Abstract
Summary
We tested the feasibility of a fracture prevention decision aid in an online patient portal. The decision aid was acceptable for patients and successfully decreased decisional conflict. This study suggests the possible utility of leveraging the patient portal to enhance patient education and decision making in osteoporosis care.
Introduction
Although interventions have improved osteoporosis screening and/or treatment for certain populations of high-risk patients, recent national studies suggest that large-scale uptake of these interventions has been limited. We aimed to determine the feasibility and potential efficacy of a patient portal-based osteoporosis decision aid (DA).
Methods
We conducted a pilot randomized controlled trial of primary care patients aged ≥55 who were enrolled in a patient portal and had a T-score of <−1. Intervention subjects were provided a link to a patient DA. The DA contained a 10-year fracture risk calculator, summary of medication risks and benefits (prescription and nonprescription), and an elicitation of values. Subjects completed questionnaires assessing the primary outcomes of decisional conflict and preparation for decision making and secondary outcomes related to feasibility and planning for a larger trial. Charts were reviewed for physician-subject interactions and medication uptake.
Results
The DA was acceptable to subjects, but 17 % of the patients in the decision aid arm incorrectly entered their T-scores into FRAX-based risk calculator. Decisional conflict was lower post-intervention for those who were randomized to the decision aid arm compared to controls (17.8 vs. 47.1, p < .001), and there was a significant difference in the percentage of patients who made a treatment decision at 3 months. No significant differences were observed in medication uptake.
Conclusions
A portal-based osteoporosis DA was acceptable and improved several measures of decision quality. Given its effect on improving the quality of patients’ decisions, future studies should examine whether it improves physician guideline adherence or medication adherence uptake among treated patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
US osteoporosis guidelines emphasize treatment based on personal fracture risk [1, 2], but many high-risk patient groups are actually less likely to have bone density testing [3] or receive treatment [4] than lower-risk patients. Only half of all patients started on medications are taking them after 1 year [5, 6], suggesting that patients also need further support to persist with their osteoporosis treatments. Although interventions have improved osteoporosis screening and/or treatment for certain populations of high-risk patients, recent national studies suggest that large-scale uptake of interventions to support high-quality care has not occurred [7–12].
Physicians and patients thus appear to need more effective tools to support high-quality targeted osteoporotic fracture prevention. Decision aids (DAs) emphasize shared decision making and include several features to support individualized treatment discussions like those needed for fracture prevention. DAs for a range of conditions have been shown to decrease decisional conflict, increase knowledge, and (when probabilities are included in the aid) improve the accuracy of risk perceptions [13]. In addition, they are increasingly web-based [14, 15], facilitating rapid updates and dissemination.
We hypothesized that a web-based decision aid delivered via an electronic health record (EHR)-based patient portal could improve key measures of patients’ preparation for decision making and decisional conflict in postmenopausal osteoporotic fracture prevention. Patient portals have become widespread [16] and offer the opportunity to deliver the decision aid before an office visit for at-home completion. EHR delivery has the potential advantages of reducing clinician office time (an important barrier to decision aid use [13]) while still providing patients assurance that the information comes from a trusted source. Current evidence for the efficacy of decision aids delivered via EHRs is limited but compelling, particularly a small randomized trial of an influenza immunization decision aid in a workplace-based EHR, which produced increases in beliefs in vaccine effectiveness and a low likelihood of side effects, as well as a nonsignificant but encouraging 25 % higher immunization rate. We conducted a pilot randomized controlled trial of our osteoporosis DA delivered via an EHR, with a goal of testing its potential efficacy in improving decision-making in osteoporotic fracture prevention (prescription and nonprescription) with minimal disruptions to care. We also examined important feasibility questions regarding design and procedures for a larger randomized trial.
Methods
Enrollment criteria and overview
Our study was targeted at any postmenopausal patients who had already been screened and found to have a T-score of <−1 or lower. Subjects were enrolled from three primary care (internal medicine and family medicine) clinics within a Midwestern multispecialty academic group practice between November 2013 and December 2014. Recruitment was stopped due to the end of the funding period. All English-speaking women who had a patient portal (Epic Systems Corporation MyChart ®) account were ≥55 years of age (to ensure postmenopausal state) and had a recent bone mineral density (BMD) test that indicated osteopenia or osteoporosis were eligible to participate. Individuals with any dementia/cognitive impairments and/or less than 1-year life expectancy were excluded.
Design
Randomization was stratified by diagnosis (osteoporosis or osteopenia) after consent and was blinded to the subject (Fig. 1 and Online Resource 1). Two predetermined block randomization schedules for osteoporosis and osteopenia were created using a computer random number generator and maintained electronically. The study coordinator was responsible for randomization and blinded to allocation until after consent was obtained. Participants in the experimental group received the decision aid, while those in the control group were directed to the National Institute on Aging homepage (www.nia.nih.gov) rather than the decision aid. This control site provided web-based information relevant to aging but not specific to osteoporosis. All study procedures were approved by the Medical College of Wisconsin Institutional Review Board, and informed consent was obtained from all individual participants included in the study.
Decision aid
The decision aid was created based on the publicly available decision aid titled “Healthy Bones” from the Agency for Healthcare Research and Quality (AHRQ) [14], which was designed to be used by patients who are considering osteoporosis screening. Since our study was targeted at postmenopausal patients who had already been screened, key adaptations were made to better address this population. The primary adaptation was the development of a personalized fracture risk calculator utilizing the publicly available probabilities from the FRAX website [17] that utilizes bone density information (Fig. 2a), with more minor adaptations made to ensure that the decision aid meet International Patient Decision Aid Standards (IPDAS) [18] for prescription and nonprescription treatment decisions (e.g., values elicitation, see below).
The final decision aid included information about osteoporosis including causes, risk factors, “how to determine if you have osteoporosis” personalized fracture risk based on FRAX, details about medication and nonprescription treatment, and a values elicitation exercise related to the treatment decision. In this exercise, patients were asked to rate how much they agree with statements like “I think that the benefits of prescription medicines outweigh the risks or side effects,” or “I feel sure about my decision right now” (Fig. 2b). A medication table containing information about clinical trials, common side effects, and characteristics of the medication (including frequency and mode of administration; Fig. 2c), recommendations for getting more dietary calcium, and what forms of exercise are beneficial for bones was also included. Two print-outs were available at the end of the decision aid that contained extensive information about treatments and a personalized summary of risk information and values.
The adapted decision aid underwent initial testing with patients (N = 7) from an academic general internal medicine clinic. Participants were observed while being asked to verbalize their impressions about the presentation and usability of the website, and changes were made based on their feedback.
Procedure
All participants who met eligibility criteria were recruited either by physician referral through the patient portal when the physician was communicating bone density results or (when recruitment was slow by the initial method) by a mailed invitation from the study team targeted to occur within 2 weeks after bone density tests were performed. Study procedures were completed semi-independently over the internet. The research coordinator reviewed the informed consent form and study procedures with the patient by phone and beyond that was available by phone as needed. Outcomes were collected pre- and post-intervention and at 3 months using emailed invitations from an internet-based survey application. Data from the risk calculator and values sections of the decision aid were scanned into the participants’ EHR and their physicians were notified by email about the availability of the information. Any logistical problems with using the web-based decision aid were noted by the research coordinator to determine how difficult it was for patients to use the web-based decision aid. Patient charts were also reviewed to collect FRAX information to calculate 10-year total fracture risk (FRAX® score; Online Resource 2).
Outcomes
Primary outcomes
Decision quality
The primary outcomes for the study were measured using the Preparation for Decision-Making Scale [19] and the Decisional Conflict Scale (DCS) [20]. The 10-item Preparation for Decision Making Scale developed by the Ottawa Hospital Research Institute (scores range from 0 to 100) [19] was measured immediately post-intervention. The DCS includes subscales that assess if patients feel informed, are clear on their values related to the decision, have enough support, and are uncertain about their decision. As in previous studies [13], the DCS was measured at baseline and post-intervention. To assess durability of response, we measured the DCS again at 3 months.
Feasibility
Throughout the course of the study, we assessed several aspects of the design and procedure to inform a future trial, with particular focus on feasibility of patients completing FRAX, including using BMD results. Physicians also completed a questionnaire at 3 months for each individual patient to assess the effect of the decision aid on the length of visit and physician/clinic staff workload. Physician-subject contact regarding osteoporosis or osteopenia was assessed at 6 months from chart review.
Secondary outcomes
Treatment decisions
Secondary outcomes included items post-intervention and at 3 months regarding whether the subject made any decision about prescription and nonprescription strategies to prevent osteoporotic fracture. In addition, if a subject reported making a decision at 3 months, she was also asked to indicate if this decision included taking prescription medications (osteoporosis only), taking supplemental calcium and/or vitamin D, or making lifestyle changes (participants could choose all three options). Details of prescription medication use were also evaluated by chart review at 6 months in the osteoporosis subgroup.
Shared decision making
Patient-reported shared decision making was evaluated at 3 months using four yes/no items adapted by Fowler [21] from the DECISIONS study by the University of Michigan [22]. These items assessed patient perceptions of any follow-up discussions with a primary care physician, including whether the subject was provided with alternative treatment options, discussed reasons for and against taking medication, and was asked what she wanted to do regarding treatment. This instrument is scored by assigning 1 point for every yes, with a maximum total score of 4 indicating the highest level of shared decision making.
Analysis
Decisional conflict and knowledge were compared using one-way ANOVA tests with a significance level of p < .05 at baseline, post-intervention, and at 3 months. Additional outcomes were compared using a two-sided student’s t-test and Chi-square analysis as appropriate for the full study cohort and the osteoporosis group, all with a significance level of p < .05. Results for each subscale were similar to the overall DCS score and are not reported here. Where appropriate, change between baseline and post-intervention and baseline and 3-month follow-up were analyzed using a two-sided student’s t-test. These analyses, which had similar findings, provide a more conservative estimate of the effect and are reported in the appendix (Online Resource 3). All analyses were computed using IBM SPSS Statistics.
Subgroup analyses
In order to account for the variability of participant risk and experience with treatment, in addition to pre-planned analyses for the osteoporosis subgroup, secondary post hoc analyses were conducted based on National Osteoporosis Foundation (NOF) guideline treatment recommendations [1] and prior experience with treatment. These included those (a) diagnosed with osteopenia with FRAX ≥ 20 or osteoporosis [1], (b) with no prior bisphosphonate use, (c) with no bisphosphonate use at the time of randomization, and (d) a combination of those with osteopenia with FRAX ≥ 20 or osteoporosis and no prior or current bisphosphonate use. These post hoc subgroup analyses are shown only in the appendix (Online Resource 4).
Results
Fifty patients from 18 primary care physicians were enrolled in the study. Participants had a median age of 79 years; 96 % were non-Hispanic white; 86 % had attended at least some college. Over 89 % had at least one fracture risk factor other than age and low BMD, and 38 % had a BMD T-score of ≤ − 2.5 (osteoporosis). Additional subject characteristics are reported in Table 1. All participants completed follow-up procedures.
Primary outcomes
Decision quality
Preparation for decision making
Subjects in the decision aid arm reported being more prepared for making decisions about their treatment (mean = 68.1, SD = 23.4) than controls (mean = 39.0, SD = 29.4) on the Preparation for Decision Making Scale (p < .001) (Table 2). Although scores were also higher in the decision aid arm among the osteoporosis subgroup (N = 19), the difference was smaller and not statistically significant [62.9 (SD = 28.6) vs. 43.3 (SD = 25.6), p = .172]. More details on responses to each item in this scale are available in the appendix (Online Resource 5).
Decisional conflict
Decisional conflict scores was significantly lower post-intervention for those who were randomized to the decision aid arm compared to controls (17.8 vs. 47.1, p < .001). Lower decisional conflict in the decision aid arm persisted at 3 months but was no longer significant (11.2 vs. 25.5, p = .078) (Table 2 and Fig. 3). Among subjects with osteoporosis, decisional conflict was also lower in the decision aid arm relative to controls post-intervention, but these differences did not reach statistical significance immediately post-intervention (18.9 vs. 43.3, p = .063) or at 3 months (16.5 vs. 30.8, p = .367) (Fig. 3).
Decisional conflict scores in main cohort and osteoporosis subgroup. This figure compares decisional conflict between decision aid and control arms at baseline, post-intervention (or control website), and at 3 months based on the Decisional Conflict Scale [23] in the full cohort and in a subgroup of patients diagnosed with osteoporosis. The asterisk indicates a significant result
Feasibility of a larger portal-based decision aid randomized controlled trial
Technical aspects of use
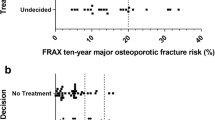
Although five (17.2 %) of decision aid arm participants needed initial troubleshooting guidance when the link to the decision aid did not work with their default browser, all participants ultimately completed all sections of the web-based decision aid with minimal assistance. No phone calls were made to the research staff for assistance related to other technical issues. However, five (17.2 %) subjects were initially provided an underestimate of their FRAX score because they did not include the negative sign in their femoral neck T-score for the risk calculator. Subjects were contacted and this was corrected by the research team.
Timing of decision aid use
Although all participants received their BMD results by electronic message within the patient portal, 7 (14 %; 5 intervention, 2 controls) were asked for further follow-up (i.e., to come in for office visit or call the clinic). At 3 months, 26 % of the cohort had an office visit with their primary care physician or a specialty physician in which a fracture prevention discussion was documented in the EHR. The proportion of patients with office visits was higher in the decision aid arm vs. control arm, but this difference was not statistically significant (27.6 vs. 23.8 %, p = .484).
Physician-reported experience
In physician survey responses (response rate = 44 %), 76.5 % reported that there were no changes in physician workload, 11.8 % reported that the decision aid decreased workload, and 11.8 % of respondents indicated that the decision aid affected their workload but did not specify if workload increased or decreased. Given the small proportion of patients who returned to the office prior to the 3-month assessment, planned formal analyses regarding whether the DA changed the perceived length of visit were not performed.
Secondary outcomes
Treatment decisions
Post-intervention 62.1 % of the decision aid arm and 40.0 % of the control arm reported having made a decision about prescription and/or nonprescription treatment for fracture prevention (p = .128). At 3 months, those in the decision aid arm were significantly more likely to report having made a decision (82.8 vs. 57.1 %, p = .046) (Table 2). Of those who reported making a decision, there were no differences by experimental arm regarding decisions to make lifestyle changes, although there may have been a trend toward decisions to increase supplemental vitamin D or calcium uptake (Table 2). There were also no significant differences in prescription medication use in the osteoporosis subgroup, though there was less medication uptake in the decision aid arm based on self-report and chart review (self-report 23.1 vs. 50.0 %, p = .241; chart review 15.4 vs. 50.0 %, p = .111).
Shared decision making
Shared decision making was assessed at 3 months. Although shared decision making scores were higher in the decision aid arm, these differences were not statistically significant in the cohort overall [Table 2; 3.19 (SD = 1.2) vs. 2.91 (SD = 1.3), p = .566] or in the osteoporosis subgroup [Table 2; 2.67 (SD = 1.5) vs. 2.25 (SD = 0.5), p = .614].
Discussion
In this pilot randomized trial, a patient portal-based decision aid for fracture prevention also improved patients’ preparation for decision making, decreased decisional conflict immediately post-intervention, and increased the likelihood that patients reported making a decision about how to prevent osteoporotic fractures. The decision aid was also acceptable to patients and feasible for them to use. These improvements in decision making occurred with little evidence of increased physician workload.
Our results regarding both increases in the percentage of patients who made a decision and improvements in decisional conflict were consistent with a systematic review of decision aids across a number of conditions [13] and extend those findings to a portal-based aid. To our knowledge, this was the first decision aid in fracture prevention to show a difference in decisional conflict with a decision aid. Decisional conflict and preparation for decision making are commonly used measures of decisional quality [13, 18] which reflect patients’ understanding of important aspects of the decision they are facing. At 3 months, we also found that an increased number of patients actually made a decision in the decision aid arm. Furthermore, the possible trend toward decisions to increase uptake of calcium and vitamin D deserves future study.
Although there were differences in other secondary outcomes of nonprescription medication uptake and intentions to take nonprescription medications between decision aid and control arms, these differences did not reach statistical significance. Furthermore, the effect upon prescription treatment decisions was not statistically significant among the osteoporosis subgroup was reversed (though not statistically so). While decision aids were initially developed for particularly value-sensitive decisions such as total joint replacement (where the decision may hinge on the value the patient places on risk of surgery vs. chronic pain), they have also been applied to preventive care, particularly to preventive care decisions that involve personalized risk estimates. Guidelines by the National Osteoporosis Foundation and several international groups recommend that shared decision making be part of standard treatment [1, 2], but as the results of this and other studies [24] suggest, patients either place larger value on avoiding medications or less value on fracture reduction than their physicians do. Therefore, even when the physician and patient see similar outcomes data, they may interpret the information presented differently. Future qualitative studies as well as studies in larger cohorts could provide insight into which values might be driving patients’ decisions and evaluate whether the aid improves the match between values and decisions. They could also examine further questions such as whether adding framing messages to reduce unrealistic expectations (e.g., “this medication reduces fractures by about the same amount as cholesterol medications reduce heart attacks”) to an osteoporosis decision aid could increase guideline adherence.
Although the higher scores for our secondary outcomes of shared decision making in the decision aid arm in our pilot study were not statistically significant, our finding of greater shared decision making in the decision aid arm is consistent with another study that used audiotapes to assess communication and found significant improvement in measures of shared decision making after an in-office osteoporosis decision aid [25]. Since perceptions of increased length of office visits with decision aids are important barriers to decision aids outside of trials [13], a larger study is needed to determine whether our portal-based decision aid (which addresses this barrier) can still support shared decision making.
Our study also showed the feasibility of several aspects for a larger randomized controlled trial like providing older patients with patient portal-based decision aids, as patients were able to navigate the electronic decision aid with minimal assistance. However, the current study suggests that for osteoporosis decision aids specifically, patients may not be able to appropriately use their BMD score in the FRAX calculator without some assistance. Unless automated entry of test results can be incorporated into portal-based decision aids, it may be necessary in future trials—or in actual office practice—to develop a hybrid protocol where patients complete the decision aid outside the physician office, but more technical sections are completed with assistance from office staff or the physician. Further, recruiting and providing the decision aid “just in time” when bone density results are sent to the patient may not be the most effective timing for recruitment in a larger trial unless bone density tests can be scheduled prior to a preventive visit. Many patients in our study did not have a formal office visit with their physician and did not have the opportunity to discuss the decision aid. A future randomized controlled trial will need to ensure that patients return to see their physician during the study period perhaps by recruiting after the return visit has been set-up in order to get a better reading on the impact of the portal-based decision aid on shared decision making and physician workload.
This study has limitations. First, this study was underpowered for treatment decisions, limiting the power to detect differences between groups, which may have prevented statistically significant results like shared decision making at 3 months and durability of results for decisional conflict. However, a strength of our study was the repeated assessment of decisional conflict scores over time. Second, neither patients nor physicians could be adequately blinded to their treatment arm. Third, our sample of patients included some with prior treatment experience or FRAX scores that did not reach guideline recommendations. Given the high rate of discontinuation of osteoporosis medications and nonprescription treatments, we believe these more inclusive criteria for use of the decision aid are appropriate, but a larger study could focus on guideline-appropriate treatments for specific subgroups. Fourth, the decision aid included only total fracture risk because of programming costs, though in the USA, guidelines utilize hip fracture risk as well. Finally, the cohort was rather homogenous and limited to one health system, and the decision aid should be studied in other settings. Despite these limitations, we were able to demonstrate a significant difference in DCS after the intervention, supporting the value of this decision aid in improving decision quality regarding osteoporosis treatment.
In conclusion, a patient portal-based decision aid was effective at decreasing decisional conflict, preparing patients to make a decision on how to prevent fractures and at increasing patients’ self-reported decision making. The promising results of this pilot study provide important evidence of the feasibility of conducting a larger randomized controlled trial of a portal-based aid in osteoporosis and support the need for larger studies of its impact on patient care.
References
National Osteoporosis Foundation (2014) Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation, Washington D.C
Watts NB, Bilezikian JP, Camacho PM, Greenspan SL, Harris ST, Hodgson SF, Kleerekoper M, Luckey MM, McClung MR, Pollack RP, Petak SM, Osteoporosis Task Force AACE (2010) American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 16(Suppl 3):1–37. doi:10.4158/EP.16.S3.1
Neuner JM, Binkley N, Sparapani RA, Laud PW, Nattinger AB (2006) Bone density testing in older women and its association with patient age. J Am Geriatr Soc 54(3):485–489. doi:10.1111/j.1532-5415.2005.00622.x
Andrade SE, Majumdar SR, Chan KA, Buist DS, Go AS, Goodman M, Smith DH, Platt R, Gurwitz JH (2003) Low frequency of treatment of osteoporosis among postmenopausal women following a fracture. Arch Intern Med 163(17):2052–2057. doi:10.1001/archinte.163.17.2052
Lo J, Pressman A, Omar M, Ettinger B (2006) Persistence with weekly alendronate therapy among postmenopausal women. Osteoporosis Int 17(6):922–928. doi:10.1007/s00198-006-0085-2
Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ (2007) Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc 82(12):1493–1501. doi:10.1016/s0025-6196(11)61093-8
Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD (2014) Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res 29(9):1929–1937. doi:10.1002/jbmr.2202
Balasubramanian A, Tosi LL, Lane JM, Dirschl DR, Ho P-R, O’Malley CD (2014) Declining rates of osteoporosis management following fragility fractures in the U.S., 2000 through 2009. J Bone Joint Surg 96(7):e52. doi:10.2106/jbjs.l.01781
Saag KG, Gehlbach SH, Curtis JR, Youket TE, Worley K, Lange JL (2006) Trends in prevention of glucocorticoid-induced osteoporosis. J Rheum 33(8):1651–1657
Kamel H, Hussain M, Tariq S, Perry HI, Morley J (2000) Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med 109:326–328. doi:10.1016/S0002-9343(00)00457-5
Neuner JM, Zhang X, Sparapani R, Laud PW, Nattinger AB (2007) Racial and socioeconomic disparities in bone density testing before and after hip fracture. J Gen Intern Med 22:123–145. doi:10.1007/s11606-007-0217-1
Neuner JM, Zimmer JK, Hamel MB (2003) Diagnosis and treatment of osteoporosis in patients with vertebral compression fractures. J Am Geriatr Soc 51(4):483–491. doi:10.1046/j.1532-5415.2003.51156.x
Stacey D, Légaré F, Col NF, Bennett CL, Barry MJ, Eden KB, Holmes-Rovner M, Llewellyn-Thomas H, Lyddiatt A, Thomson R (2014) Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev (1). doi:10.1002/14651858.CD001431.pub4
Agency for Healthcare Research and Quality (2012) Healthy bones: a decision aid for women after menopause. http://www.effectivehealthcare.ahrq.gov/ehc/decisionaids/osteoporosis/. Accessed: 2013
Ottawa Hospital Research Institute (2011) Osteoporosis: Should I have a dual x-ray absorptiometry (DEXA) test? http://204.187.39.28/AZsumm.php?ID=1128. Accessed February 2013
Neuner J, Fedders M, Caravella M, Bradford L, Schapira M (2015) Meaningful use and the patient portal: patient enrollment, use, and satisfaction with patient portals at a later-adopting center. Am J Med Qual 30(2):105–113. doi:10.1177/1062860614523488
Charts to download (2011) World Health Organization Collaborating Centre for Metabolic Bone Diseases. http://www.shef.ac.uk/FRAX/charts.aspx. Accessed 2013
Elwyn G, O’Connor A, Stacey D, Volk R, Edwards A, Coulter A, Thomson R, Barratt A, Barry M, Bernstein S, Butow P, Clarke A, Entwistle V, Feldman-Stewart D, Holmes-Rovner M, Llewellyn-Thomas H, Moumjid N, Mulley A, Ruland C, Sepucha K, Sykes A, Whelan T (2006) Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. Brit Med J 333(7565):417. doi:10.1136/bmj.38926.629329.AE
Graham I, O’Connor A (1996) Preparation for decision making scale. http://decisionaid.ohri.ca/docs/develop/Tools/PrepDM.pdf. Accessed February 2013
O’Connor A (1993) User manual- decisional conflict scale. http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf. Accessed February 2013
Fowler FJ Jr, Gallagher PM, Drake KM, Sepucha KR (2013) Decision dissonance: evaluating an approach to measuring the quality of surgical decision making. Joint Comm J Quality Patient Saf 39(3):136–144
Zikmund-Fisher BJ, Couper MP, Singer E, Levin CA, Fowler FJ, Ziniel S, Ubel PA, Fagerlin A (2010) The DECISIONS study: a nationwide survey of United States adults regarding 9 common medical decisions. Med Decis Mak 30(5 suppl):20S–34S. doi:10.1177/0272989x09353792
O’Connor AM (1995) Validation of a decisional conflict scale. Med Decis Mak 15(1):25–30. doi:10.1177/0272989X9501500105
Neuner JM, Schapira MM (2014) Patient perceptions of osteoporosis treatment thresholds. J Rheumatol 41(3):516–522. doi:10.3899/jrheum.130548
Montori VM, Shah ND, Pencille LJ, Branda ME, Van Houten HK, Swiglo BA, Kesman RL, Tulledge-Scheitel SM, Jaeger TM, Johnson RE, Bartel GA, Melton Iii LJ, Wermers RA (2011) Use of a decision aid to improve treatment decisions in osteoporosis: the osteoporosis choice randomized trial. Am J Med 124(6):549–556. doi:10.1016/j.amjmed.2011.01.013
Acknowledgments
A special thanks to Glenn Bushee for his invaluable assistance in the development of the decision aid and to the Agency for Healthcare Research and Quality and staff at John M. Eisenberg Center for Clinical Decisions and Communications Science for making their decision aid publically available.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This study was funded by the Clinical and Translational Science Institute of Southeast Wisconsin (project number 5,520,204).
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Smallwood, A.J., Schapira, M.M., Fedders, M. et al. A pilot randomized controlled trial of a decision aid with tailored fracture risk tool delivered via a patient portal. Osteoporos Int 28, 567–576 (2017). https://doi.org/10.1007/s00198-016-3767-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3767-4