Abstract
Summary
We evaluate 38 elderly women who had received long-term denosumab treatment after stopping the drug. Taking into account the gain during treatment and the loss after stopping treatment, they lost 35.5% of the total gain in the spine, 44.6% of the total gain in the femoral neck, and 103.3% in the total hip.
Introduction
Denosumab (DMAb) is a soluble inhibitor of the receptor activator of nuclear factor-kappaB ligand (RANKL) and, therefore, does not incorporate into the bone matrix. Consistently, DMAb discontinuation is associated with reversal of the effects attained with treatment.
Purpose
The aim of this study is to assess changes in BMD after a year of discontinuation of DMAb in a group of postmenopausal women treated with DMAb for 7 or 10 years. Secondly, is to evaluate the occurrence of fragility fractures.
Methods
Women who had participated in the FREEDOM study and its extension were invited to participate in this follow-up study. BMD at LS and hip and spine X-rays were obtained. Results were compared to the last value obtained while in treatment to assess changes after discontinuation.
Results
Thirty-eight women, mean age: 81 ± 3.4 years completed study procedures; none had received bisphosphonates after stopping DMAb. Mean gap time between DMAb last dose and the follow-up visit was 17 months (range 16–20 months). Bone mineral density (BMD) decreased significantly in all regions: − 8.1% in LS, − 6% in FN, and − 8.4% in TH. Five (5/38, 13.15%) patients had a fragility fracture, one suffered a wrist fracture, and four experienced vertebral fractures. Three patients suffered one vertebral fracture and one of them had two vertebral fractures. Laboratory results showed the following mean values: CTX = 996 ± 307 pg/ml (normal values 550 ± 226 pg/ml); osteocalcin = 55.2 ± 18.6 ng/ml (normal value 42 ng/ml); and 25 OH vitamin D = 23.7 ± 6.9 ng/ml.
Conclusion
Our results describe the rapid bone loss occurring after cessation of denosumab treatment. Further studies are needed to assess if patients have a higher risk of fracture after stopping DMAb and if so, which patients have the highest risk, and assess the role of transitioning to bisphosphonates in the long term.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Denosumab, a fully human monoclonal antibody that binds to and inhibits the receptor activator of nuclear factor-kappaB ligand (RANKL), is an effective treatment for osteoporosis in postmenopausal women and men and for prevention of bone loss associated with hormone ablation therapy in patients with breast or prostate cancer [1,2,3,4]. Its biannual dose is well accepted, and patients find it easy to adhere to treatment which is essential in a chronic condition like osteoporosis [5].
Throughout 10 years of treatment, denosumab (DMAb) has shown to steadily increase, without a therapeutic plateau, not only trabecular but also cortical bone [6]. In addition, it is an adequate choice for patients with renal impairment who are at high risk of fracture and have very limited treatment options [7].
After stopping DMAb, several studies have shown that bone remodeling increased significantly, to higher than baseline levels and that bone mineral density decreased significantly, and rapidly in lumbar spine and hip [8,9,10]. Fracture incidence after denosumab therapy cessation was evaluated in several clinical trials [8,9,10,11]. These trials did not demonstrate an excess in fracture risk after discontinuation. However, they were not correctly designed to evaluate vertebral fractures after stopping the drug and no routine X-rays were performed [12]. In December 2015, case reports of multiple vertebral fractures after stopping DMAb were published [13,14,15,16,17,18]. This clinical scenario was described by Popp et al. as the presence of multiple new clinical vertebral fractures, associated with either no or low trauma, in a context of high bone turnover (elevated biochemical markers), and rapid loss of lumbar spine BMD, occurring after discontinuation (loss-of-effect) of a reversible antiresorptive therapy [15]. Secondary causes of bone loss or fractures had to be excluded. To our knowledge, a total of 24 case reports have been described so far [18]. Concerns have been raised regarding possible increased vertebral fragility following denosumab discontinuation due to a rebound in bone turnover to values above pre-treatment levels and accompanying rapid bone loss.
Regarding these concerns, we decided to assess postmenopausal women who had participated in the pivotal FREEDOM study and its extension in our center. The aim of the study was to evaluate BMD changes and fracture occurrence after at least 1 year of stopping DMAb in this group of patients.
Material and methods
The methodologies of both the FREEDOM study (Fracture REduction Evaluation of Denosumab in Osteoporosis every 6 months) and the extension study have already been published in detail [1, 6].Women who had participated in the FREEDOM study and its extension in our center—IDIM, Buenos Aires, Argentina—were invited to take part in this follow-up study. Inclusion criteria were female gender, treatment with denosumab (60 mg six-monthly) for at least seven consecutive years without skipping more than two doses, ambulatory and willing to attend a visit at our center, no bone active treatment after discontinuation of denosumab, except for calcium and vitamin D, and no medical conditions which, according to the investigator’s judgment, could have an influence on bone health disorder (including but not limited to cancer, primary hyperparathyroidism, decompensated cardiovascular disease).
Study procedures
The study protocol was reviewed and approved by an institutional review board and all patients signed an informed consent form before entering the study.
Medical interview and anamnesis
Data were collected regarding clinical and bone health risk factors (occurrence of incidental fractures, calcium and vitamin D supplementation, use of drugs, and other conditions which might affect bone health).
Bone mineral density assessment
Lumbar spine (LS) (L1-L4), total hip (TH), and femoral neck (FN) BMD were measured using dual X-ray equipment (Lunar Prodigy Advance, software 13.6). Bone mineral density (BMD) was compared to the last value obtained during the last visit of the pivotal study/extension, which took place 6 months after the last DMAb injection. In order to assess changes upon discontinuation, dual-energy x-ray absorptiometry (DXA) values were compared to those obtained while under treatment with DMAb.
Vertebral fracture assessment: spine X-ray
In order to assess changes upon discontinuation, lateral thoracic and lumbar X-rays were performed and compared to the last X-rays obtained in the last study visit, which took place 6 months after the last DMAb injection. All X-rays were performed and analyzed by the same specialist using the Genant semi-quantitative method for vertebral fracture assessment.
Laboratory tests
Serum calcium (NM-BAPTA; reference range: 8.8–10.5 mg/dl), serum phosphate (phosphomolydate; reference range: 2.7–4.5 mg/dl), parathyroid hormone (PTH) (electrochemiluminescence; reference range: 10.0–65.0 pg/ml), 25(OH) vitamin D (chemiluminescence; reference range: > 30 ng/mL), serum C-telopeptide (CTx) (electrochemiluminescence; reference range: 556 pg/ml ± 226), total alkaline phosphatase (AP) (kinetic at 37 °C; reference range: 35–104 IU/l), bone-specific alkaline phosphatase (BAP) (chemiluminescence; reference range: ≤ 21.3 μg/l), and osteocalcin (electrochemiluminescence; reference range: 11.0–43.0 ng/ml) were obtained. Although this bone profile testing was performed to assess bone remodeling upon discontinuation, the results could not be compared to the last previous tests obtained while under treatment because samples had been analyzed by different laboratories and not all patients had previous bone marker measurements.
Statistical analysis
A formal statistical hypothesis was not tested at the end of this 1-year observation study. All analyses were descriptive in nature. For this study, all parameters are expressed as mean ± SD. The comparison of DXA values between the FREEDOM/extension study data and those of the follow-up visit was analyzed using the paired t test or Wilcoxon signed-rank test depending on data distribution. Differences between independent groups were analyzed using the t test or Wilcoxon rank sum test depending on data distribution. Comparisons of proportions were performed using the Fisher’s exact test. The relationships were studied using Pearson correlation. P values (two-sided) ≤ 0.05 were considered significant. All statistical analyses were performed on STATISTIX 7.0 Copyright ©1995, 2000 Analytical software (Statsoft).
Results
A total of 56 women completed participation in the FREEDOM study and its extension at our center. After completing the study, all patients were monitored outside this center by their primary physicians. Thirteen patients were excluded, four had been diagnosed with cancer, one with hyperparathyroidism, two were unable to travel to the center, one had Parkinson’s disease, one had a psychiatric condition, one was not ambulatory, one had severe cardiovascular disease, and two had died. Forty-three women were invited to participate in a follow-up visit and four of them canceled for personal reasons. From January to April 2016, 39 women agreed to participate and signed the informed consent form. One patient was excluded for current bisphosphonate use, so, finally 38 women were included in the study.
The mean age was 81 ± 3.4 years (range: 76–89 years) and BMI was 28.4 ± 5.4 (Table 1). All subjects were of Hispanic-Latino origin. Seventeen patients had received DMAb treatment during 7 years and 21 during 10 years. Mean gap time between DMAb last dose and the follow-up visit was 17 months (range 16–20 months). Most of these patients were treatment naïve before entering the FREEDOM study. According to the study inclusion criteria, patients should not have received more than 3 years of bisphosphonates. Only two patients had received bisphosphonates in a clinically significant manner (for approximately 2 years with a wash-out period of 2 years). None of them reported other conditions potentially affecting bone metabolism. Half of the patients were receiving calcium and vitamin D supplementation (≥ 1 g calcium daily; ≥ 800 IU vitamin D daily).
Bone mineral density
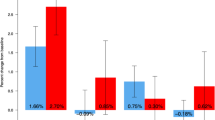
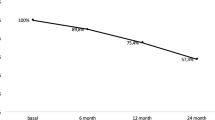
BMD had decreased significantly in all regions; − 8.1% in LS, − 6.0% in FN, and − 8.4% in TH (Table 2). Mean BMD and T-score at the lumbar spine were 0.923 mg/cm2 and − 2.1, respectively, while the average BMD and T-score had been 1.005 mg/cm2 and − 1.5 in the last visit of the pivotal study/extension (p < 0.01). Mean BMD and T-score at the femoral neck were 0.770 mg/cm2 and − 1.7, respectively, while the average BMD and T-score had been 0.820 mg/cm2 and − 1.3 (p < 0.01). Mean BMD and T-score at the total hip were 0.794 mg/cm2 and − 1.7, respectively, while the average BMD and T-score had been 0.866 mg/cm2 and − 1.1 (p < 0.01) in the last visit of the pivotal study/ extension.
The were no significant differences in any region in the amount of bone loss between the group treated with DMAb for 7 years and that treated for 10 years (− 7.3% in LS, − 5.5% in FN, and − 7.9% in TH compared to − 8.5, − 6.5, and −8.7%, respectively; p = 0.32, 0.5, and 0.59, respectively).
Only two patients had received ibandronate in a clinically significant manner before receiving DMAb, one during 12 months and the other during 30 months with a wash-out period of 2 years. They had similar changes to the rest of the patients (total group − 8.1% in LS and − 8.4% in TH vs. these two patients − 6. 1% in LS and – 5. 6% in TH). One of them worsened a previous vertebral fracture (from mild to moderate) after stopping the drug.
According to the follow-up visit evaluation, 17 (44.7%) women were categorized as osteoporotic, 9 of whom had densitometric osteoporosis only in the lumbar spine, 4 in the hip only, and 4 in both regions. According to the last FREEDOM/extension study data, only 4 (10.5%) patients had previous osteoporotic values (at least one region with T-score ≤ − 2.5).
Mean BMD gain during the FREEDOM/extension study in LS was 0.231 ± 0.089 g/cm2 accounting for a 29.9 ± 11.9% increase in BMD (7 years: 25.6 ± 9.8%; 10 years: 33.4 ± 12.5%). For the femoral neck, the mean BMD gain was 0.112 ± 0.054 g/cm2 (16.6 ± 8.7%, − 7 years: 12.7 ± 7.5%; 10 years: 19.7 ± 8.6% –). As for the total hip, the mean BMD gain was 0.070 ± 0.055 g/cm2 (9.3 ± 7.6% – 7 years: 5.2 ± 5.5%; 10 years: 12.6 ± 7.5% –). For the overall group, taking into account the gain during treatment and the loss after stopping treatment, women lost 35.5% of the total gain in the LS, 44.6% of the total gain in the FN, and 103.3% in the TH.
After evaluating the correlation between mean BMD change during treatment (gain) and change after stopping DMAb treatment (BMD bone loss), we found a significant negative correlation in LS (r = −0.42; p < 0.01) and TH (r = −0.46; p < 0.01). In addition, an assessment of the correlation between baseline BMD values and those after treatment cessation was carried out. Statistically significant positive correlations were observed in all regions: LS, r = 059; p < 0.01; FN, r = 0.52; p < 0.01 y TH, r = 084; p < 0.01.
Fracture assessment
After stopping denosumab, 5/38 (13.15%) patients suffered a fragility fracture. one suffered a wrist fracture, and four suffered vertebral fractures. None of the fractured patients (and any patient in the study) had received bisphosphonate treatment after stopping DMAb. The patient with the wrist fracture had a prevalent wrist fracture before receiving 10 years of denosumab. Out of the four patients who suffered vertebral fractures after stopping denosumab, two had no history of fragility fractures, but two had prevalent vertebral fractures. Of these last two patients with prevalent vertebral fractures, one had fractured before entering the FREEDOM trial and again during the study and the other had a vertebral and a wrist fracture before receiving DMAb (see Table 3 for fractured patients details).
Of the four patients who had vertebral fractures after stopping treatment, three patients suffered an asymptomatic, unique vertebral fracture, and the other patient fractured two vertebrae after a fall from her own height (clinical).
All fractured patients had all turnover bone markers (osteocalcin, ALK, and c-telopeptides) above reference range (Table 3) and 25OH vitamin D levels higher than 20 ng/ml. BMD loss was significant in every region, especially in the LS. Every fractured patient had osteoporosis in the LS before entering the FREEDOM study and none of them had it when finishing the study. During the FREEDOM study/extension trial, 7/38 (18.4%) patients suffered a fragility fracture compared to 5/38 (13%) (p 0.25) after stopping the drug (Table 4).
In order to identify risk factors for fracture after discontinuation, we compared age, BMI, biochemical parameters, prevalent fractures, number of years on DMAb, BMD values,and BMD loss between fractured (n = 5), and non-fractured (n = 33) patients. There were no statistically significant differences between the groups (data not shown). Sixty percent of the fractured patients (3/5) had a prevalent fragility fracture vs. 42.4% (14/33) of the non-fractured patients; this difference did not reach statistical significance (p = 0.64).
Biochemical parameters
In our patients, mean laboratory results were CTX: 996 ± 307 pg/ml (normal value premenopausal women 550 ± 226 pg/ml), osteocalcin 55.2 ± 18.6 ng/ml (normal value up to 42 ng/ml), 25 OH vitamin D 23.7 ± 6.9 ng/ml, PTH 44.7 ± 12.1 pg/ml, calcium 9.8 ± 0.3 mg/dl, and P 4 ± 0.4 mg/dl. In fractured patients, CTX values were 1064.2 ± 462 vs. 986 ± 286 pg/ml (p 0.73) in non-fractured.
Discussion
We assessed 38 women who had participated in the FREEDOM trial and its extension, and found that BMD decreased significantly after treatment cessation in all women while bone turnover markers evidenced a high remodeling state. Moreover, five of the 38 patients (13.15%) experienced one or more fragility fractures. Our study had the strength that these patients had been followed in our center for almost 12 years; each patient was individually evaluated by a specialized physician and had BMD, biochemical and X-ray assessment. None of them had been prescribed another osteoporosis treatment after stopping DMAb. This was an aged population; all women were older than 76 years, and even in these elderly women DMAb cessation triggers a high remodeling state.
The most common fractures observed after stopping DMAb were vertebral fractures. These occurred in 4/38 patients. Only one of these fractured patients had two clinical vertebral fractures. The other three were unique, asymptomatic and only diagnosed after X-ray assessment. Half of these patients had a history of prevalent vertebral fractures; one before and during denosumab treatment, and the other before DMAb treatment. Fractures happened in the context of high bone turnover and rapid and significant bone loss.
Published case reports had described patients with multiple clinical fractures with an acute onset of symptoms [13,14,15,16,17,18]. Anastasilakis et al. recently analyzed characteristics of 24 patients who had experienced 112 vertebral fractures after stopping DMAb [18]. No other types of fractures were reported. Most patients experienced multiple vertebral fractures (mean number 4.7). However, in our group, only one patient had more than one vertebral fracture. Similarly to our patients, most women had not received treatment prior or after DMAb therapy, while only 33% (8/24) had prevalent vertebral fractures. They found that patients with ≤ 2 years of denosumab treatment had fewer fractures than those with > 2 years (mean ± fractures 3.2 ± 0.7 vs. 5.2 ± 1.4, p = 0.055). However, our group had been treated for a very long time and suffered mostly unique asymptomatic fractures.
Risk of fracture after cessation of denosumab in women with osteoporosis was analyzed in the FREEDOM pivotal study [10]. Women (N = 327) who discontinued denosumab after two to five doses reported no excess of fractures during the off-treatment period. However, the median off-treatment follow-up duration was only 8 months; the study was not designed to capture vertebral fractures and at least one third of the patients had begun an osteoporosis treatment. A more thorough analysis of the data was presented by Brown et al. during the 2016 ASBMR Annual Meeting in Atlanta [11]. Women who had received at least two doses of denosumab and stayed in the study at least 7 months after the last denosumab injection were included (n = 1001). Fifty-six of them sustained new vertebral fractures (5.6%), a percentage comparable to those who had discontinued placebo. However, a greater percentage experienced multiple vertebral fractures in the group that discontinued denosumab. Prevalent vertebral fractures, before or during the treatment period, were the strongest predictor of new vertebral fractures following discontinuation. This study had the same limitations described above and additional vertebral fractures may have been missed due to the relatively short duration of off-treatment follow-up. Our study highlights the importance of monitoring patients with spine X-rays after treatment cessation. Up to now, real fracture prevalence after stopping DMAb is unknown and more scientific evidence is needed to address an important clinical question: whether this interval of high turnover and rapid bone loss results in an abnormally high fracture risk or just a return to the pre-treatment fracture status.
In our study, all patients lost BMD in all regions evaluated (− 8% in the LS and the total hip and − 6% in the FN). In the phase 2 study of Miller et al., follow-up after discontinuing denosumab treatment was available in 50 patients [8]. After 2 years of treatment with 210 mg denosumab every 6 months or 30 mg every 3 months, BMD in the LS and TH had increased, on average, by about 8 and 5%, respectively, but fell back to or near baseline within 1 year of stopping therapy. Similar responses to treatment withdrawal, loss of the 6% gain in the LS BMD within 1 year, were observed in the phase 3 prevention study in which women had received the clinical denosumab dose of 60 mg every 6 months for 2 years [9]. Finally, in the observational study of McClung et al. [19], a subgroup of 42 women received denosumab for 8 years, and was evaluated 12 months after treatment cessation, without having received another medication. They had lost 7.4% in the LS and 7.8% in the TH; the spine remained above baseline but the TH values returned to baseline 1 year after denosumab discontinuation. The rate of bone loss observed in our patients (6 to 8%) was similar to the above mentioned in earlier studies, although our patients had received therapy much longer. This shows that long-term denosumab therapy, as expected, does not protect patients from bone loss when therapy is discontinued. However, because our patients had experienced such large increases in BMD during long-term treatment, BMD remained well above baseline in the spine and FN at 17 months off-treatment. The TH BMD fell back to baseline value after discontinuation, and this can be related to a lesser magnitude in the TH BMD gain during treatment. Correlations show the amount of bone loss is related to the amount that had been gained: the greater the gain, the greater the loss after discontinuation. This relation between bone loss and bone gained was first shown by Miller et al. [8]. However, regardless of the length of exposure to the drug and different patient’s characteristics (age and BMD for example), the amount of bone loss seems similar in most studies: around 6 to 8% in the LS and TH and a bit lower in the FN (5 to 6%) in the first year.
Although we do not have previous results during treatment to confirm a rebound in bone turnover markers, values were higher than premenopausal reference range and much higher than expected in aged postmenopausal women. These values were obtained more than a year after the last DMAb dose (17 ± 1 months, range 16–20 months—11 months without DMAb treatment –). Previous studies have shown that after stopping DMAb, bone turnover rises above baseline at 9 months with a peak at 12 months and a progressive decrease thereafter, reaching again baseline levels approximately 30 months after the last dose [8,9,10]. According to these results, our patients still need more time to reach normal values again. While all fractured patients had high bone turnover, there were no significant differences between these patients’ values and those of the ones who did not sustained a fracture.
Despite the fact that the increase in bone turnover and decrease in BMD are universal after denosumab discontinuation, only a minority of patients sustained vertebral fractures. We did not find any significant differences in age, BMI, biochemical parameters, prevalent fractures, number of years on DMAb, BMD values, and BMD loss between fractured and non-fractured patients. The mechanism responsible for the development of these fractures is unknown, but it is hypothesized that they are due to transient increases in bone turnover and rapid loss of BMD following cessation of denosumab [20]. This phenomenon is expected to affect trabecular bone to a greater extent and more rapidly than cortical bone; a hypothesis that could explain the multiple vertebral fractures reported in clinical cases [4,5,6,7,8]. Anastasilakis et al. recently measured biochemical parameters including markers of bone and mineral metabolism and serum microRNAs and gene expression of mRNAs of factors regulating formation and activity of osteoclasts in three different groups of women. The first cases involved five women who suffered vertebral fractures after stopping DMAb, the second, women who had vertebral fractures but were treatment naïve, and the third group comprised five women who had stopped DMAb but had not sustained vertebral fractures. Compared to treatment-naïve fractured women, the first group had higher serum P1NP and CTX levels, and significantly lower serum miR-503, and miR-222-2 that downregulate osteoclastogenesis and osteoclast activity, and higher RANK (13-fold) and catepsin K (2.6-fold) mRNA. The respective values of the third group were in the same direction as the first group but of a lesser magnitude. They conclude that bone fragility in women with clinical vertebral fractures after stopping denosumab therapy is pathophysiologically different from that of treatment-naïve women with osteoporosis and clinical vertebral fractures and can be associated with upregulation of markers of osteoclast formation and activity.
However, to date, no risk factors have been identified that could predict with certainty which patients have the highest risk of fracture after stopping treatment. Meanwhile, in the everyday clinical practice, physicians should highlight to their patients the importance of strict adherence to the biannual dose of this treatment and advise them not to discontinue the drug without medical supervision. Also, taking an osteoporosis medication after stopping denosumab appeared to attenuate the decline in BMD [19]. In the DAPS study, postmenopausal women with low bone mass who had received denosumab for 1 year were switched to weekly alendronate therapy [5]. The increase in BMD that had occurred with denosumab therapy was preserved during 1 year of alendronate therapy. As we already routinely do in patients who stop anabolic treatment like teriparatide, it seems very prudent that therapy with another antiresorptive agent, such as a bisphosphonate, should be initiated to maintain what had been gained [12]. It has been suggested that patients with previous bisphosphonate exposure may have a very mild rebound in bone turnover after DMAb cessation [18]. Future studies may show if this strategy is useful in the long term and can prevent fragility fractures.
Besides the lack of bone turnover markers on treatment, we have to recognize that our study has limitations, including its small size, observational design, and lack of a placebo control group.
Our results describe what has been previously published: a high remodeling state evidenced by bone turnover markers and the consequent bone loss that occurs after cessation of denosumab. This study is too small to evaluate fracture outcomes. More studies are needed to try to identify which patients have higher risk of fracturing after stopping DMAb and to assess the role of transitioning to bisphosphonates in the long term. Meanwhile, it is important to increase physicians’ awareness that after denosumab discontinuation another antiresorptive treatment should be administered to avoid rapid bone loss.
References
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, ZoogHB AM, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C, Trial F (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Langdahl BL, Teglbjærg CS, Ho PR, Chapurlat R, Czerwinski E, Kendler DL, Reginster JY et al (2015) A 24-month study evaluating the efficacy and safety of denosumab for the treatment of men with low bone mineral density: results from the ADAMO trial. J Clin Endocrinol Metab 100(4):1335–1342. https://doi.org/10.1210/jc.2014-4079
Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L et al (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377(9768):813–822. https://doi.org/10.1016/S0140-6736(10)62344-6
Stopeck A., Martin M., Ritchie D., Body J.J., Paterson A., Viniegra M., et al. (2010) Effect of denosumab versus zoledronic acid treatment in patients with breast cancer and bone metastases: results from the extended blinded treatment phase. Cancer Res 2010: P6-14-01–P6-14-01. https://doi.org/10.1016/j.ejca.2015.09.011
Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, Borenstein J et al (2012 Jan) Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 23(1):317–326. https://doi.org/10.1007/s00198-011-1780-1
Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, Czerwiński E, Fahrleitner-Pammer A, Kendler DL, Lippuner K, Reginster JY, Roux C, Malouf J, Bradley MN, Daizadeh NS, Wang A, Dakin P, Pannacciulli N, Dempster DW, Papapoulos S (2017) 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 5(7):513–523. https://doi.org/10.1016/S2213-8587(17)30138-9
Jamal SA, Ljunggren O, Stehman-Breen C, Cummings SR, McClung MR, Goemaere S, Ebeling PR, Franek E, Yang YC, Egbuna OI, Boonen S, Miller PD (2011) Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res 26(8):1829–1835. https://doi.org/10.1002/jbmr.403
Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, Liu Y, San Martin J, AMG 162 Bone Loss Study Group (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 43:222–229
Bone HG, BologneseMA YCK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 96:972–980
Brown JP, Roux C, Törring O, Ho PR, Beck Jensen JE, Gilchrist N, Recknor C, Austin M, Wang A, Grauer A, Wagman RB (2013) Discontinuation of denosumab and associated fracture incidence: analysis from the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial. J Bone Miner Res 28:746–752
Brown JP, Ferrari S, Gilchrist N, Beck Jensen JE, Pannacciulli N, Recknor C, et al. (2016) Discontinuation of denosumab and associated fracture incidence: analysis from FREEDOM and its extension. J Bone Miner Res 31 (Suppl 1):Available at https://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=51d4e88b-f79d- 47e2-a15b-134f0c57b52e. Accessed September 27, 2016
McClung MR (2016) Cancel the denosumab holiday. Osteoporos Int 27(5):1677–1682
Anastasilakis AD, Makras P (2016) Multiple clinical vertebral fractures following denosumab discontinuation. Osteoporos Int 27(5):1929–1930
Aubry-Rozier B, Gonzalez-Rodriguez E, Stoll D, Lamy O (2016) Severe spontaneous vertebral fractures after denosumab discontinuation: three case reports. Osteoporos Int 27(5):1923–1925
Popp AW, Zysset PK, Lippuner K (2016) Rebound-associated vertebral fractures after discontinuation of denosumab-from clinic and biomechanics. Osteoporos Int 27(5):1917–1921
Polyzos SA, Terpos E (2016) Clinical vertebral fractures following denosumab discontinuation. Endocrine 54(1):271–272
Lamy O, Gonzalez-Rodriguez E, Stoll D, Hans D, Aubry-Rozier B (2016) Severe rebound-associated vertebral fractures after denosumab discontinuation: nine clinical cases report. J Clin Endocrinol Metab
Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O (2017) Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res. https://doi.org/10.1002/jbmr.3110
McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM (2011) Observations following discontinuation of long-term denosumab therapy. J Bone Miner Res 26(8):1829–1835. https://doi.org/10.1002/jbmr.403
Anastasilakis AD, Yavropoulou MP, Makras P et al (2017) Increased osteoclastogenesis in patients with vertebral fractures following discontinuation of denosumab treatment. Eur J Endocrinol 176(6):677–683
Acknowledgements
We acknowledge Amgen’s support in data sharing and review of this manuscript.
We thank Susana Carballo for editorial assistance on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
María Belén Zanchetta, Juan Boailchuk, Fabio Massari, Fernando Silveira, Cesar Bogado, and José R. Zanchetta declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zanchetta, M.B., Boailchuk, J., Massari, F. et al. Significant bone loss after stopping long-term denosumab treatment: a post FREEDOM study. Osteoporos Int 29, 41–47 (2018). https://doi.org/10.1007/s00198-017-4242-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4242-6