Abstract
Studies have suggested that vitamin D supplementation may increase serum fibroblast growth factor 23 (FGF23) among vitamin D-deficient patients although the results were inconsistent across the studies. This systematic review and meta-analysis was conducted to summarize all available data. A systematic review was conducted using MEDLINE and EMBASE database from inception to February 2019 to identify studies that provided oral vitamin D3 supplement to vitamin D-deficient participants (25-hydroxyvitamin D < 20 ng/mL). Mean serum FGF23 concentration and standard deviation of participants at baseline and after vitamin D3 supplementation were extracted to calculate standard mean difference (SMD). Pooled SMD was calculated by combining SMDs of each study using random effects model. Nine studies were eligible for the meta-analyses. Seven studies measured serum intact FGF23, and two studies measured serum C-terminal FGF23. The meta-analyses found that serum intact FGF23 increased significantly after oral vitamin D3 supplementation in vitamin D-deficient participants with the pooled SMD of 0.36 (95%CI, 0.14, 0.57; p = 0.001; I2 of 36%). Serum C-terminal FGF23 also increased after vitamin D3 supplementation in vitamin D-deficient participants with the pooled SMD of 0.28 although without reaching statistical significance (95%CI, − 0.08, 0.65; p = 0.13; I2 of 0%). Funnel plot of the meta-analysis of serum intact FGF23 did not provide a suggestive evidence for publication bias. Vitamin D supplementation leads to a significant increase in serum intact FGF23 among vitamin D-deficient patients. An increase in serum C-terminal FGF23 was also observed although the number of included studies was too small to demonstrate statistical significance. The present systematic review and meta-analysis revealed that serum intact FGF23 concentration increased significantly after oral vitamin D3 supplementation in vitamin D-deficient participants. An increase in serum C-terminal FGF23 concentration was also observed although the number of included studies was too small to demonstrate statistical significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D is a steroid hormone responsible for maintaining calcium and phosphate homeostasis. Humans endogenously synthesize vitamin D in the skin upon exposure to ultraviolet B radiation and exogenously derive vitamin D from dietary sources and supplements. Once entering the circulation, vitamin D undergoes liver hydroxylation by 25-hydroxylase enzyme and turns into 25-hydroxyvitamin D (25(OH)D), which is generally measured for assessment of vitamin D status. 25(OH)D is then converted by 1α-hydroxylase enzyme in the kidney into the active from of 1,25-dihydroxyvitamin D (1,25(OH)2D) that exerts physiological functions by promoting intestinal calcium and phosphate absorption, renal tubular reabsorption of calcium, and bone resorption and formation [1]. Vitamin D deficiency, defined as serum 25(OH)D concentration of less than 20 ng/mL [2], can cause transient hypocalcemia as a result of impairment of intestinal calcium absorption. Decreased serum ionized calcium can subsequently lead to secondary hyperparathyroidism as a compensatory mechanism to maintain serum calcium concentration in a physiologically acceptable range [1, 3].
FGF23 is a phosphaturic hormone secreted by osteocytes. It inhibits renal tubular reabsorption of phosphate causing increased urinary phosphate excretion [4]. In addition, FGF23 suppresses the expression of 1α-hydroxylase enzyme in the kidney and induces the expression of 25-hydroxyvitamin D-24-hydroxylase enzyme, resulting in a decrease in conversion of 25(OH)D into 1,25(OH)2D and an increase in catabolism of 25(OH)D and 1,25(OH)2D into inactive carboxylic acids [5, 6]. Increased serum phosphate concentration is the known major physiologic regulator that stimulates FGF23 production by osteocyte [4, 6]. In addition, in vivo studies revealed that 1,25(OH)2D and parathyroid hormone (PTH) can also directly stimulate synthesis of FGF23 in osteocyte [5, 7, 8]. High concentration of serum FGF23 can be observed as a physiologic reaction to impaired renal function when renal phosphate excretion is compromised [6, 9]. Serum FGF23 can also be pathologically elevated in rare inherited diseases such as X-linked hypophosphatemic rickets as well as acquired disorders such as tumor-induced osteomalacia [10].
Although studies on the vitamin D–PTH–FGF23 axis are rising in number, the effect of vitamin D supplementation on serum FGF23 concentration in individuals with vitamin D deficiency is still not known. Treatment of vitamin D deficiency is known to recover intestinal calcium and phosphate absorption, leading to resolution of secondary hyperparathyroidism and decreased urinary loss of phosphate [3]. However, the effects of vitamin D supplementation on serum FGF23 are inconsistent across studies [11,12,13,14,15,16,17,18,19]. The current systematic review and meta-analysis was conducted with the aim to gather all available data to better describe the effect of vitamin D3 (cholecalciferol) supplementation on changes in serum FGF23 among vitamin D-deficient patients.
Methods
Search strategy
Three investigators (P.U., N.C., P.R.) independently searched for published studies indexed in MEDLINE and EMBASE from inception (1950 for MEDLINE and 1947 for EMBASE) to February 2019. Search terms derived from terms related to fibroblast growth factor 23 and vitamin D. The detailed search strategy is provided in the Supplemental Material 1. No language limitation was applied.
Inclusion criteria
Studies that were eligible to be included into the meta-analysis must be either prospective interventional single-arm study or randomized controlled study that gave oral vitamin D3 supplement to participants with vitamin D deficiency (defined as serum 25(OH)D concentration of less than 20 ng/mL, using assay methodology that measures the total 25(OH)D [25(OH)D2 and 25(OH)D3] [2]. Eligible studies must clearly define dosage and duration of vitamin D3 supplementation and must also report mean concentration of serum intact and/or C-terminal FGF23 of participants and its standard deviation (SD) or standard error of the mean at baseline and after oral vitamin D3 supplementation.
Study eligibility was independently determined by the two investigators (N.C. and P.R.). Different opinions were resolved by conference with the senior investigator (P.U.).
Data extraction
A standardized data collection form was used for extracting the following details: last name of the first author, country where the study was conducted, study design, year of publication, number of participants, recruitment of participants, dosage and duration of oral vitamin D3 supplementation, average age of participants, percentage of female, baseline serum 25(OH)D concentration, serum FGF23 at baseline and after vitamin D3 supplementation, and duration of serum FGF23 follow-up measurement.
Statistical analysis
Mean serum FGF23 and SD of participants before and after vitamin D3 supplementation were extracted from each study and the standard mean difference (SMD) was calculated. Pooled SMD was then calculated by combining SMDs of each study using random-effects model. The heterogeneity of the SMDs across the included studies was quantified using the Q statistic, which is complimented with I2 statistics. A value of I2 of 0–25% indicates insignificant heterogeneity, 26–50% low heterogeneity, 51–75% moderate heterogeneity, and 76–100% high heterogeneity [20]. Visual inspection of funnel plots was used to assess for the presence of publication bias. Data analysis was performed using Review Manager 5.3 software from the Cochrane Collaboration (London, UK).
Results
Search results
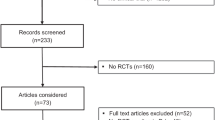
A total of 4404 articles were retrieved from MEDLINE and EMBASE databases in which duplicated articles were removed, leaving 3259 articles for title and abstract review. Based on title and abstract review, a total of 3217 articles were excluded as they clearly did not fulfill the inclusion criteria on the basis of type of article and study design. A total of 42 articles underwent full-length article review in which 33 articles were excluded as they did not report the outcome of interest. Finally, a total of nine studies consisting of seven randomized-controlled studies and two prospective interventional single arm studies met the inclusion criteria and were included into the meta-analysis [11,12,13,14,15,16,17,18,19]. Seven of the nine included studies measured serum intact FGF23 [11,12,13,14,15,16,17], while the other two studies measured serum C-terminal FGF23 [18, 19]. Therefore, two meta-analyses were performed separately; one for the studies that measured serum intact FGF23, and the other for the studies that measured serum C-terminal FGF23. Please note that the study by Alshayeb et al. [11] reported concentration of FGF23 among individual subgroup (which was based on renal function status) but did not report concentration of FGF23 for the entire cohort. Therefore, data from each subgroup were used for the calculation of pooled SMD. Please also note that the study by De Niet et al. [13] randomized their participants into two groups and both groups received oral vitamin D3 supplementation (the difference was in dosage and frequency). Therefore, both subgroups were eligible and included in this meta-analysis. The study review and selection process are described in Fig. 1. The basic characteristics of the included studies are summarized in Table 1.
Change in serum intact FGF23 concentration after vitamin D3 supplement in vitamin D-deficient patients
The meta-analysis found that serum intact FGF23 concentration increased significantly after oral vitamin D3 supplementation in vitamin D-deficient participants with the pooled SMD of 0.36 (95%CI, 0.14, 0.57; p = 0.001). The statistical heterogeneity of this meta-analysis was low with I2 of 36% (Fig. 2).
Change in serum C-terminal FGF23 concentration after oral vitamin D3 supplementation in vitamin D-deficient patients
The meta-analysis found that serum C-terminal FGF23 concentration increased after vitamin D3 supplementation in vitamin D-deficient participants with the pooled SMD of 0.28 although without reaching statistical significance (95%CI, − 0.08, 0.65; p = 0.13). The statistical heterogeneity of this meta-analysis was insignificant with I2 of 0% (Fig. 3).
Evaluation for publication bias
Funnel plot was used to assess for publication bias in the meta-analysis of change in serum intact FGF23. The plot was reasonably symmetric and did not show a suggestive evidence for the presence of publication bias (Fig. 4).
Discussion
The present study is the first systematic review and meta-analysis that summarizes the results of all available prospective interventional single-arm and randomized controlled studies that gave oral vitamin D3 supplement to participants with vitamin D deficiency and measured serum intact or C-terminal FGF23 at baseline and after vitamin D3 supplementation. The meta-analysis of both intact and C-terminal FGF23 revealed an increase in concentration of FGF23 after vitamin D3 supplementation in vitamin D-deficient patients. However, statistical significance was not achieved in C-terminal FGF23 analysis, probably due to limited number of participants as only two studies were included in this meta-analysis. PTH is a known sensitive biomarker of vitamin D and calcium status and normalization of serum PTH reflects response to vitamin D supplementation among vitamin D-deficient patients [1, 3]. The results of this study may suggest that FGF23 could be another possible surrogate marker for vitamin D status and response to vitamin D supplementation.
A few possible explanations exist for the observed increase in serum FGF23 in response to vitamin D3 supplementation in vitamin D-deficient patients. The first proposed mechanism is based on the evidence from vivo studies that 1,25(OH)2D can independently stimulate mRNA expression of FGF23 in osteocyte by interacting with vitamin D responsive element in the promotor region of the gene encoding for FGF23 [8, 21, 22]. Serum concentration of 1,25(OH)2D is usually normal even in the presence of vitamin D deficiency as a result of secondary hyperparathyroidism that enhances renal conversion of 25(OH)D into 1,25(OH)2D [1,2,3]; however, tissue and cellular level of 1,25(OH)2D in osteocyte could still be low [23]. Therefore, repletion of vitamin D might lead to an increase in intracellular 1,25(OH)2D concentration in the osteocyte which would then increase synthesis and secretion of FGF23. The second possible explanation is that vitamin D-deficient state is associated with impaired intestinal phosphate absorption and secondary hyperparathyroidism, which would lead to a decrease in renal tubular reabsorption of phosphate [1, 3]. Both will cause a relative phosphate-deficient state and, thus, suppression of osteocyte production of FGF23 [4, 6]. Correction of vitamin D deficiency will improve intestinal phosphate absorption and reverse secondary hyperparathyroidism, resulting in normalization of phosphate status and, subsequently, serum FGF23 concentration. This explanation is also supported by the fact that eight of the nine included studies reported a decrease in serum PTH concentration after vitamin D3 supplementation [11,12,13,14,15,16,17,18]. Theoretically, secondary hyperparathyroidism seen in vitamin D-deficient individuals should accelerate urinary phosphate loss, giving rise to hypophosphatemia. However, serum phosphate concentration of vitamin D-deficient individuals is usually within the normal range. One potential explanation is related to this suppression of FGF23 production, resulting in compensatory decreased urinary phosphate excretion [24].
In addition, it is worth noting that in vivo studies have demonstrated that PTH can directly stimulate osteocytic production of FGF23 by activating nuclear receptor-associated protein-1 to induce FGF23 transcription [7]. Therefore, the lower level of PTH as a result of treatment of vitamin D deficiency could theoretically decrease the concentration of FGF23. However, the current meta-analysis found an increase in serum FGF23 after vitamin D supplementation, suggesting that change in phosphate status or change in cellular/tissue concentration of 1,25(OH)2D in osteocyte plays a more vital role to regulate level of FGF23 concentration than the direct effect of PTH.
Since the physiologic effect of vitamin D2 (ergocalciferol) on calcium and phosphate metabolism is very similar to vitamin D3 [25], it is possible that prescribing vitamin D2 to vitamin D-deficient patients would also increase osteocytic production of FGF23. In fact, increased serum FGF23 concentration after treatment with vitamin D2 has been observed by some interventional studies although the number of included participants was relatively small [26, 27].
This systematic review and meta-analysis has some limitations and the results must be interpreted with caution. First, there was some between-study heterogeneity in the intact FGF23 analysis which was probably due to the difference in dosage, frequency, and duration of vitamin D supplementation across the included studies. Second, the analysis did not have comparators and, therefore, we cannot be certain that the change in serum FGF23 concentration was a result of vitamin D3 supplementation. It is still possible that serum FGF23 concentration will spontaneously increase over time without any intervention. This particular concern exists for studies that included participants with chronic kidney disease and end-stage renal disease [11, 14] as serum FGF23 in those participants might increase because of their declining renal function and worsening phosphate retention over time [28].
In summary, this study found that vitamin D3 supplementation leads to a significant increase in serum intact FGF23 among patients with vitamin D deficiency. An increase in serum C-terminal FGF23 was also observed although the number of included studies was too small to demonstrate statistical significance.
References
Holick MF (2007) Vitamin D Deficiency. N Engl J Med 357(3):266–281. https://doi.org/10.1056/NEJMra070553
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(7):1911–1930. https://doi.org/10.1210/jc.2011-0385
Holick MF (2006) Resurrection of vitamin D deficiency and rickets. J Clin Invest 116(8):2062–2072. https://doi.org/10.1172/jci29449
Bonewald LF, Wacker MJ (2013) FGF23 production by osteocytes. Pediatr Nephrol 28(4):563–568. https://doi.org/10.1007/s00467-012-2309-3
Prié D, Friedlander G (2010) Reciprocal control of 1,25-Dihydroxyvitamin D and FGF23 formation involving the FGF23/Klotho system. Clin J Am Soc Nephrol 5(9):1717–1722. https://doi.org/10.2215/CJN.02680310
Erben RG (2018) Physiological actions of fibroblast growth factor-23. Front Endocrinol 9:267–267. https://doi.org/10.3389/fendo.2018.00267
Lanske B, Razzaque MS (2014) Molecular interactions of FGF23 and PTH in phosphate regulation. Kidney Int 86(6):1072–1074. https://doi.org/10.1038/ki.2014.316
Yu X, Sabbagh Y, Davis SI, Demay MB, White KE (2005) Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone 36(6):971–977. https://doi.org/10.1016/j.bone.2005.03.002
Wahl P, Wolf M (2012) FGF23 in chronic kidney disease. Adv Exp Med Biol 728:107–125. https://doi.org/10.1007/978-1-4614-0887-1_8
Kinoshita Y, Fukumoto S (2018) X-linked hypophosphatemia and FGF23-related hypophosphatemic diseases: prospect for new treatment. Endocr Rev 39(3):274–291. https://doi.org/10.1210/er.2017-00220
Alshayeb H, Showkat A, Wall BM, Gyamlani GG, David V, Quarles LD (2014) Activation of FGF-23 mediated vitamin D degradative pathways by cholecalciferol. J Clin Endocrinol Metab 99(10):E1830–E1837. https://doi.org/10.1210/jc.2014-1308
Carvalho JTG, Schneider M, Cuppari L, Grabulosa CC, Aoike DT, Redublo BMQ, Batista MC, Cendoroglo M, Moyses RM, Dalboni MA (2017) Cholecalciferol decreases inflammation and improves vitamin D regulatory enzymes in lymphocytes in the uremic environment: a randomized controlled pilot trial. PLoS One 12(6). https://doi.org/10.1371/journal.pone.0179540
De Niet S, Coffiner M, Da Silva S, Jandrain B, Souberbielle JC, Cavalier E (2018) A randomized study to compare a monthly to a daily administration of vitamin D3 supplementation. Nutrients 10(6). https://doi.org/10.3390/nu10060659
Garcia-Lopes MG, Pillar R, Kamimura MA, Rocha LA, Canziani MEF, Carvalho AB, Cuppari L (2012) Cholecalciferol supplementation in chronic kidney disease: restoration of vitamin D status and impact on parathyroid hormone. Ann Nutr Metab 61(1):74–82. https://doi.org/10.1159/000339618
Kamelian T, Saki F, Jeddi M, Dabbaghmanesh MH, Omrani GHR (2018) Effect of cholecalciferol therapy on serum FGF23 in vitamin D deficient patients: a randomized clinical trial. J Endocrinol Investig 41(3):299–306. https://doi.org/10.1007/s40618-017-0739-2
Nygaard B, Frandsen NE, Brandi L, Rasmussen K, Oestergaard OV, Oedum L, Hoeck HC, Hansen D (2014) Effects of high doses of cholecalciferol in normal subjects: a randomized double-blinded, placebo- controlled trial. PLoS One 9(8):e102965. https://doi.org/10.1371/journal.pone.0102965
Turrini F, Scarlini S, Giovanardi P, Messora R, Roli L, Chester J, Mussi C, Bertolotti M, Trenti T, Bondi M (2017) Effects of cholecalciferol supplementation in patients with stable heart failure and low vitamin D levels (eCSPloiT-d): a double-blind, randomized, placebo-controlled pilot study. Minerva Cardioangiol 65(6):553–562. https://doi.org/10.23736/S0026-4725.17.04340-7
Mesinovic J, Mousa A, Wilson K, Scragg R, Plebanski M, de Courten M, Scott D, Naderpoor N, de Courten B (2019) Effect of 16-weeks vitamin D replacement on calcium-phosphate homeostasis in overweight and obese adults. J Steroid Biochem Mol Biol 186:169–175. https://doi.org/10.1016/j.jsbmb.2018.10.011
Trummer C, Schwetz V, Pandis M, Grübler MR, Verheyen N, Gaksch M, Zittermann A, März W, Aberer F, Steinkellner J, Friedl C, Brandenburg V, Voelkl J, Alesutan I, Obermayer-Pietsch B, Pieber TR, Tomaschitz A, Pilz S (2018) Effects of vitamin D supplementation on FGF23: a randomized-controlled trial. Eur J Nutr 58:1–7. https://doi.org/10.1007/s00394-018-1672-7
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD (2006) Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 17(5):1305–1315. https://doi.org/10.1681/ASN.2005111185
Ito M, Sakai Y, Furumoto M, Segawa H, Haito S, Yamanaka S, Nakamura R, Kuwahata M, Miyamoto K-i (2005) Vitamin D and phosphate regulate fibroblast growth factor-23 in K-562 cells. Am J Physiol Endocrinol Metab 288(6):E1101–E1109. https://doi.org/10.1152/ajpendo.00502.2004
Chesney RW, Zimmerman J, Hamstra A, DeLuca HF, Mazess RB (1981) Vitamin D metabolite concentrations in vitamin D deficiency: are calcitriol levels normal? JAMA Pediatr 135(11):1025–1028. https://doi.org/10.1001/archpedi.1981.02130350029010
Fukumoto S (2014) Phosphate metabolism and vitamin D. BoneKEy Rep 3:497–497. https://doi.org/10.1038/bonekey.2013.231
Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD (2008) Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 93(3):677–681. https://doi.org/10.1210/jc.2007-2308
Turner C, Dalton N, Inaoui R, Fogelman I, Fraser WD, Hampson G (2013) Effect of a 300 000-IU loading dose of ergocalciferol (vitamin D2) on circulating 1,25(OH)2-vitamin D and fibroblast growth Factor-23 (FGF-23) in vitamin D insufficiency. J Clin Endocrinol Metab 98(2):550–556. https://doi.org/10.1210/jc.2012-2790
Burnett-Bowie S-AM, Leder BZ, Henao MP, Baldwin CM, Hayden DL, Finkelstein JS (2012) Randomized trial assessing the effects of ergocalciferol administration on circulating FGF23. Clin J Am Soc Nephrol 7(4):624–631. https://doi.org/10.2215/CJN.10030911
Ketteler M (2011) Phosphate metabolism in CKD stages 3-5: dietary and pharmacological control. Int J Nephrol 2011:970245–970245. https://doi.org/10.4061/2011/970245
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nipith Charoenngam, Pongprueth Rujirachun, and Patompong Ungprasert declare that they have no conflict of interest. Michael F. Holick is a consultant for Quest Diagnostics Inc. and Ontometrics Inc., and on the speaker’s Bureau for Abbott Inc.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Charoenngam, N., Rujirachun, P., Holick, M. et al. Oral vitamin D3 supplementation increases serum fibroblast growth factor 23 concentration in vitamin D-deficient patients: a systematic review and meta-analysis. Osteoporos Int 30, 2183–2193 (2019). https://doi.org/10.1007/s00198-019-05102-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-019-05102-7