Abstract
Summary
A 6-month randomized controlled trial of spine-strengthening exercise and posture training reduced both radiographic and clinical measures of kyphosis. Participants receiving the intervention improved self-image and satisfaction with their appearance. Results suggest that spine-strengthening exercise and postural training may be an effective treatment option for older adults with hyperkyphosis.
Introduction
The purpose of the present study is to determine in a randomized controlled trial whether spine-strengthening exercises improve Cobb angle of kyphosis in community-dwelling older adults.
Methods
We recruited adults ≥60 years with kyphosis ≥40° and enrolled 99 participants (71 women, 28 men), mean age 70.6 ± 0.6 years, range 60–88, with baseline Cobb angle 57.4 ± 12.5°. The intervention included group spine-strengthening exercise and postural training, delivered by a physical therapist, 1-h, three times weekly for 6 months. Controls received four group health education meetings. The primary outcome was change in the gold standard Cobb angle of kyphosis measured from standing lateral spine radiographs. Secondary outcomes included change in kyphometer-measured kyphosis, physical function (modified Physical Performance Test, gait speed, Timed Up and Go, Timed Loaded Standing, 6-Min Walk), and health-related quality of life (HRQoL) (PROMIS global health and physical function indexes, SRS-30 self-image domain). ANCOVA was used to assess treatment effects on change from baseline to 6 months in all outcomes.
Results
There was a −3.0° (95% CI −5.2, −0.8) between-group difference in change in Cobb angle, p = 0.009, favoring the intervention and approximating the magnitude of change from an incident vertebral fracture. Kyphometer-measured kyphosis (p = 0.03) and SRS-30 self-esteem (p < 0.001) showed favorable between-group differences in change, with no group differences in physical function or additional HRQoL outcomes, p > 0.05.
Conclusions
Spine-strengthening exercise and posture training over 6 months reduced kyphosis compared to control. Our randomized controlled trial results suggest that a targeted kyphosis-specific exercise program may be an effective treatment option for older adults with hyperkyphosis.
Trial registration number and name of trial register
ClinicalTrials.gov; identifier NCT01751685
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Age-related hyperkyphosis, an excessive forward curvature in the thoracic spine, is a common progressive deformity that affects up to 40% of adults over the age of 65 years [1,2,3]. Kyphosis tends to progress with age [2, 4] and is associated with significant health impairments. Kyphosis greater than 40° is commonly defined as hyperkyphosis [1, 2], and once kyphosis progresses beyond 50°, the risk for falls [5, 6] and fractures [7] is increased. Multiple studies have reported that older persons with hyperkyphosis suffer from poor and worsening health-related quality of life (HRQoL) [1] and physical function [2, 8,9,10]. Despite these adverse effects on health, hyperkyphosis is only recently becoming recognized by health care providers as a health concern [11], and to date, there is no standard of care.
Given the expected increased prevalence and incidence of hyperkyphosis in an aging population, effective preventative and therapeutic interventions are required. Greater kyphosis has been associated with underlying osteoporosis, vertebral fractures, diffuse idiopathic skeletal hyperostosis (DISH), degenerative disc disease, and spinal extensor muscle weakness and density, and treatment interventions may need to be tailored accordingly [3, 12,13,14,15]. Treatment for osteoporosis may have limited utility for preventing hyperkyphosis, given that only a third of patients with hyperkyphosis have evidence of underlying vertebral fractures [16]. Furthermore, no currently approved FDA medication for osteoporosis has an indication for prevention or treatment of hyperkyphosis. To explore another potential avenue for intervention, our research group conducted a pilot study targeting spinal muscle strength among older women with hyperkyphosis. After a 3-month exercise intervention, we observed a significant improvement in the clinical kyphometer measure of kyphosis, Biodex spinal extension peak torque and the modified Physical Performance Test (PPT) [17]. Moreover, a systematic review including seven randomized controlled trials reported that exercise interventions targeting back extensor muscle strength resulted in modest improvements in clinical measures of kyphosis [18]. However, small sample sizes, heterogeneity of the study subjects, lack of inclusion of men, varied and unvalidated kyphosis assessments, and sparse information on physical function outcomes precluded making any conclusive treatment recommendations. Given the promising results of these earlier exercise intervention trials, we conducted an adequately powered randomized controlled trial to determine whether targeted spine-strengthening could reduce hyperkyphosis in community-dwelling older adults. Additionally, we included secondary outcome measures of physical function and HRQoL that are important correlates of age-related hyperkyphosis. Finally, we hypothesized that if exercise improves kyphosis, it may work through improving muscle strength and/or quality and thus included both isometric and computed tomography measures of muscle strength and quality as possible mediators of the effect.
Methods
Study design and participants
We conducted a priori power calculations based upon the results of our previous pilot study [17]. After accounting for a loss to follow-up of 20%, the randomized sample of 100 participants provided 80% power in two-sided tests with a type 1 error rate of 5% to detect a between-group difference of 1.9° in the primary outcome of kyphosis. We were also powered to detect between-group differences of 0.06 m/s in gait speed and 0.98 points in the modified PPT in the secondary outcomes of physical function.
Participants were recruited from January 2013 through June 2015 from local senior centers, outpatient medical clinics, physician referrals, and medical center databases. Once screened online or by telephone, a baseline screening exam was scheduled, at which time written informed consent was obtained as well as permission from the potential participant’s primary care provider.
Inclusion criteria were proficiency in English, age ≥60 years, kyphosis angle ≥40° by the Debrunner kyphometer measured at the screening visit, and ability to walk one block without the use of an assistive device, climb one flight of stairs independently, and rise from a chair without the use of one’s arms. Participants were excluded for inability to straighten the thoracic spine at least 5°, cognitive impairment (unable to draw a normal clock or recall any words on the Mini-Cog) [19], inability to pass safety tests in the screening examination or any disorder or disease likely to prevent or interfere with safe participation in a group-based exercise class (see methods paper for details on safety tests, disorders, and diseases) [20].
The study protocol was approved by the University of California San Francisco and Kaiser Permanente Northern California Institutional Review Boards.
Randomization
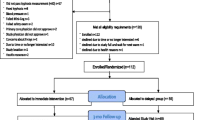
The study enrolled five waves of 20 participants each (Fig. 1). Following baseline testing, participants were randomized to the active or the control group in randomly permuted blocks of two and four, stratified by age (<75 vs 75+) and sex (male vs female). The random allocation sequence was generated by the study biostatistician. The study coordinator placed the assignments in sealed consecutively numbered envelopes to conceal allocation, and the study participant was assigned the next available ID number for the appropriate age and sex stratum. The envelope was opened after completing baseline testing.
Intervention
Active participants attended a group (n = 10) exercise program for 1 h three times per week for 6 months. A licensed physical therapist led the exercise intervention, assisted by a trained research assistant to ensure a ratio of five participants to each instructor. The intervention was a multimodal group-based kyphosis-specific exercise program that targeted multiple musculoskeletal impairments known to be associated with hyperkyphosis, including spinal extensor muscle weakness [12, 21], impaired recruitment and activation of the spinal extensor muscles [22], decreased spinal mobility [23], and poor postural alignment (see methods paper for detailed exercises) [17, 18, 20]. Exercises were progressed in intensity during the study, with an emphasis on good-quality movement while maintaining a Borg Scale intensity of 4–5, based upon 70–80% of perceived exertion [1, 24].
The instructors used postural training [25] that included auditory, visual, and tactile feedback to participants to teach them to develop and maintain neutral spinal alignment during the group exercise program. Participants were instructed to align their head over the pelvis and base of support and stabilize the spine in a neutral position while bending with the hips and knees during functional activities. Participants were provided a study manual including pictures of ideal neutral spinal alignment during functional activity, including sitting, standing, sit to stand, bending, and sleeping, and were instructed to practice ideal posture at least three times during the day outside of study visits. Participants reported compliance with this home program to the study coordinator on a weekly basis (completed a checklist reporting the number of days a week and the number of times a day that they practiced).
Control
Control participants received monthly health education group (n = 10) classes to provide social interaction for 1 h once a month for 4 months. Topics included bone health, urinary incontinence, fall prevention, and stress management.
Other study contact
After their 6-month testing visit, all study participants received a DVD of the study exercise program and exercise equipment (a foam roller and theraband). Additionally, the control participants received one-on-one instruction in the kyphosis-specific exercise program from the study physical therapist and a manual with pictures of ideal spinal alignment during functional activity including sitting, standing, sit to stand, bending, and sleeping.
Outcome assessments
Primary outcome: change in Cobb angle of kyphosis
A baseline assessment was conducted before randomization and included all primary and secondary outcome measurements. The primary outcome of change in kyphosis was assessed between baseline and 6 months using the gold standard, the Cobb angle derived from standing lateral spine radiographs, and a standardized protocol for measurement of thoracic kyphosis (T4–T12) (Fig. 2) [26]. Participants stood barefoot with knees straight and arms supported at 90° of flexion; they were instructed to hold full inhalation for the duration of the scan. Measurements were made by a trained radiologist (BF) who read the radiographs paired by participant but blinded to group allocation. A greater Cobb angle indicates more kyphosis severity. Test-retest reliability for repeated measurement of Cobb angle from the same radiograph was estimated as ICC = 0.90. Standard error of the measurement was estimated as 1.4°.
Cobb angle of kyphosis (57°) measured from the intersection of lines drawn from the superior endplate of T4 and the inferior endplate of T12 Line a is drawn from the superior endplate of T4, line b is drawn from the inferior endplate of T12, and lines c and d are perpendicular lines drawn from lines a and b. Cobb angle is where lines c and d intersect
Secondary outcomes: change in kyphometer-derived kyphosis, physical function, spinal extensor muscle strength and density, and HRQoL
All secondary outcomes that included change in kyphometer-derived kyphosis, physical function, spinal extensor muscle strength and density, and HRQoL were assessed between baseline and 6 months in both groups. Clinical measurements were made by a trained staff member at the UCSF Clinical and Translational Science Institute who was blinded to group allocation. The Debrunner kyphometer (Techmedica Inc., Camarillo, CA) was used for an external measurement of kyphosis using the T2/3 spinous process interspace as the superior landmark and T11/T12 spinous process interspace as the inferior landmark. The modified Physical Performance Test (modified PPT) [27] included seven timed standardized tasks: 50-ft floor walk, putting on and removing a laboratory coat, picking up a penny from the floor, standing up five times from a 41-cm-high chair without the use of one’s arms, lifting a 7-lb. book to a shelf, climbing one flight of stairs, and standing with feet together, as well as two additional untimed tasks: climbing up and down four flights of stairs and performing a 360° turn. A timed walk was administered over a 4-m marked course and gait speed (m/s) was calculated [28]. The Timed Up and Go test (TUG) measured the time in seconds to rise from a 41-cm-height armchair, walk 3 m, turn, and return to a fully seated position in the chair [29]. Timed Loading Standing measured the time in seconds that a participant was able to stand while holding a 2-lb. dumbbell in each hand with the arms at 90° of shoulder flexion and the elbows extended [30]. The 6-Min Walk test measured the distance in meters covered while walking in a long hallway for 6 min [31]. To monitor activity throughout the intervention, physical activity level was measured using the Physical Activity Scale for the Elderly (PASE) questionnaire [32] and step count was collected with an Omron step counter for 7 days before each testing visit. Finally, participants completed the Scoliosis Research Society (SRS-30) instrument, self-image domain [33], and the PROMIS global health (with mental and physical health components) and physical function quality-of-life questionnaires [34].
Complementing the physical function measures, isometric spinal extensor muscle strength and CT paraspinal muscle density were assessed. We used a standardized protocol for isometric spinal extensor muscle strength [17] with the Biodex 3 (Biodex Medical Systems Inc.) computerized dynamometer and the spine attachment (RSI Systems, Boulder, CO) and determined peak torque normalized to body weight. We acquired a single-slice computed tomography scan at L4–L5, and an experienced reader (BA) processed trunk muscle contours of the paraspinal extensor muscles including the erector spinae and transversospinalis. Scans were analyzed in Analyze 12.0 (Analyze, Biomedical Imaging Resource, Rochester, MN) for cross-sectional area (mm2) and density (Hounsfield units (HU)) and averaged left and right paraspinal muscles.
At 12 months, the active group completed an additional study visit where all kyphosis, physical function, and HRQoL measurements were repeated.
Other measures
At baseline, we measured height and weight using standard methods, and calculated body mass index (BMI). Bone mineral density of the hip and spine was measured using the GE Lunar Prodigy Dual X-ray Absorptiometer. An experienced radiologist (BF) assessed prevalent vertebral fractures in T4 to L4 vertebrae from baseline standing lateral spine radiographs using the Genant semiquantitative (SQ) method grading fractures 0–3, where 0 = none (normal), 1 = mild, 2 = moderate, and 3 = severe [35]. We defined a prevalent vertebral fracture as SQ ≥2. Another experienced radiologist (LN) read the radiographs and determined presence of diffuse idiopathic skeletal hyperostosis (DISH) from T4 to L4 using the Resnick criteria [36].
Adverse events were monitored by the study coordinator, who administered a standardized questionnaire on a weekly basis in the active group and in short monthly phone interviews in the control group. We documented any pain using the visual analog pain scale [37] and occurrence of falls and other injuries. Events were recorded as occurring during or outside of a study visit and as preexisting or a new event.
Statistical methods
Baseline characteristics of the active and control groups were compared using t tests, Wilcoxon, chi-squared, and exact tests as appropriate. ANCOVA was used to assess effects of the intervention on changes from baseline to 6 months in the primary and secondary endpoints in an intention-to-treat analysis. The models included fixed effects for treatment, the baseline value of the outcome, and wave of recruitment. p values <0.05 were considered statistically significant. In sensitivity analyses, we used a Bonferroni correction for the 12 secondary endpoint comparisons. In exploratory subgroup analyses, we assessed differences in the treatment effect by baseline kyphosis (lower 3 vs upper quartile), sex (male vs female), presence of diffuse idiopathic skeletal hyperostosis (DISH) (yes vs no), age (<75 vs ≥75), number of comorbidities (0–1 vs ≥2), and number of baseline vertebral fractures (0–1 vs ≥2). In these analyses, we tested for interactions with p values <0.1 considered statistically significant.
Results
Subject characteristics and clinical variables
We screened 598 potentially eligible individuals, and 99 participants were enrolled in the study (Fig. 1). At baseline, average age was 70.6 years, range 60–88, and Cobb angle was 57.4 ± 12.5° (data not shown). Half of the study participants had two or more comorbidities (Table 1). Overall, average participant gait speed was 1.21 ± 0.21 m/s, average TUG was 7.7 ± 1.3 s, and average PPT was 33.3 ± 1.7 points. Eighteen percent of the total cohort was categorized as “mild frailty” (PPT ≤31) by the modified PPT (data not shown). Randomization assigned 51 participants to the active group and 48 to the control. Subject characteristics did not differ between groups at baseline, except that more participants in the active group compared to the control group had vertebral fractures (24 vs 8.5%, p = 0.04) and DISH (30 vs 15%, p = 0.09) (Tables 1 and 2). Mean bone mineral density (BMD) at the hip and spine were normal (t-scores ≥−1.0), and there were no differences in BMD between the groups, p = 0.96 for the hip and p = 0.82 for the spine.
Intervention adherence
In the active group, participants attended an average of 75 ± 23% of the 72 scheduled exercise classes, 72% completed the daily home program, and 98% completed the home program at least three or more days a week. Participants in the control group attended a mean 63.5 ± 29% of the four monthly health education classes.
Change in primary outcome: Cobb angle of kyphosis
There was a statistically significant between-group difference in mean change in Cobb angle of −3.0° (95% CI −5.2, −0.8), p < 0.009 (Table 3). Within the active group, Cobb angle decreased by 3.3° (95% CI −4.9, −1.7), compared to a decrease of 0.3° (95% CI −1.9, 1.2) among controls (Table 3).
The effects of treatment were greater (p = 0.05 for interaction) among participants without DISH (adjusted between-group difference in change in Cobb angle = −4.0°, 95% CI −6.7, −1.3, p = 0.004), as compared to the complementary subgroup with DISH (1.6°, 95% CI −2.2, 5.5, p = 0.37) (Fig. 3). Similarly, treatment effects on Cobb angle were larger (p = 0.08 for interaction) among participants ≥75 years old (−6.9°, 95% CI −11.3, −2.6, p = 0.004), as compared to younger participants (−2.0°, 95% CI −5.0, 0.6, p = 0.12). We found no statistically significant evidence for heterogeneity of the treatment effect as measured by change in Cobb angle by sex (not shown), prevalent vertebral fracture, or number of comorbidities (all p > 0.1).
Change in secondary outcomes: Kyphometer-derived kyphosis, spinal muscle strength and density, physical function, and HRQoL
There was a statistically significant between-group difference in mean change in kyphometer-derived kyphosis of −3.0 (95% CI −5.6, −0.3) (Table 3). Within the active group, kyphosis decreased by 3.8° (95% CI −5.7, −2.0), compared to a decrease of 0.9° (95% CI −2.8, 1.0) among controls (Table 3). There were no statistically significant between-group differences in change in spinal extensor muscle strength or density or any physical function outcomes, p > 0.05. Among the quality-of-life measures, there was a statistically significant 0.43 point (95% CI 0.24, 0.61) difference between groups in change in the self-image score, p < 0.001, favoring the active group. The SRS-30 result remained statistically significant after Bonferroni correction for 12 comparisons. There were no statistically significant differences between groups in the other quality-of-life measures, including PROMIS global health (physical and mental health components) and physical function scores.
In the treated group, we assessed stability between 6 and 12 months of the outcomes affected by treatment in the first 6 months of the study. Cobb angle increased 1.6° (95% CI 0.1, 3.1), and kyphometer-derived kyphosis and SRS-30 did not change significantly, 1.3° (95% CI −0.7, 3.4) and −0.12 (95% CI −0.26, 0.02) points, respectively.
Adverse events
There were no serious adverse events (death, life-threatening adverse experiences, or related inpatient hospitalization) and no reportable adverse events associated with the study in either group according to federal regulations and UCSF Institutional Review board criteria. There were numerous non-reportable events in both groups, which included pain and stiffness felt in muscles several hours to days after testing or exercise and resolved within an expected duration [38]. Thirty-seven active participants receiving the exercise intervention (72.5%) reported a total of 76 different non-reportable events, with 13 falls and 30 reports of musculoskeletal pain, and six participants reported both a fall and musculoskeletal pain. The majority of the musculoskeletal complaints (90%) were preexisting. Seventeen control participants (35.4%) reported 21 different non-reportable events including 8 falls and 12 reports of musculoskeletal pain. There were no significant group differences in mean pain score at baseline and 6 months, p > 0.05, and no between-group differences in change in pain at 6 months, p > 0.05.
Discussion
In our randomized clinical trial among 99 community-dwelling older adults, a 6-month three times a week kyphosis-specific spine-strengthening exercise program reduced radiographic Cobb angle of kyphosis by 3°, relative to controls who received monthly health education classes. Average kyphosis angle decreased within the active group and was stable in controls. Treatment effects on kyphometer-derived kyphosis were similar. Self-image, which is a domain of self-efficacy that reflects greater physical self-confidence, also improved in the active group relative to controls. However, we did not observe expected beneficial treatment effects on any measure of physical function or other measures of HRQoL.
On average, the Cobb angle progresses slowly with age, less than 1° a year [13], so that reducing kyphosis even a small amount may be important, particularly if the treatment effects are maintained over time. The change in kyphometer-derived kyphosis is consistent with previous studies reporting improvement in clinical measures of kyphosis after targeted spine strengthening [17, 39]. In addition, the 3° reduction in thoracic kyphosis appreciated in our study approximates the magnitude (but not the direction) of change that might be expected with an incident vertebral fracture [a 3.8° (95% CI 2.7, 4.8)] increase in kyphosis] [13]. The 0.43 point (95% CI 0.24, 0.61) improvement in the self-image domain of the SRS-30 is comparable to the change after surgery for scoliosis [33]. We note that self-image is a component of self-efficacy [40], which may have longer-term benefits and contribute to higher levels of physical functioning, physical performance, and exercise adherence in patients with arthritis and other chronic conditions [41].
The twofold greater prevalence of DISH in the active group may have resulted in an underestimation of the overall treatment effects on Cobb angle of kyphosis. The exercise intervention did not improve kyphosis among participants with DISH (n = 22), in contrast to the 4.0° difference in reduction in Cobb angle among those without it. DISH is a prevalent degenerative syndrome, observed more in older men than women, and is caused by calcification of the anterior longitudinal ligaments that attach to the spine [41]. DISH restricts spine mobility and reduced the effect of the exercise intervention in this subgroup. Similarly, Al-Herz et al. [42] reported a lack of spinal muscle strengthening in an exercise intervention that was designed specifically to improve mobility and strength in DISH subjects. The presence of DISH may have restricted the mobility in the spine and reduced the effect of the exercise intervention in this subgroup, similar to the limited response to an exercise intervention that was designed specifically to improve mobility for DISH [42]. Also, greater prevalence of vertebral fractures in the active group may have affected change in Cobb angle. Among those with two or more vertebral fractures (n = 16), there was a non-significant 2.1° increase in Cobb angle, although the confidence intervals were wide and the test for interaction was not significant (p = 0.15). We did find a more robust response to the intervention of −6.9° among participants 75 years and older (n = 19), in contrast to the −2.0° difference in change in Cobb angle among the younger participants aged 60–75 years (n = 80). Thus, participants who were older or without DISH and vertebral fractures appeared to respond better to the intervention, although the treatment effects were very imprecisely estimated among these small subgroups of participants. Additional studies are required to confirm or refute these observations in subjects with DISH, vertebral fractures, older age, and kyphosis.
Observational data have shown that hyperkyphosis is associated with impaired physical function [5, 6, 8, 9, 43, 44]. Moreover, in our earlier pilot trial [17], we reported improvements in physical function in the modified PPT among treated participants. These results suggested that an exercise program that reduced hyperkyphosis could have beneficial effects on physical function. However, our randomized controlled trial did not show statistically significant treatment benefits for any physical function outcome, and in many of the outcomes, the 95% confidence intervals of the active and controls overlapped. It is possible that a larger change in kyphosis is needed in order to have an effect on physical function. Additionally, we did not design the intervention to improve physical function per se, and it is also plausible that this explains why physical function did not change.
We hypothesized that change in spinal extensor muscle strength and/or muscle density would mediate the effect of the intervention on kyphosis, but we did not find a significant change in either spinal muscle strength or density. Mean baseline spinal extension peak torque to body weight was 72% in our SHEAF cohort at baseline, a measure of strength that was double what we reported at baseline in our pilot trial (35%). In the pilot trial, we observed a 21% increase in peak torque to body weight after the intervention, but it is possible that the SHEAF intervention was not of sufficient intensity to yield a meaningful improvement in strength or density, in part because participants were already strong at baseline. Instead, the observed kyphosis change may be due to the postural training that was provided, which may have improved spinal extensor muscle activation patterns that are known to be adversely affected in hyperkyphotic posture [22, 45]. However, we did not measure muscle activation during the study, and this may be worth investigating with electromyography in future trials. Treatment effects in Cobb angle of kyphosis that were observed after the 6-month intervention in the active group were attenuated at the 12-month study visit, whereas the treatment effects in kyphometer-derived kyphosis and self-image were maintained. It is likely that ongoing postural training practice may be necessary to maintain the improvement in Cobb angle.
Strength and limitations
Our study was powered to detect a fairly small difference in primary outcome, the gold standard Cobb angle of kyphosis derived from radiographs [26]. In addition, it included men, broadening its generalizability, and we found no evidence for modification of the treatment effect by sex. However, the principal limitation of the study is that the observed effect of treatment on the primary outcome over 6 months was small. Given that Cobb angle usually progresses slowly over time, future studies are needed to understand whether this intervention affects progression of kyphosis particularly among those with kyphosis >50° and vertebral fractures. Second, we did not find any changes in physical function. Our cohort was also extremely robust at baseline, with normal bone mineral density t-scores, and gait speed and TUG scores better than age-matched norms [46, 47], which could have impacted the effects of the intervention on physical function. Third, muscle quality was measured by computed tomography in the lumbar spine but we did not find a change in muscle density after the intervention. It is possible that the kyphosis-specific intervention had a positive effect on the muscle density in the thoracic spine, but this was not measured. Fourth, we were unable to disentangle possible effects of hands-on care from the exercise intervention itself given that there were more clinic visits for the treatment versus controls. In addition, the sample size was small-to-moderate; this is reflected in the confidence intervals for treatment effects on several of the secondary outcomes, which include substantial effects in both directions. Finally, now that we have demonstrated that Cobb angle is modifiable, next steps may be to explore less expensive exercise/postural training options.
Conclusions
A targeted spine-strengthening exercise and posture training program for 6 months reduced both radiographic and clinical measures of kyphosis in older women and men, compared to the controls. Participants who received the intervention also improved their self-image and satisfaction with their appearance. However, the intervention did not significantly improve secondary outcomes of physical function and other aspects of HRQoL. Moreover, there was no improvement in spinal muscle strength or density, which we hypothesized would mediate the effects of the targeted exercise on kyphosis. Nonetheless, even the narrower benefits that we observed on kyphosis and self-image suggest that a targeted spine-strengthening exercise with postural training should be considered as a viable and safe treatment option for older adults.
References
Takahashi T, Ishida K, Hirose D, Nagano Y, Okumiya K, Nishinaga M et al (2005) Trunk deformity is associated with a reduction in outdoor activities of daily living and life satisfaction in community-dwelling older people. Osteoporos Int 16(3):273–279. doi:10.1007/s00198-004-1669-3
Kado DM, Huang MH, Karlamangla AS, Barrett-Connor E, Greendale GA (2004) Hyperkyphotic posture predicts mortality in older community-dwelling men and women: a prospective study. J Am Geriatr Soc 52(10):1662–1667
Katzman W, Cawthon P, Hicks GE, Vittinghoff E, Shepherd J, Cauley JA et al (2011) Association of spinal muscle composition and prevalence of hyperkyphosis in healthy community-dwelling older men and women. J Gerontol A Biol Sci Med Sci. doi:10.1093/gerona/glr160
Kobayashi T, Atsuta Y, Matsuno T, Takeda N (2004) A longitudinal study of congruent sagittal spinal alignment in an adult cohort. Spine (Phila Pa 1976) 29(6):671–676
McDaniels-Davidson C, Davis A, Wing D, Nichols J, Kado D (2016) editors. Kyphosis, balance dynamics, and incident falls in community dwelling older adults. J Am Geriatr Soc
van der Jagt-Willems H, de Groot M, van Campen J, Lamoth C, Lems W (2015) Associations between vertebral fractures, increased thoracic kyphosis, a flexed posture and falls in older adults: a prospective cohort study. BMC Geriatr 15(1):34
Kado DM, Miller-Martinez D, Lui LY, Cawthon P, Katzman WB, Hillier TA et al (2014) Hyperkyphosis, kyphosis progression, and risk of non-spine fractures in older community dwelling women: the study of osteoporotic fractures (SOF). J Bone Miner Res 29(10):2210–2216. doi:10.1002/jbmr.2251
Kado DM, Huang MH, Barrett-Connor E, Greendale GA (2005) Hyperkyphotic posture and poor physical functional ability in older community-dwelling men and women: the Rancho Bernardo study. J Gerontol A Biol Sci Med Sci 60(5):633–637
Katzman WB, Harrison SL, Fink HA, Marshall LM, Orwoll E, Barrett-Connor E et al (2014) Physical function in older men with hyperkyphosis. J Gerontol A Biol Sci Med Sci. doi:10.1093/gerona/glu213
Katzman WB, Huang MH, Lane NE, Ensrud KE, Kado DM (2013) Kyphosis and decline in physical function over 15 years in older community-dwelling women: the Study of Osteoporotic Fractures. J Gerontol A Biol Sci Med Sci. doi:10.1093/gerona/glt009
Bayliss M, Miltenburger C, White M, Alvares L (2013) A conceptual and disease model framework for osteoporotic kyphosis. Osteoporos Int 24(9):2423–2432. doi:10.1007/s00198-013-2317-6
Hongo M, Miyakoshi N, Shimada Y, Sinaki M (2012) Association of spinal curve deformity and back extensor strength in elderly women with osteoporosis in Japan and the United States. Osteoporos Int 23(3):1029–1034. doi:10.1007/s00198-011-1624-z
Kado DM, Huang MH, Karlamangla AS, Cawthon P, Katzman W, Hillier TA et al (2013) Factors associated with kyphosis progression in older women: 15 years’ experience in the study of osteoporotic fractures. J Bone Miner Res 28(1):179–187. doi:10.1002/jbmr.1728
Katzman WB, Parimi N, Mansoori Z, Nardo L, Kado DM, Cawthon PM et al (2016) Cross-sectional and longitudinal associations of diffuse idiopathic skeletal hyperostosis (DISH) and thoracic kyphosis in older men and women. Arthritis Care Res. doi:10.1002/acr.23115
Katzman WB, Miller-Martinez D, Marshall LM, Lane NE, Kado DM (2014) Kyphosis and paraspinal muscle composition in older men: a cross-sectional study for the Osteoporotic Fractures in Men (MrOS) research group. BMC Musculoskelet Disord 15:19. doi:10.1186/1471-2474-15-19
Kado D, Browner W, Palmero L, Nevitt M, Genant H, Cummings S (1999) Vertebral fractures and mortality in older women. A prospective study. Arch Intern Med 159:1215–1220
Katzman WB, Sellmeyer DE, Stewart AL, Wanek L, Hamel KA (2007) Changes in flexed posture, musculoskeletal impairments, and physical performance after group exercise in community-dwelling older women. Arch Phys Med Rehabil 88(2):192–199. doi:10.1016/j.apmr.2006.10.033
Bansal S, Katzman WB, Giangregorio LM (2014) Exercise for improving age-related hyperkyphotic posture: a systematic review. Arch Phys Med Rehabil 95(1):129–140. doi:10.1016/j.apmr.2013.06.022
Borson S, Scanlan JM, Chen P, Ganguli M (2003) The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc 51(10):1451–1454
Katzman WB, Vittinghoff E, Kado DM, Schafer AL, Wong SS, Gladin A et al (2016) Study of hyperkyphosis, exercise and function (SHEAF) protocol of a randomized controlled trial of multimodal spine-strengthening exercise in older adults with hyperkyphosis. Phys Ther 96(3):371–381. doi:10.2522/ptj.20150171
Itoi E, Sinaki M (1994) Effect of back-strengthening exercise on posture in healthy women 49 to 65 years of age. Mayo Clin Proc 69(11):1054–1059
Greig AM, Briggs AM, Bennell KL, Hodges PW (2014) Trunk muscle activity is modified in osteoporotic vertebral fracture and thoracic kyphosis with potential consequences for vertebral health. PLoS One 9(10):e109515. doi:10.1371/journal.pone.0109515
Hinman MR (2004) Comparison of thoracic kyphosis and postural stiffness in younger and older women. Spine J 4(4):413–417
Pollock ML, Wenger NK (1998) Physical activity and exercise training in the elderly: a position paper from the Society of Geriatric Cardiology. Am J Geriatr Cardiol 7(4):45–46
Van Dillen LR, Norton BJ, Sahrmann SA, Evanoff BA, Harris-Hayes M, Holtzman GW et al (2016) Efficacy of classification-specific treatment and adherence on outcomes in people with chronic low back pain. A one-year follow-up, prospective, randomized, controlled clinical trial. Man Ther 24:52–64. doi:10.1016/j.math.2016.04.003
Lundon KM, Li AM, Bibershtein S (1998) Interrater and intrarater reliability in the measurement of kyphosis in postmenopausal women with osteoporosis. Spine 23(18):1978–1985
Reuben DB, Siu AL (1990) An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc 38(10):1105–1112
Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E et al (2003) Physical performance measures in the clinical setting. J Am Geriatr Soc 51(3):314–322
Podsiadlo D, Richardson S (1991) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39(2):142–148
Shipp KM, Purse JL, Gold DT, Pieper CF, Sloane R, Schenkman M et al (2000) Timed loaded standing: a measure of combined trunk and arm endurance suitable for people with vertebral osteoporosis. Osteoporos Int 11(11):914–922
Laboratories ATSCoPSfCPF (2002) ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166(1):111–117. doi:10.1164/ajrccm.166.1.at1102
Washburn RA, Smith KW, Jette AM, Janney CA (1993) The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46(2):153–162
Asher MA, Min Lai S, Burton DC (2000) Further development and validation of the Scoliosis Research Society (SRS) outcomes instrument. Spine (Phila Pa 1976) 25(18):2381–2386
Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D (2010) The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. Journal of applied measurement 11(3):304–314
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8(9):1137–1148
Resnick D, Shapiro RF, Wiesner KB, Niwayama G, Utsinger PD, Shaul SR (1978) Diffuse idiopathic skeletal hyperostosis (DISH) [ankylosing hyperostosis of Forestier and Rotes-Querol]. Semin Arthritis Rheum 7(3):153–187
Childs JD, Piva SR, Fritz JM (2005) Responsiveness of the numeric pain rating scale in patients with low back pain. Spine (Phila Pa 1976) 30(11):1331–1334
Armstrong RB (1984) Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc 16(6):529–538
Greendale GA, Huang MH, Karlamangla AS, Seeger L, Crawford S (2009) Yoga decreases kyphosis in senior women and men with adult-onset hyperkyphosis: results of a randomized controlled trial. J Am Geriatr Soc. doi:10.1111/j.1532-5415.2009.02391.x
Judge TA, Erez A, Bono JE, Thoresen CJ (2002) Are measures of self-esteem, neuroticism, locus of control, and generalized self-efficacy indicators of a common core construct? J Pers Soc Psychol 83(3):693–710
Strecher VJ, DeVellis BM, Becker MH, Rosenstock IM (1986) The role of self-efficacy in achieving health behavior change. Health Educ Q 13(1):73–92
Al-Herz A, Snip JP, Clark B, Esdaile JM (2008) Exercise therapy for patients with diffuse idiopathic skeletal hyperostosis. Clin Rheumatol 27(2):207–210. doi:10.1007/s10067-007-0693-z
Katzman WB, Huang MH, Lane NE, Ensrud KE, Kado DM (2013) Kyphosis and decline in physical function over 15 years in older community-dwelling women: the Study of Osteoporotic Fractures. J Gerontol A Biol Sci Med Sci 68(8):976–983. doi:10.1093/gerona/glt009
Lorbergs AL, O’Connor GT, Zhou Y, Travison TG, Kiel DP, Cupples LA et al (2016) Severity of kyphosis and decline in lung function: the Framingham study. J Gerontol A Biol Sci Med Sci. doi:10.1093/gerona/glw124
Solomonow M, Zhou BH, Baratta RV, Burger E (2003) Biomechanics and electromyography of a cumulative lumbar disorder: response to static flexion. Clin Biomech (Bristol, Avon) 18(10):890–898
Bohannon RW (2006) Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther. 29(2):64–68
Bohannon RW (2008) Population representative gait speed and its determinants. J Geriatr Phys Ther 31(2):49–52
Acknowledgments
This research was supported by the National Institute on Aging (NIA) RO1AG041921 and by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors wish to acknowledge the Data Safety Monitoring Board for their study oversight, Nicole King and Scott Puracchio for their assistance in data collection, and Lorenzo Nardo for data acquisition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study protocol was approved by the University of California San Francisco and Kaiser Permanente Northern California Institutional Review Boards.
Conflicts of interest
None.
Funding
This research was supported by the National Institute on Aging (NIA) RO1AG041921.
Additional information
D. M. Kado and N. E. Lane are joint senior authors.
Rights and permissions
About this article
Cite this article
Katzman, W.B., Vittinghoff, E., Lin, F. et al. Targeted spine strengthening exercise and posture training program to reduce hyperkyphosis in older adults: results from the study of hyperkyphosis, exercise, and function (SHEAF) randomized controlled trial. Osteoporos Int 28, 2831–2841 (2017). https://doi.org/10.1007/s00198-017-4109-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4109-x