis indicated that diets with high pro-inflammatory components might increase the risk of osteoporosis and fractures and lower BMD of lumbar spine and total hip. More prospective studies involving populations of diverse ages and genders are expected to further verify the universality of the results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a systemic metabolic disease characterized by decreased bone mass, degenerative variations of bone microstructure, and increased bone fragility, thereby elevating the risk of fracture and even death [1]. Based on the World Health Organization, the osteoporosis is indicated when bone mineral density (BMD) values are 2.5 standard deviations (SDs) or more below the mean values of young, healthy females aged 20 to 29 years [2]. BMD is also used as an independent predictor of fracture risk. Osteoporosis has affected approximately 200 million people worldwide and led to a high disability rate as well as aggravating social costs, thus becoming a serious global burden [3].
Chronic inflammation plays a critical role in tissue damage and is closely related to multiple chronic conditions, including osteoporosis and fractures [4, 5]. A number of studies have indicated that diet, as the key source of biologically active ingredients, could mediate inflammation response [6]. Certain nutrients, such as vitamins A, C, E, and carotenoids, and minerals like selenium and zinc, may have anti-inflammatory features, as well as drinks/foods like green tea, fatty fish rich in omega-3 polyunsaturated fatty acids [7,8,9]. In contrast, consumption of red meat (rich in cholesterol and saturated fats) may result in pro-inflammation outcomes [10,11,12]. Therefore, it may contribute to the development of public health strategies by investigating the potential effects of diet-related inflammation in bone health.
The Dietary Inflammatory Index (DII) refers to a novel scoring system developed to quantify the inflammatory potential of diets among different populations [13]. Such index was created by assigning a score for each of 45 food parameters reported to regulate the levels of 6 specific inflammatory biomarkers. It has been verified that a higher DII score was correlated with high concentrations of inflammatory biomarkers (e.g., C-reactive protein [CRP] and tumor necrosis factor alpha [TNF-α]) [14, 15], suggesting that DII may be conducive to clarify the relationship between the inflammatory potential of diets and chronic diseases. Actually, higher DII score has been shown to be associated with elevated risk of various cancers [16], cardiovascular diseases [17], obesity [18], and depressive disorders [19] in systematic reviews and meta-analyses.
For DII and bone health, several studies reported positive associations between elevated DII score and the risk of fractures [20, 21]. However, one multi-racial cohort study of 92,694 postmenopausal women exhibited an inverse association [22]. Besides, it was reported that the elevated DII score was associated with lower BMD of lumbar spine and total hip [23, 24]. However, Cervo et al. found no statistically significant association between DII and BMD among older Australian men [25]. Accordingly, the relationship between DII and bone diseases remains controversial for inconsistent results among different epidemiological studies. To our knowledge, no systematic review and meta-analysis has been conducted to conclude this issue.
Thus, this systematic review and meta-analysis aimed to comprehensively pool available data and precisely ascertain the association between the inflammatory potential of diet, as measured by the DII score and BMD, osteoporosis risk, and fracture risk.
Materials and methods
The whole process of the present meta-analysis complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [26].
Search strategy
We conducted a comprehensive literature search for all potentially relevant observational studies in PubMed and Web of Science up to May 1, 2020. The following keywords were adopted: (“bone mineral density” OR “bone density” OR “bone loss” OR “osteoporosis” OR “osteopenia” OR “fracture” OR “broken bone”) AND (“inflammatory” OR “inflammation” OR “anti-inflammatory” OR “pro-inflammatory”) AND (“diet-related” OR “diet” OR “dietary”). The search was restricted to studies written in English. The reference lists of all relevant reviews and full-text papers were manually checked to ensure the recall ratio of the retrieval.
Inclusion criteria
Studies were considered eligible according to the following explicit criteria: (1) cohort, case-control, or cross-sectional designs were adopted; (2) the exposure of interest was categorical DII (the highest versus the lowest category of DII) or continuous DII, as computed at baseline; (3) the primary outcome of interest was BMD or osteoporosis or fracture among the population having not diagnosed with major chronic diseases; (4) studies providing beta coefficient for BMD; (5) those reporting risk estimates (risk ratio [RR], hazard ratio [HR], or odds ratio [OR]) and corresponding 95% confidence interval (CI) for the risk of osteoporosis and fractures, or sufficient data was presented for the relevant calculation. If several reports were from the same study, only the most comprehensive one was included. The eligibility of all potentially relevant studies was determined by two independent investigators. Any discrepancies were discussed and resolved with a third investigator.
Assessment of study quality
The methodological quality of eligible studies was assessed by two investigators independently with the Newcastle-Ottawa Quality Assessment Scale (NOS). Briefly, this scale is divided into three parameters of quality, namely, selection (four points), comparability (one point), and outcome (three points) with a scoring from 0 to 16 [27]. A higher score indicates better quality. Any discrepancies were discussed and resolved with a third investigator.
Data extraction
Data were extracted from each eligible study by a standard data extraction form. The following information was recorded, including first author’s name, publication year, country, study design, duration of follow-up, BMD or osteoporosis or fracture site, DII measurement, distribution of DII, sample size, age, gender, variables adjusted or matched, relevant effect sizes (ESs), and corresponding 95% CIs. If the studies provide more than one multivariable ESs, the model with the most comprehensive confounders adjusted for analysis was only adopted. The food components adopted to calculate DII in each study were also extracted (Supplementary Table 1). If necessary, the corresponding authors will be contacted for additional information.
Statistical analysis
The risk estimates and their 95% CIs of the highest versus the lowest category were pooled to investigate the relationships between the DII and the risk of osteoporosis and fractures. In DII and BMD analysis, the beta coefficients with their 95% CIs were converted to ORs for synthesis. For the original studies not reporting the multivariable-adjusted risk estimates, we calculated the unadjusted risk estimates with the original data. Inconsistency index (I2) and Cochran Q test were adopted to assess the statistical heterogeneity among studies [28]. I2 > 50% or P < 0.05 was considered significantly heterogeneous. The fixed-effects model was employed to combine study-specific results when no statistically significant heterogeneity existed among studies; otherwise, a random-effects model would be used for more conservative estimates [29].
Given the significant heterogeneity between studies investigating fractures, we conducted subgroup analysis and meta-regression, stratified by fracture site, gender, study design, major confounders adjusted or not, and geographic location to delve into the sources of heterogeneity.
Sensitivity analysis was conducted by excluding each study to explore the potential impact of a single study on the summary risk estimates. Publication bias was evaluated by Egger’s linear regression test and Begg’s rank correlation test with funnel plots [30, 31]. All statistical analyses were conducted with STATA software, version 15.1 (Stata Corp, College Station, TX). P < 0.05 was considered the level of statistical significance.
Results
Literature search and study characteristics
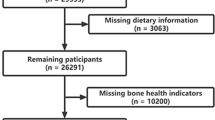
The process of literature screening is provided in the flow chart (Fig. 1). A total of 2224 studies were identified by database searching, while 5 additional records were taken from reference lists of retrieved papers. After duplicates and obviously irrelevant articles were excluded, 16 full-text articles were reviewed for detailed assessment. Next, 5 studies were excluded for the following reasons: one of them provided insufficient data, one was a review, and three focused on the lactating women, adolescent children, and young adults, respectively. Finally, 11 articles (4 cohort, 1 case-control, and 6 cross-sectional) satisfied the inclusion criteria in this meta-analysis [20,21,22,23,24,25, 32,33,34,35,36].
Baseline characteristics of covered studies are elucidated in Table 1. All articles were published from 2015 to 2019. A total of 127,769 participants with a mean age (SD) of 61.8 (8.30) were involved. The DII scores ranged from − 9.13 to 7.11. Three studies reported BMD, three reported osteoporosis, and five reported fractures. Several countries were involved: two studies were from Australia, three from Korea, three from America, one from Brazil, one from China, and one from Iran. One study was conducted on males, four on females, and six on both males and females. All included studies acquired information on food intake via food frequency questionnaires (FFQ) and calculated DII using Shivappa’s method. According to the NOS scoring system, the quality scores of included studies ranged from 10 to 15 with a median of 12 (Supplementary Table 2).
DII and BMD
As is shown in Fig. 2, per one-unit increase in DII score showed associations with a 1% reduction in lumbar spine BMD (pooled OR: 0.990; 95% CI: 0.984, 0.995; I2 = 44.5%, P-heterogeneity = 0.144) and 0.5% reduction in total hip BMD (pooled OR: 0.995; 95% CI: 0.990, 0.999; I2 = 0.0%, P-heterogeneity = 0.392), respectively. However, no statistically significant association between DII and BMD was found in femoral neck (pooled OR: 0.998; 95% CI: 0.994, 1.002). Meta-regression analysis indicated that there was no statistically significant heterogeneity across strata of gender (P-regression > 0.05).
DII and osteoporosis risk
Figure 3 presents the forest plot for osteoporosis risk. A considerable positive association was identified between the highest category of DII and osteoporosis risk (pooled ES: 1.31; 95% CI: 1.16, 1.48), compared with the lowest category of DII. No evidence of heterogeneity was observed among studies (I2 = 0.0%, P-heterogeneity = 0.525).
DII and fracture risk
The forest plot of DII and fracture risk is presented in Fig. 4. For the highest versus the lowest category of DII, the pooled ES was 1.26 (95% CI: 1.03, 1.59) from five studies, with significant heterogeneity (I2 = 87.3%, P-heterogeneity < 0.001). Subsequently, subgroup analysis was performed to seek possible sources of heterogeneity. Table 2 lists the results among different subgroups stratified by potential modifying factors. Statistically significant heterogeneity was observed across strata of study design (P-regression = 0.029). The results revealed that the association between DII and fracture appeared to be more noticeable in cross-sectional studies (pooled ES: 1.30; 95% CI: 1.15, 1.45) and case-control study (ES: 2.44; 95% CI: 1.73, 3.45) than that in cohort studies (pooled ES: 1.06; 95% CI: 0.8, 1.41).
Sensitivity analysis and publication bias
As is revealed from sensitivity analysis, none of studies evidently affected the pooled results. For limited studies reporting on BMD and osteoporosis, only publication bias was tested for fracture. Begg’s tests indicated no statistical evidence of publication bias (P > 0.05).
Discussion
To the best of our knowledge, this is the first meta-analysis concerned with the relationships between DII and BMD, osteoporosis risk, and fracture risk. Current evidence was exploited from 11 studies with 127,769 participants to obtain the result that highly pro-inflammatory diets, as calculated by higher DII scores, are negatively associated with BMD of lumbar spine and total hip, but not the femoral neck. Moreover, the findings revealed that the highest DII score was associated with a 31% increased risk of osteoporosis and 28% increased risk of fracture compared with the lowest DII, respectively.
The potential effect of pro-inflammatory diets on bone disease was interpreted by several biological mechanisms. Long-term intake of pro-inflammatory ingredients could elevate the level of inflammation in the body. It was suggested that the high saturated fatty acid diet might facilitate the secretion of interleukin-1 (IL-1) and IL-6 [10]. Red meat intake might result in concentrations of plasma CRP and other pro-inflammatory cytokines [6]. Moreover, existing studies demonstrated that pro-inflammatory factors were related to bone erosion and subsequent bone mass loss by suppressing osteoblast functions and stimulating osteoclast activity [37, 38].
With the in-depth exploration of the mechanism of bone diseases, studies on inflammatory biomarkers and BMD at different sites have been leaping forward. A population-based cohort study including 365 older adults suggested robust associations of serum IL-8, IL-10, and TNF levels with change in lumbar spine BMD. However, no inflammatory biomarkers were found to be associated with femoral neck BMD [39]. Likewise, in another cross-sectional study among patients with metabolic syndrome, log-transformed high-sensitivity-CRP (hs-CRP) concentrations were significantly associated with lumbar spine BMD in postmenopausal females. There was no evidence in favor of an association between hs-CRP and femoral neck BMD [39]. The definitive reason for this difference remains unclear. One possible explanation is that the lumbar spine with bone trabeculae as the major part is highly vascular and might be more susceptible to inflammation-induced bone fragility. Moreover, the higher surface-to-volume ratio of the lumbar spine than the femoral neck could contribute to increased metabolic activity. These may partially explain our findings that elevated DII was negatively associated with BMD of lumbar spine, but not femoral neck.
Our findings were broadly compatible with existing similar studies, in which food components that regulate inflammation are associated with bone disease. Nutritional epidemiology studies have demonstrated that high intake of carbohydrate and fat might contribute to osteoporosis or fracture [40, 41]. These food items are generally considered to have pro-inflammatory potential. In contrast, food components that are conceivable to have anti-inflammatory features, such as favored fruits, vegetables, and whole grains, have been proved as the protective factors of bone loss [22]. The abovementioned studies stressed the effects of single nutrients or food ingredients on bone health. Furthermore, predefined dietary patterns considering the interactions of several food items have been indicated to be relevant [42]. For instance, a Mediterranean diet rich in plant-based foods, olive oil, and fish was associated with reduced risk of fractures or low BMD [43], whereas a Western-style diet characterized by high intake of processed and red meat, refined grains, sugars, and fat may have more deleterious effects on bone health [44]. As a different approach, DII focuses on inflammatory potential of diet, providing an opportunity to assess the individual’s dietary intakes of different pro- or anti-inflammatory components as a whole. Food items included in the DII with their inflammatory potentials are showed in Supplementary Table 3. Our findings further emphasized the significance of a healthy dietary pattern containing anti-inflammatory nutrients (e.g., some vitamins, minerals, and polyunsaturated fatty acids) in bone health.
As is revealed from the results of meta-regression analysis, association between DII and fracture risk was impacted by study design. A significantly stronger association between DII and fracture risk was revealed among cross-sectional or case-control studies than prospective cohort studies. It has been acknowledged that cross-sectional and case-control studies are particularly vulnerable to recall and selection biases. This thereby leads to a weaker causal argument. Accordingly, the potential effect of highly pro-inflammatory diets on fracture risk may be overestimated. Of note, only 1 of the 5 studies included in the analysis investigated osteoporotic fracture [20], while the remaining 4 reported total fractures. We found that higher DII scores were more significantly associated with osteoporotic fractures than total fractures. Although osteoporosis is the major risk factor for fractures in the elderly [45], there are still some fractures caused by other factors (e.g., accidents), which may not be closely related to diets. Therefore, no statistically significant association was found in the cohort studies, probably because the included fracture cases were not completely induced by osteoporosis. This inconsistence should be interpreted cautiously, since only a few studies were included in the meta-regression.
Some limitations of this meta-analysis should be considered. Firstly, the included studies generally proved an unfavorable effect of pro-inflammatory diets on bone outcomes. However, 8 out of 11 studies were conducted on postmenopausal females or elderly groups, and thus, the findings of this study may more applicable to those populations. Secondly, though FFQ is a standard tool for obtaining dietary information, it is difficult for participants to accurately recall food intake over a long period of time. The dietary parameters of DII calculation in different studies ranged from 19 to 41, and those nutrients regulating inflammation but not included in analysis might affect the results as well. Thirdly, in the analysis of DII and fracture risk, our results showed statistically significant heterogeneity, probably due to the variation in study design. However, the use of random-effects model was allowed to consider the heterogeneity among studies. Lastly, we are unable to conduct dose-response meta-analysis because of the insufficient data in included studies.
Despite the limitations, this study also has some strengths. Above all, this is the first meta-analysis that comprehensively assessed the relation between DII score and bone health. Besides, all included studies that calculated DII score complied with Shivappa’s method, thereby enhancing the comparability. Moreover, both subgroup analysis and meta-regression were conducted to detect potential sources of heterogeneity; sensitivity analysis and tests for publication bias testified the stability of the main outcomes.
Conclusions
In summary, the present meta-analysis indicates that high pro-inflammatory diets, as measured by higher DII scores, are significantly related to lower BMD of lumbar spine and total hip, as well as elevated risk of osteoporosis and fractures. Transitioning to diets with less pro-inflammatory or more anti-inflammatory components should be suggested to prevent adverse bone outcomes, especially in the older. Nevertheless, more prospective studies involving populations of diverse ages and genders are required to further verify the universality of the results.
References
Kanis JA, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for C, Economic Aspects of O, the Committees of Scientific A, National Societies of the International Osteoporosis F (2019) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 30(1):3–44. https://doi.org/10.1007/s00198-018-4704-5
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 4(6):368–381. https://doi.org/10.1007/BF01622200
Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, Liu S, Looker AC, Wallace TC, Wang DD (2016) Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int 27(1):367–376. https://doi.org/10.1007/s00198-015-3386-5
Calder PC, Albers R, Antoine JM, Blum S, Bourdet-Sicard R, Ferns GA, Folkerts G, Friedmann PS, Frost GS, Guarner F, Lovik M, Macfarlane S, Meyer PD, M’Rabet L, Serafini M, van Eden W, van Loo J, Vas Dias W, Vidry S, Winklhofer-Roob BM, Zhao J (2009) Inflammatory disease processes and interactions with nutrition. Br J Nutr 101 Suppl 1:S1–45. https://doi.org/10.1017/S0007114509377867
Barbour KE, Boudreau R, Danielson ME, Youk AO, Wactawski-Wende J, Greep NC, LaCroix AZ, Jackson RD, Wallace RB, Bauer DC, Allison MA, Cauley JA (2012) Inflammatory markers and the risk of hip fracture: the Women’s Health Initiative. J Bone Miner Res 27(5):1167–1176. https://doi.org/10.1002/jbmr.1559
Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC (2007) Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr 137(4):992–998. https://doi.org/10.1093/jn/137.4.992
Neale EP, Batterham MJ, Tapsell LC (2016) Consumption of a healthy dietary pattern results in significant reductions in C-reactive protein levels in adults: a meta-analysis. Nutr Res 36(5):391–401. https://doi.org/10.1016/j.nutres.2016.02.009
Hodges JK, Zhu J, Yu Z, Vodovotz Y, Brock G, Sasaki GY, Dey P, Bruno RS (2020) Intestinal-level anti-inflammatory bioactivities of catechin-rich green tea: rationale, design, and methods of a double-blind, randomized, placebo-controlled crossover trial in metabolic syndrome and healthy adults. Contemp Clin Trials Commun 17:100495. https://doi.org/10.1016/j.conctc.2019.100495
Sánchez-Moreno C, Cano MP, de Ancos B, Plaza L, Olmedilla B, Granado F, Martín A (2004) Consumption of high-pressurized vegetable soup increases plasma vitamin C and decreases oxidative stress and inflammatory biomarkers in healthy humans. J Nutr 134(11):3021–3025. https://doi.org/10.1093/jn/134.11.3021
Dumas JA, Bunn JY, Nickerson J, Crain KI, Ebenstein DB, Tarleton EK, Makarewicz J, Poynter ME, Kien CL (2016) Dietary saturated fat and monounsaturated fat have reversible effects on brain function and the secretion of pro-inflammatory cytokines in young women. Metabolism 65(10):1582–1588. https://doi.org/10.1016/j.metabol.2016.08.003
Stock JK (2019) Cholesterol and inflammatory risk: insights from secondary and primary prevention. Atherosclerosis 280:192–193. https://doi.org/10.1016/j.atherosclerosis.2018.10.009
Samraj AN, Pearce OM, Läubli H, Crittenden AN, Bergfeld AK, Banda K, Gregg CJ, Bingman AE, Secrest P, Diaz SL, Varki NM, Varki A (2015) A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci U S A 112(2):542–547. https://doi.org/10.1073/pnas.1417508112
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR (2014) Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 17(8):1689–1696. https://doi.org/10.1017/S1368980013002115
Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Marcos A, Huybrechts I (2015) Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr 113(4):665–671. https://doi.org/10.1017/S000711451400395X
Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR (2014) A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr 17(8):1825–1833. https://doi.org/10.1017/S1368980013002565
Jayedi A, Emadi A, Shab-Bidar S (2018) Dietary inflammatory index and site-specific cancer risk: a systematic review and dose-response meta-analysis. Adv Nutr 9(4):388–403. https://doi.org/10.1093/advances/nmy015
Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, Grosso G (2018) Dietary inflammatory index and cardiovascular risk and mortality-a meta-analysis. Nutrients 10(2). https://doi.org/10.3390/nu10020200
Farhangi MA, Vajdi M (2020) The association between dietary inflammatory index and risk of central obesity in adults: an updated systematic review and meta-analysis. Int J Vitam Nutr Res:1–18. doi:https://doi.org/10.1024/0300-9831/a000648
Tolkien K, Bradburn S, Murgatroyd C (2019) An anti-inflammatory diet as a potential intervention for depressive disorders: a systematic review and meta-analysis. Clin Nutr 38(5):2045–2052. https://doi.org/10.1016/j.clnu.2018.11.007
Zhang ZQ, Cao WT, Shivappa N, Hebert JR, Li BL, He J, Tang XY, Liang YY, Chen YM (2017) Association between diet inflammatory index and osteoporotic hip fracture in elderly Chinese population. J Am Med Dir Assoc 18(8):671–677. https://doi.org/10.1016/j.jamda.2017.02.011
Mazidi M, Shivappa N, Wirth MD, Hebert JR, Vatanparast H, Kengne AP (2017) The association between dietary inflammatory properties and bone mineral density and risk of fracture in US adults. Eur J Clin Nutr 71(11):1273–1277. https://doi.org/10.1038/ejcn.2017.133
Orchard T, Yildiz V, Steck SE, Hebert JR, Ma Y, Cauley JA, Li W, Mossavar-Rahmani Y, Johnson KC, Sattari M, LeBoff M, Wactawski-Wende J, Jackson RD (2017) Dietary inflammatory index, bone mineral density, and risk of fracture in postmenopausal women: results from the Women’s Health Initiative. J Bone Miner Res 32(5):1136–1146. https://doi.org/10.1002/jbmr.3070
Cervo MM, Shivappa N, Hebert JR, Oddy WH, Winzenberg T, Balogun S, Wu F, Ebeling P, Aitken D, Jones G, Scott D (2020) Longitudinal associations between dietary inflammatory index and musculoskeletal health in community-dwelling older adults. Clin Nutr 39(2):516–523. https://doi.org/10.1016/j.clnu.2019.02.031
Shivappa N, Hebert JR, Karamati M, Shariati-Bafghi SE, Rashidkhani B (2016) Increased inflammatory potential of diet is associated with bone mineral density among postmenopausal women in Iran. Eur J Nutr 55(2):561–568. https://doi.org/10.1007/s00394-015-0875-4
Cervo MMC, Scott D, Seibel MJ, Cumming RG, Naganathan V, Blyth FM, Le Couteur DG, Handelsman DJ, Ribeiro RV, Waite LM, Shivappa N, Hebert JR, Hirani V (2020) Proinflammatory diet increases circulating inflammatory biomarkers and falls risk in community-dwelling older men. J Nutr 150(2):373–381. https://doi.org/10.1093/jn/nxz256
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012. https://doi.org/10.1016/j.jclinepi.2009.06.005
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu Y, Du L (2015) The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 8(1):2–10. https://doi.org/10.1111/jebm.12141
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Park S, Na W, Sohn C (2018) Relationship between osteosarcopenic obesity and dietary inflammatory index in postmenopausal Korean women: 2009 to 2011 Korea National Health and Nutrition Examination Surveys. J Clin Biochem Nutr 63(3):211–216. https://doi.org/10.3164/jcbn.18-10
Na W, Park S, Shivappa N, Hebert JR, Kim MK, Sohn C (2019) Association between inflammatory potential of diet and bone-mineral density in Korean postmenopausal women: data from fourth and fifth Korea National Health and Nutrition Examination Surveys. Nutrients 11(4). https://doi.org/10.3390/nu11040885
Veronese N, Stubbs B, Koyanagi A, Hebert JR, Cooper C, Caruso MG, Guglielmi G, Reginster JY, Rizzoli R, Maggi S, Shivappa N (2018) Pro-inflammatory dietary pattern is associated with fractures in women: an eight-year longitudinal cohort study. Osteoporos Int 29(1):143–151. https://doi.org/10.1007/s00198-017-4251-5
Morimoto M, Shivappa N, de Souza GP, Martini LA, Schuch NJ, Hebert JR, Pinheiro MM (2019) Lack of association between dietary inflammatory index and low impact fractures in the Brazilian population: the Brazilian Osteoporosis Study (BRAZOS). Adv Rheumatol 59(1):16. https://doi.org/10.1186/s42358-019-0059-7
Kim HS, Sohn C, Kwon M, Na W, Shivappa N, Hebert JR, Kim MK (2018) Positive association between dietary inflammatory index and the risk of osteoporosis: results from the KoGES_Health Examinee (HEXA) cohort study. Nutrients 10(12). https://doi.org/10.3390/nu10121999
Sponholtz TR, Zhang X, Fontes JD, Meigs JB, Cupples LA, Kiel DP, Hannan MT, McLean RR (2014) Association between inflammatory biomarkers and bone mineral density in a community-based cohort of men and women. Arthritis Care Res 66(8):1233–1240. https://doi.org/10.1002/acr.22270
Ehnert S, Baur J, Schmitt A, Neumaier M, Lucke M, Dooley S, Vester H, Wildemann B, Stockle U, Nussler AK (2010) TGF-beta1 as possible link between loss of bone mineral density and chronic inflammation. PLoS One 5(11):e14073. https://doi.org/10.1371/journal.pone.0014073
Fuggle NR, Westbury LD, Syddall HE, Duggal NA, Shaw SC, Maslin K, Dennison EM, Lord J, Cooper C (2018) Relationships between markers of inflammation and bone density: findings from the Hertfordshire Cohort Study. Osteoporos Int 29(7):1581–1589. https://doi.org/10.1007/s00198-018-4503-z
Wong SK, Chin KY, Suhaimi FH, Ahmad F, Jamil NA, Ima-Nirwana S (2018) Osteoporosis is associated with metabolic syndrome induced by high-carbohydrate high-fat diet in a rat model. Biomed Pharmacother 98:191–200. https://doi.org/10.1016/j.biopha.2017.12.042
Ilich JZ, Kelly OJ, Kim Y, Spicer MT (2014) Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arh Hig Rada Toksikol 65(2):139–148. https://doi.org/10.2478/10004-1254-65-2014-2541
Haring B, Crandall CJ, Wu C, LeBlanc ES, Shikany JM, Carbone L, Orchard T, Thomas F, Wactawaski-Wende J, Li W, Cauley JA, Wassertheil-Smoller S (2016) Dietary patterns and fractures in postmenopausal women: results from the Women’s Health Initiative. JAMA Intern Med 176(5):645–652. https://doi.org/10.1001/jamainternmed.2016.0482
Jennings A, Mulligan AA, Khaw KT, Luben RN, Welch AA (2020) A Mediterranean diet is positively associated with bone and muscle health in a non-Mediterranean region in 25,450 men and women from EPIC-Norfolk. Nutrients 12(4). https://doi.org/10.3390/nu12041154
Melaku YA, Gill TK, Adams R, Shi Z (2016) Association between dietary patterns and low bone mineral density among adults aged 50 years and above: findings from the North West Adelaide Health Study (NWAHS). Br J Nutr 116(8):1437–1446. https://doi.org/10.1017/s0007114516003366
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R, National Osteoporosis F (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25(10):2359–2381. https://doi.org/10.1007/s00198-014-2794-2
Funding
This work was supported by National Natural Science Foundation of China (grant number 81703289).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics approval
Yaqing Fang, Jiahao Zhu, Jiayao Fan, Lingling Sun, Shaofang Cai, Chunhong Fan, Yaohong Zhong, and Yingjun Li confirmed that this work complied with ethical standards.
Consent for publication
Yaqing Fang, Jiahao Zhu, Jiayao Fan, Lingling Sun, Shaofang Cai, Chunhong Fan, Yaohong Zhong, and Yingjun Li confirmed that the paper is being submitted for consideration for publication in Osteoporosis International; that this paper is approved by all co-authors and explicitly by the responsible authorities where the work was carried out. If accepted, it will not be published elsewhere.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fang, Y., Zhu, J., Fan, J. et al. Dietary Inflammatory Index in relation to bone mineral density, osteoporosis risk and fracture risk: a systematic review and meta-analysis. Osteoporos Int 32, 633–643 (2021). https://doi.org/10.1007/s00198-020-05578-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05578-8